Abstract
Enhancing the effectiveness of exercise for long-term body weight management and overall health benefits may be aided through complementary dietary strategies that help to control acute postexercise energy compensation. Inulin-type fructans (ITFs) have been shown to induce satiety through the modified secretion of appetite-regulating hormones. This study investigated the acute impact of oligofructose-enriched inulin (OI) consumption after exercise on objective and subjective measures of satiety and compensatory energy intake (EI). In a randomized crossover study, following the completion of a 45 min (65–70% VO2peak) evening exercise session, participants (BMI: 26.9 ± 3.5 kg/m2, Age: 26.8 ± 6.7 yrs) received one of two beverages: (1) sweetened milk (SM) or (2) sweetened milk + 20 g OI (SM+OI). Perceived measures of hunger were reduced in SM+OI relative to SM (p = 0.009). Within SM+OI, but not SM, plasma concentrations of GLP-1 and PYY were increased and acyl-ghrelin reduced from pre-exercise to postexercise. EI during the ad libitum breakfast in the morning postexercise tended to be lower in SM+OI (p = 0.087, d = 0.31). Gastrointestinal impacts of OI were apparent with increased ratings of flatulence (p = 0.026, d = 0.57) in participants the morning after the exercise session. Overall, the ingestion of a single dose of OI after an exercise session appears to induce subtle reductions in appetite, although the impact of these changes on acute and prolonged EI remains unclear.
1. Introduction
Over the past 3 decades, there has been a steady increase in adipose-related chronic disease [1,2]. Approximately 40% of the adult population employs personal weight control efforts to lower and manage body weight [3]. With the widespread belief that exercise is highly effective for weight loss [4], exercise is one of the most utilized strategies employed by individuals to control body weight [3]. Despite the increased energy expenditure induced with exercise, exercise training has not been shown to yield anticipated weight loss [5,6]. Based on measures of fat free and fat mass after long-term exercise interventions, weight loss that is achieved represents only a fraction of exercise-induced energy expenditure, suggesting that physical activity is associated with a high degree of energy compensation [7].
The mechanisms underlying energy compensation are not completely understood. Evidence suggests that energy compensation may be due to either a reduction in non-exercise activity thermogenesis (NEAT) or an increase in EI following exercise. Despite the reputed transient anorectic effects associated with a bout of exercise [8], long-term exercise interventions paradoxically result in increased appetite and hunger with exercise training. Myers et al. observed that after a 12-week exercise intervention in 24 women with overweight, total energy intake and pre-meal hunger were significantly increased [6]. Based on the level of energy expenditure from the exercise sessions and anticipated weight reduction in response to the energy expenditure, energy compensation limited weight reduction to only 22% of expected values.
With evidence of compensatory EI following exercise, there is current interest in strategies that assist with postexercise appetite management. Research has demonstrated that the consumption of prebiotics, specifically inulin-type fructans (ITF), have the potential to induce satiety through enhanced secretion of glucagon-like peptide-1 (GLP-1) and peptide YY(PYY) as well as reduced secretion of orexigenic hormone acyl-ghrelin [9]. The release of GLP-1 and PYY from enteroendocrine L-cells in the distal gut can induce satiety through activation of appetite-suppressing regions within the arcuate nucleus of the hypothalamus [10]. In contrast, acyl-ghrelin, released from gastric cells, increases neuropeptides within the hypothalamic arcuate nucleus that stimulate appetite. This action is mediated through vagal–brainstem–hypothalamic signaling or direct activation through movement of hormones across the incomplete blood–brain barrier via the median eminence [10]. ITFs are a fermentable fibre consisting of linear fructans linked with β1–2 bonds. Inulin, commonly derived from chicory, has a degree of polymerization (DP) between 2 and 60, whereas oligofructose is a shorter chain molecule with a DP < 10 [9]. As evidenced by acute increases in breath hydrogen shortly after ingestion, fermentation of inulin is initiated within hours of consumption [11]. Oligofructose, being a smaller molecule with a lower DP relative to inulin, is purported to undergo a more rapid fermentation within the GI tract [9]. The rapid fermentation of ITFs increases the production of SCFAs, which are key molecules linked to the secretion of gut-derived, satiety signals [12,13,14,15,16,17,18].
To date, there remains limited research investigating the acute impact of ITFs on satiety hormone secretion and energy intake during the postexercise period. Given the appetite-suppressing qualities of ITFs, there is a potential role for these dietary agents to help manage postexercise appetite. The objective of this study was to assess the acute influence of oligofructose-enriched inulin (OI) consumption after the completion of a 45 min session of moderate to vigorous exercise on appetite-regulating hormones and self-reported measures of appetite over a 12 h postexercise period in adults.
2. Materials and Methods
2.1. Participants and Ethical Approval
A total of 14 participants (8 male and 6 female) between the ages of 18–50 were recruited from the University of Lethbridge and the surrounding area. Eligibility was assessed using a screening questionnaire and interview. All participants completed the physical activity readiness questionnaire plus (PAR-Q+) to determine their ability to complete the exercise sessions [19]. If a prospective participant answered ‘yes’ to any of the questions in Section 1 and Section 2 of the PAR-Q+, participation in the study was not permitted. Recreational physical activity was assessed using a modified Godin’s leisure time exercise questionnaire (GLTEQ) [20]. To ensure that participants were accustomed to regular exercise, recreational activity was set as a minimum of 3 sessions of moderate to vigorous activity a week to the minimum recommended 150 min per week. Exclusion criteria included smoking, taking medication that could influence appetite, consumption of prebiotic or probiotic supplements, lactose intolerance, irritable bowel syndrome, and restrictive eating behaviour. Restrictive eating behaviour was assessed using the Three-Factor Eating Questionnaire Revised 18-item (TFEQ-R18) [21,22,23]. To control for appetite fluctuations throughout the menstrual cycle [24,25], female participants were tested in the early follicular phase (day 1–10), based on self-reported menstruation. All participants provided written informed consent prior to participating in the study. Ethical approval was obtained by the University of Lethbridge Human Research Committee and was conducted in accordance with the ethical principles of the 1964 Declaration of Helsinki. The results from the control session used in this study have been reported previously [26].
2.2. Baseline Testing
For 24 h prior to baseline testing, participants were requested to refrain from alcohol, caffeine, and moderate to vigorous exercise. Height (nearest 0.1 cm) and weight (nearest 0.1 kg) were measured using a mechanical beam scale (Heath-o-Meter Professional, McCook, IL, USA). An incremental test to exhaustion on an electromagnetically braked cycle ergometer (Velotron, QUARQ, Spearfish, SD, USA) was used to assess peak oxygen uptake (VO2peak). Following a 5 min warm-up at a self-selected pace, participants initiated the test at 50 W for 4 min at 80 rpm. After 4 min, the power output was increased by 25 W/min for females or 30 W/min for males. The test was terminated when participants reached volitional failure or could no longer maintain 80 rpm. Oxygen consumption was assessed through breath-by-breath analysis using a metabolic cart (Quark CPET, COSMED, Chicago, IL, USA). Heart rate was measured continuously throughout the test by a Garmin heart rate monitor (HRM-Dual, Garmin, Olathe, KS, USA). At the end of the VO2peak test, VO2peak was determined to be the greatest 30 s rolling average.
2.3. Experimental Conditions
In random order, participants completed two experimental sessions. Participants were advised to arrive at the laboratory at ~1900 h. Both sessions were performed at the same time of day. Each session included a standardized 45 min evening exercise session at 65–70% of VO2peak on the cycling ergometer. VO2 was spot monitored during each session using the metabolic cart. Immediately after the completion of the exercise session, participants received a beverage of either (1) sweetened milk (SM) (Vanilla Vibe, Milk2Go, QC, Canada) or (2) SM with 20 g of a 50/50 mixture of oligofructose and inulin (SM+OI) (Prebiotin, Jackson GI Medical, PA, USA). For each session, the volume of SM was 10 mL/kg body weight, providing an average 495 ± 25 kcals. A 1–4 week washout period was provided between all trials. Female participants completed both experimental sessions during the early follicular phase of the menstrual cycle. All participants were asked to follow the same meal and activity patterns for the 24 h leading into the exercise sessions. Food intake was assessed using three-day food records starting the morning of the exercise session day. Participants were free to leave the laboratory after completion of the 1 h postexercise bloodwork and requested to return to the laboratory the following morning. Apart from water consumption, no food or beverage was permitted during the time period away from the laboratory. The following morning, participants reported to the laboratory around the time they would typically consume their first meal. After confirming that participants maintained the fast throughout the evening, participants received an ad libitum buffet breakfast meal consisting of instant oatmeal, orange juice, and coffee or black tea with 18% cream and/or sugar. Participants were told to consume as much or as little as they would like until they were satisfied. The meal was provided in a distraction-free office. Post-meal, all remaining food was weighed in order to calculate food consumed and EI. Venous blood samples were collected via venipuncture from the antecubital area at pre-exercise (PRE-EX), 1 h post-drink (POST-EX), and the following morning in a fasted state (PRE-BFST). Appetite and gastrointestinal perceptions were assessed using a visual analogue scale (VAS) at PRE-EX, POST-EX, PRE-BFST, and post ad libitum meal (POST-BFST). An overview of the timeline for each experimental condition is shown in Figure 1.
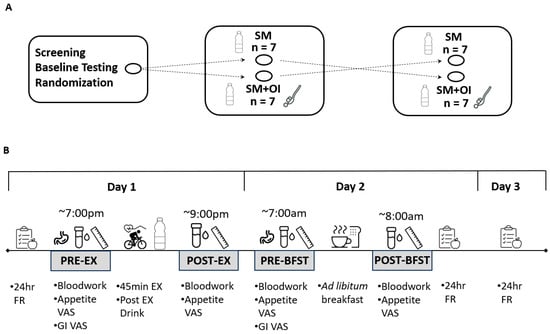
Figure 1.
Study design. (A) Crossover experimental sessions, separated by 1–4 weeks. (B) Experimental protocol for each session. GI: Gastrointestinal; FR: food record; PRE-EX: pre-exercise; OI: oligofructose-enriched inulin; PRE-BFST: pre-breakfast; POST-BFST: post ad libitum breakfast; POST-EX; postexercise; SM: Sweetened Milk; VAS: Visual Analogue Scale.
2.4. Appetite Perceptions
Appetite perceptions were assessed using a validated 100 mm VAS [27]. Each category of appetite perceptions was anchored at 0 mm and 100 mm with opposing statements that answered each question. There were eight aspects of appetite assessed: hunger (anchored by “I am not hungry at all” and “I have never been more hungry”), satisfaction (anchored by “I am completely empty” and “I cannot eat another bite”), fullness (anchored by “Not at all full” and “Totally full”), and prospective food consumption (PFC) (anchored by “Nothing at all” and “A lot”). An additional measure of subjective appetite was calculated by taking the mean appetite rating using the 4 measures of appetite from the VAS (hunger, satisfaction, fullness, and PFC) through the composite satiety score (CSS) [28]. CSS was calculated using the equation: CSS (mm) = (satisfaction + fullness + (100 − PFC) + (100 − hunger))/4 [29]. Previous studies have utilized the CSS [28,29,30,31] as it provides a mean appetite rating of the 4 subjective measures of hunger, satisfaction, fullness, and PFC.
2.5. Gastrointestinal Perceptions
Gastrointestinal perceptions were assessed using a 100 mm VAS. This questionnaire assessed how gastrointestinal feelings surrounding 4 separate categories have, on average, felt over the past 12 h. Each category was anchored by opposing statements at 0 mm and 100 mm. Abdominal comfort was anchored by “Greatly improved” and “Greatly decreased”. Abdominal distention and Bloating, Flatulence or Passage of Gas, and Rumbling of your Stomach were all anchored by “No problem” and “Very Strong”.
2.6. Three-Day Food Diaries and Physical Activity
Three-day food diaries included the day of exercise and 2 days afterwards. Participants could track intake via an online resource (MyFitnessPal™) or by written food diaries. To ensure compliance, email and verbal reminders were implemented. Participants were given a digital food scale and instructions on completing a food diary to increase the accuracy of the diet records. Once finished participants brought their food diaries to the lab. Diaries were entered into FoodWorks 14 Software (The Nutritional Company, Long Valley, NJ, USA). EI from all three days for each condition was added together to examine the total energy intake (TEI). Weekly physical activity during the week of each experimental condition was evaluated using a modified GLTEQ.
2.7. Blood Collection and Analysis
Blood samples were collected into 6 mL EDTA vacutainers via venipuncture. Immediately after collection, a protease inhibitor cocktail containing dipeptidyl peptidase IV inhibitor (10 μL/mL blood; MilliporeSigma Corp., Burlington, MA, USA), sigma protease inhibitor (1 mg/mL blood; SigmaFast, MilliporeSigma Corp.) and pefabloc (1 mg/mL blood; MilliporeSigma Corp.) was added to the sample to prevent degradation of appetite-related hormones. Samples were centrifuged at 2000 rpm for 10 min at 4 °C. Plasma aliquots were stored at −80 °C for later analysis. The concentration of plasma hormones was determined using the Human PYY (Total) kits, High Sensitivity GLP-1 Active Chemiluminescent kits, and Human Ghrelin (Active) ELISA kits (MilliporeSigma Corp., Burlington, MA, USA). Inter- and intra-assay variations were 3.09 ± 3.29% and 2.39 ± 1.14% for PYY, 6.20 ± 6.71% and 6.75 ± 5.46% for GLP-1, and 2.73 ± 3.11% and 2.11 ± 1.50% for acyl-ghrelin. All samples were assayed in duplicate and samples from one participant session were analyzed in the same assay to minimize the effects of inter-assay variation.
2.8. Statistical Analysis
SPSS software v25.0 for Windows was used for data analysis. PD blood sample concentrations were normalized to BL values and analyzed as an absolute change in concentration. Differences in concentrations of the hormone levels, appetite perceptions, and gastrointestinal feelings across time were examined using two-way repeated-measures analysis of variance (ANOVA). When there was a condition x time interaction, pairwise comparisons were used to examine significant differences at individual time points. Within-condition differences were examined using a one-way RM-ANOVA followed by a post hoc Bonferroni analysis. Data was assessed for normality using the Shapiro–Wilk test. Cohen’s d [32] was calculated for all pairwise comparisons to examine the effect size. A small effect size is defined as <0.2, moderate effect size 0.20 ≥ 0.80, and large effect size > 0.80. Statistical significance was set at p < 0.05. All results are presented as mean ± standard deviation (SD). A sample size estimation of 14 was based on previously published studies using similar, postexercise research designs [33,34,35,36] and calculated in G*Power with input values of α = 5%, β = 80%, and effect size = 0.7 (moderate) using the repeated-measures ANOVA, between factor statistical test. The results from the control session used in this study have been reported previously [26].
3. Results
3.1. Participants
A total of 14 participants (8 male and 6 female) completed the study. Participants were 26.8 ± 6.7 years old with a BMI of 26.9 ± 3.5 kg/m2, height of 174.5 ± 11.1 cm, and weight of 82.5 ± 15.8 kg. Female participants were tested on day 6.6 ± 1.8 of the menstrual cycle. The average VO2peak was 37.3 ± 7.5 mL O2·kg−1·min−1, with the average exercise intensities being 68.0 ± 4.9% and 69.1 ± 3.7% for SM and SM+OI, respectively. Average restrained eating scores were 12.2 ± 3.9.
3.2. Energy Intake
In SM, there was a main effect of time for three-day energy intake (p = 0.001). On Day 2 (the day after exercise), SM consumed more energy than on Day 1 (p = 0.005; d = 0.96) and Day 3 (p = 0.021; d = 0.85) (Figure 2A). Within SM+OI, the difference between Day 1 and Day 2 was not significant (p = 0.099, d = 0.67). The ad libitum breakfast consumption difference between SM+OI and SM did not reach statistical significance (SM: 673 ± 227 kcal; SM+OI: 606 ± 205 kcal; p = 0.087; d = 0.31) (Figure 2B). Total EI remained the same between conditions (p = 0.667) (Figure 2C). Macronutrient distribution between the experimental conditions did not differ.
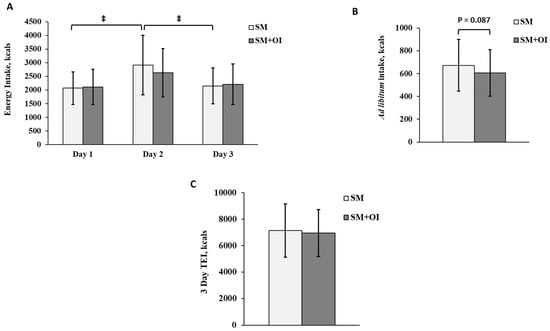
Figure 2.
(A) Daily energy intake over 3 days in the SM versus SM+OI: Day 1–day of exercise; (B) Ad libitum breakfast energy intake on Day 2; (C) total energy intake over 3 days. ‡: significant main effect of time for SM, where p < 0.050. Values are means ± standard deviation, n = 14. SM: Sweetened milk; SM+OI: Sweetened milk + oligofructose-enriched inulin; TEI: Total energy intake.
3.3. Appetite-Related Hormones
Baseline (PRE-EX) concentrations of GLP-1, PYY, and acyl-ghrelin did not differ between conditions (p < 0.050). There was a main effect of time for all hormones (p < 0.01) (Figure 3). Within SM+OI, concentrations of GLP-1 (p = 0.003; d = 0.88) and PYY (p = 0.050; d = 0.34) were greater at POST-EX compared to PRE-EX while the concentration of acyl-ghrelin (p = 0.003, d = 0.97) was reduced at POST-EX compared to PRE-EX. In contrast, the change between PRE-EX to POST-EX in SM was not significant for GLP-1 (p = 0.392, d = 0.44), PYY (p = 0.252, d = 0.27), and acyl-ghrelin (p = 0.061, d = 0.65). In both conditions, GLP-1 and PYY were reduced and acyl-ghrelin increased from PRE-EX to PRE-BFST as well as POST-EX to PRE-BFST (p < 0.050).
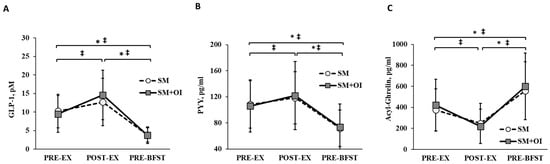
Figure 3.
Hormone concentrations throughout experimental sessions between SM and SM+OI. (A) GLP-1, (B) PYY, and (C) Acyl-Ghrelin. ‡: significant within condition difference for SM where p < 0.050. *: significant within condition difference for SM+OI where p < 0.050. Values are means ± standard deviation, n = 14. GLP-1: glucagon-like peptide-1; PRE-EX: pre-exercise session; PRE-BFST: pre-breakfast; POST-BFST: post ad libitum breakfast; POST-EX; postexercise; PYY: peptide YY; SM: Sweetened milk; SM+OI: Sweetened milk + oligofructose-enriched inulin.
3.4. Appetite Perceptions
Measures of hunger, satisfaction, fullness, PFC, and CSS did not differ at PRE-EX. There was a main effect of time for subjective measures of appetite (p < 0.001) (Figure 4). Condition affected the hunger rating, with SM+OI demonstrating an overall reduction in hunger relative to SM (p = 0.009) (Figure 4A). There was a trend towards a reduction in satisfaction with SM+OI (p = 0.080) (Figure 4B). No differences were observed for measures of fullness (p = 0.916), PFC (p = 0.302), or CSS (p = 0.102) between SM and SM+OI.
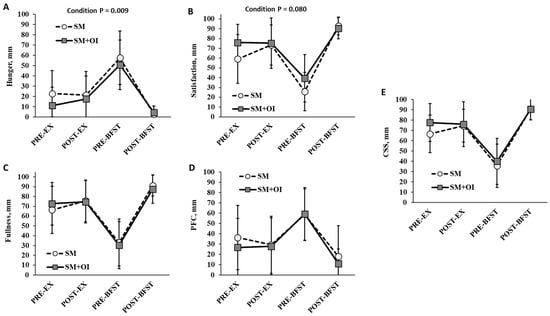
Figure 4.
Change in perceived appetite ratings throughout experimental sessions for (A) Hunger, (B) Satisfaction, (C) Fullness, (D) PFC, and (E) CSS. Values are means ± standard deviation, n = 14. CSS, Composite Satiety Score; PFC, Prospective Food Consumption; PRE-EX: pre-exercise session; PRE-BFST: pre-breakfast; POST-BFST: post ad libitum breakfast; POST-EX; postexercise; SM: Sweetened milk; SM+OI: Sweetened milk + oligofructose-enriched inulin.
3.5. Gastrointestinal Perceptions
Condition and time interacted to affect measures of flatulence (p = 0.031) (Figure 5C). Flatulence ratings were elevated between conditions at PRE-BFST, where SM+OI reported increased flatulence compared to SM (p = 0.026; d = 0.57). SM+OI had within-condition changes, including an increase in abdominal discomfort (p = 0.005, d = 1.27) and increase in flatulence (p < 0.001; d = 1.67) and rumbling (p = 0.001; d = 1.16) from PRE-EX to PRE-BFST. Time impacted ratings of flatulence and rumbling (p < 0.005).
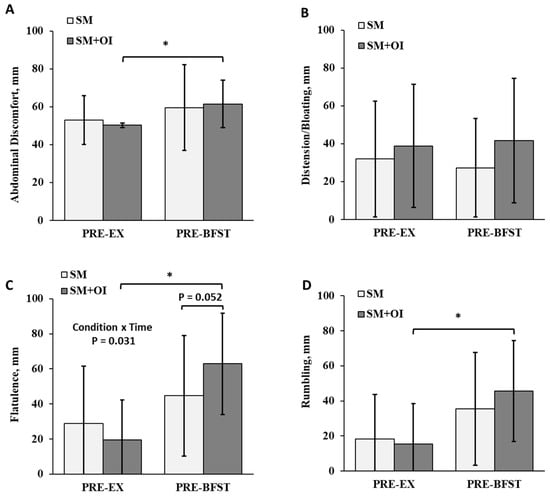
Figure 5.
Comparison of gastrointestinal perceptions between the pre-exercise and pre-breakfast period in the SM and SM+OI conditions. (A) Abdominal comfort, (B) Distention and bloating, (C) Flatulence, and (D) Rumbling. *: significant within condition difference for SM+OI where p < 0.050. Values are means ± standard deviation, n = 14. PRE-EX: pre-exercise session; PRE-BFST: pre-breakfast; POST-BFST: post ad libitum breakfast; POST-EX; postexercise; SM: Sweetened milk; SM+OI: Sweetened milk + oligofructose-enriched inulin.
4. Discussion
Although exercise commonly induces a transient reduction in appetite following a session of exercise, long-term exercise is associated with increased hunger and energy compensation [7]. There is growing interest in the use of dietary strategies to manage appetite during the postexercise period [6,37]. While previous studies have noted the satiating impacts of OI, studies to date have not examined the acute impact of OI after exercise. This study evaluated the influence of OI, added to a sweetened milk beverage, on postexercise EI, appetite-regulating hormones, and subjective appetite. Participants reported reduced feelings of hunger in the SM+OI condition. Coupled with these changes, there was an elevation in GLP-1 and PYY and reduction in acyl-ghrelin from pre-exercise to postexercise in SM+OI. The fermentative action of OI within the gastrointestinal tract was evident, with increased ratings of flatulence the morning after the exercise session. Despite these noted changes in markers of appetite, there were no clear reductions in EI associated with OI intake the day following the acute exercise bout.
Dietary fibre is one of the primary components of food associated with enhanced satiety [38,39]. In our study, we identified a hunger-reducing effect of a 20 g dose of OI over an ~15 h period following a bout of exercise. The finding of reduced hunger following acute ingestion of ITFs contrasts with previously conducted research. Prior studies examining single doses of ITF, ranging from 5–24 g, have failed to find modification of subjective appetite ratings [40,41,42,43,44,45,46]. Of note, none of the previous studies have included an exercise session prior to ingestion of the ITF. It is possible that the incorporation of the exercise session interacts with the OI ingestion to reduce subjective hunger. Although exercise commonly induces transient reductions in appetite following a session of exercise, several studies suggest that appetite may be elevated in the hours following an exercise session [47,48,49,50,51]. With an increase in postexercise hunger, there may be an enhanced sensitivity to the appetite-reducing effects of OI. Unfortunately, given that we did not include a non-exercise condition in our study, it was not possible to assess the interaction between exercise and OI on perceived hunger.
Despite a reduction in subjective hunger ratings, we did not identify a decrease in EI between the two conditions. The day following the exercise session, approximately 12 h after the bout of exercise, we identified a trend towards a 67 kcal reduction in ad libitum breakfast intake with OI. Our study was unique with the assessment of an ad libitum breakfast meal following an evening exercise session. Previously conducted research has traditionally investigated the impact of an acute dose of ITF, consumed in the morning, on ad libitum intake with a lunch meal, assessed ~4 h after fibre ingestion [40,42]. In one study conducted by Harrold et al., it was reported that a 15 g dose of inulin, consumed with a fixed load breakfast, reduced ad libitum lunch intake by 80 kcal [45]. In another study, in a group of healthy men, 16 g of inulin lowered ad libitum lunch intake by 266 kcal relative to control [46]. Furthermore, Hess et al. reported that 16 g of short-chain fructooligosaccharide reduced EI by ~370 kcal over a 24 h period. This finding, however, was only observed in females [44]. While we did not find a difference in 24 h EI between groups, assessed through food record analysis, we did observe an increase in EI from day 1 to day 2 in the SM condition that was not present in the SM+OI condition. Thus, it is possible that OI may have helped to reduce energy compensation the day following the exercise session. TEI, however, was identical between conditions over the 3-day period of study. Of note, we did not observe any differences between males and females. While short-term, acute studies have yielded mixed findings, longer-term interventions using ITFs have highlighted the effectiveness of the prebiotic supplement for inducing satiety and reducing EI [39]. In a 2 week study, Cani et al. showed that supplementation with 16 g of ITFs per day decreased total daily EI and subjective hunger [52]. Furthermore, in a 12 week study in healthy individuals with overweight or obesity, 21 g/day of oligofructose was demonstrated to reduce EI and increase weight loss [53]. Whether longer-term consumption of OI, in conjunction with exercise, would help to overcome the orexigenic effects of exercise and elicit sustained reductions in EI remains unclear and requires further examination.
Exercise sessions have been demonstrated to transiently alter appetite-regulating hormones, with increases in GLP-1 and PYY and reductions in acyl-ghrelin [54]. Similarly, ITFs have been found to increase satiety hormones and reduce orexigenic signals [55]. Here, despite no between-condition changes, we show that there was an elevation in GLP-1 and PYY and reduction in acyl-ghrelin one hour postexercise that was not evident in SM. This trend suggests that OI may elicit subtle postexercise effects within the gastrointestinal tract, leading to the altered secretion of appetite-regulating hormones. Although the mechanisms by which FOS mediates these changes remain unclear, the relatively rapid fermentation of FOS by gut microbiota, and subsequent production of SCFAs, may be in part responsible [13,56,57]. Increases in breath hydrogen, a marker of ITF fermentation, have been demonstrated to increase in a dose-dependent manner within 2 h of ITF ingestion [16,40,41,42]. In our study, with the use of oligofructose, a shorter-chain ITF, some of the OI would have fermented rapidly, potentially contributing to the subtle effects on the appetite-regulating hormones that we observed. Unfortunately, without a blood draw 2 h postexercise, we could not assess whether appetite hormones were trending up or down during this postexercise period. Several reports have indicated that the acute ingestion of inulin, consumed with a breakfast meal, is insufficient to modify GLP-1 and PYY for up to 8 h [41,42,58]. At ~12 h after the initial OI ingestion, prior to the consumption of the breakfast meal the day following exercise, our data show no differences in the concentrations of appetite hormones. The failure of ITFs to influence fasting concentrations of appetite hormones in the postexercise context is consistent within the literature. In a study conducted by Malkova et al., wherein women with overweight consumed an inulin-propionate supplement in conjunction with exercise training for a 4 week period, no changes in fasting concentrations of GLP-1 and PYY were observed [59]. Overall, the ability of ITF and associated metabolic by-products to influence appetite-regulating hormones appears to be subtle and transient.
Relative to changes in appetite, the impact of OI on gastrointestinal feelings was more pronounced. Compared to baseline measures, OI increased flatulence and led to within-condition increases in abdominal discomfort and rumbling the morning after the exercise session. Similar findings have been reported in other acute studies. In 22 healthy women, given 4 different types of fermentable fibre, bloating and flatulence increased 24 h post-consumption, with oligofructose inducing the greatest increase in GI symptoms [40]. In another study, Hess et al. reported that flatulence was the most cited GI effect associated with the consumption of a 16 g dose of short-chain fructooligosaccharide [44]. Bonnema et al. found that both 5 g and 10 g doses of inulin and oligofructose produced mild gastrointestinal bloating and flatulence within 4 h, with the symptoms tapering off after 48 h [60]. Longer-term prebiotic studies indicate that most study participants will experience some gastrointestinal side effects, ranging in severity [61,62]. It is reported that gastrointestinal symptoms associated with ITF consumption dissipate within a few days of use [63]. From our study, it is unclear if there were any interactions between exercise-induced GI effects and OI consumption. Some individuals are susceptible to exercise-induced gastrointestinal symptoms [64]. Acute ingestion of OI would likely exacerbate these symptoms, given that OI is classified as a FODMAP (fermentable oligosaccharide, disaccharide, monosaccharide, and polyols) [65]. Thus, individualized assessment of dose and tolerance would be warranted with a population utilizing postexercise ITFs as an appetite management strategy.
This study is not without limitations. The first limitation centres on the absence of a non-exercise control condition. Without this condition, it was not possible to assess interaction effects between the exercise session and ITF consumption. Secondly, this study incorporated 3-day food records to assess EI. Food records are subject to misreporting and recall bias [66], which may have affected the accuracy of the EI data collected for this study. Furthermore, given that ITFs are believed to mediate many of the impacts on appetite through gastrointestinal fermentation, our study was limited by the fact that we did not include direct measurements of prebiotic fermentation or measurement of SCFAs during the postexercise period. Measures of breath hydrogen in conjunction with the postexercise blood draws would have been beneficial. Lastly, with restrictions on exercise timing, postexercise drink volumes, fasting periods, eating time frames, and selected ad libitum breakfast food items, the conditions of this study deviate from accustomed free-living conditions. It is possible that these study restrictions influenced subjective ratings of appetite and ad libitum intake with the breakfast meal.
5. Conclusions
Our findings suggest that an acute dose of OI can help lower subjective measures of hunger during the postexercise period. Alongside changes in hunger, OI tended to lower subsequent EI with an ad libitum meal; however, any influence of OI on EI appears to be relatively transient. Based on these findings, postexercise OI ingestion may provide some assistance to individuals susceptible to heightened postexercise energy compensation. Presently, there remains a lack of postexercise nutritional guidelines for individuals using exercise to achieve weight loss goals. The coupling of exercise with nutrition-based strategies using prebiotics to curb postexercise appetite may serve to promote exercise adherence and help individuals with obesity and obesity-related chronic disease attain the important cardiometabolic benefits of exercise. Future studies are warranted to investigate whether repeated OI exposure after exercise limits overall energy compensation and aids in sustainable weight management with exercise training.
Author Contributions
C.C.H. recruited participants, collected, and analyzed data, and wrote the manuscript. M.R.B. designed the study, obtained funding, and had final responsibility for the study. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a research grant from the University of Lethbridge. C.C.H. was supported through a CIHR-CGS-M.
Institutional Review Board Statement
The study was conducted in accordance with the ethical principles of the 1964 Declaration of Helsinki and approved by the University of Lethbridge Human Participant Research Committee on 31 December 2017 (Protocol #2017-094).
Informed Consent Statement
Informed consent was obtained from all participants involved in the study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Acknowledgments
The authors would like to thank all participants who volunteered their time for this study.
Conflicts of Interest
C.C.H. and M.R.B. declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
References
- Wharton, S.; Lau, D.C.W.; Vallis, M.; Sharma, A.M.; Biertho, L.; Campbell-Scherer, D.; Adamo, K.; Alberga, A.; Bell, R.; Boulé, N.; et al. Obesity in adults: A clinical practice guideline. Can. Med. Assoc. J. 2020, 192, E875. [Google Scholar] [CrossRef]
- Wadden, T.A.; Brownell, K.D.; Foster, G.D. Obesity: Responding to the global epidemic. J. Consult. Clin. Psychol. 2002, 70, 510–525. [Google Scholar] [CrossRef] [PubMed]
- Santos, I.; Sniehotta, F.F.; Marques, M.M.; Carraça, E.V.; Teixeira, P.J. Prevalence of personal weight control attempts in adults: A systematic review and meta-analysis. Obes. Rev. 2017, 18, 32–50. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.M.; Kyle, T.K.; Stanford, F.C. The gap between expectations and reality of exercise-induced weight loss is associated with discouragement. Prev. Med. 2015, 81, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Wirth, A.; Wabitsch, M.; Hauner, H. The prevention and treatment of obesity. Dtsch. Arztebl. Int. 2014, 111, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Myers, A.; Dalton, M.; Gibbons, C.; Finlayson, G.; Blundell, J. Structured, aerobic exercise reduces fat mass and is partially compensated through energy intake but not energy expenditure in women. Physiol. Behav. 2019, 199, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Riou, M.È.; Jomphe-Tremblay, S.; Lamothe, G.; Stacey, D.; Szczotka, A.; Doucet, E. Predictors of Energy Compensation during Exercise Interventions: A Systematic Review. Nutrients 2015, 7, 3677–3704. [Google Scholar] [CrossRef]
- Thivel, D.; Rumbold, P.L.; King, N.A.; Pereira, B.; Blundell, J.E.; Mathieu, M.E. Acute post-exercise energy and macronutrient intake in lean and obese youth: A systematic review and meta-analysis. Int. J. Obes. 2016, 40, 1469–1479. [Google Scholar] [CrossRef]
- Hughes, R.L.; Alvarado, D.A.; Swanson, K.S.; Holscher, H.D. The Prebiotic Potential of Inulin-type Fructans: A Systematic Review. Adv. Nutr. 2021, 13, 492–529. [Google Scholar] [CrossRef]
- Suzuki, K.; Simpson, K.A.; Minnion, J.S.; Shillito, J.C.; Bloom, S.R. The role of gut hormones and the hypothalamus in appetite regulation. Endocr. J. 2010, 57, 359–372. [Google Scholar] [CrossRef]
- Rahat-Rozenbloom, S.; Fernandes, J.; Cheng, J.; Gloor, G.B.; Wolever, T.M. The acute effects of inulin and resistant starch on postprandial serum short-chain fatty acids and second-meal glycemic response in lean and overweight humans. Eur. J. Clin. Nutr. 2017, 71, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Roberfroid, M.; Gibson, G.R.; Hoyles, L.; McCartney, A.L.; Rastall, R.; Rowland, I.; Wolvers, D.; Watzl, B.; Szajewska, H.; Stahl, B.; et al. Prebiotic effects: Metabolic and health benefits. Br. J. Nutr. 2010, 104 (Suppl. 2), S1–S63. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.S.; Chambers, E.S.; Morrison, D.J.; Frost, G. The role of short chain fatty acids in appetite regulation and energy homeostasis. Int. J. Obes. 2015, 39, 1331–1338. [Google Scholar] [CrossRef]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 2012, 61, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Samuel, B.S.; Shaito, A.; Motoike, T.; Rey, F.E.; Backhed, F.; Manchester, J.K.; Hammer, R.E.; Williams, S.C.; Crowley, J.; Yanagisawa, M.; et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc. Natl. Acad. Sci. USA 2008, 105, 16767–16772. [Google Scholar] [CrossRef] [PubMed]
- Daud, N.M.; Ismail, N.A.; Thomas, E.L.; Fitzpatrick, J.A.; Bell, J.D.; Swann, J.R.; Costabile, A.; Childs, C.E.; Pedersen, C.; Goldstone, A.P.; et al. The impact of oligofructose on stimulation of gut hormones, appetite regulation and adiposity. Obesity 2014, 22, 1430–1438. [Google Scholar] [CrossRef] [PubMed]
- Verhoef, S.P.; Meyer, D.; Westerterp, K.R. Effects of oligofructose on appetite profile, glucagon-like peptide 1 and peptide YY3-36 concentrations and energy intake. Br. J. Nutr. 2011, 106, 1757–1762. [Google Scholar] [CrossRef]
- Tarini, J.; Wolever, T.M. The fermentable fibre inulin increases postprandial serum short-chain fatty acids and reduces free-fatty acids and ghrelin in healthy subjects. Appl. Physiol. Nutr. Metab. 2010, 35, 9–16. [Google Scholar] [CrossRef]
- Jamnik, V.K.; Warburton, D.E.; Makarski, J.; McKenzie, D.C.; Shephard, R.J.; Stone, J.A.; Charlesworth, S.; Gledhill, N. Enhancing the effectiveness of clearance for physical activity participation: Background and overall process. Appl. Physiol. Nutr. Metab. 2011, 36 (Suppl. 1), S3–S13. [Google Scholar] [CrossRef] [PubMed]
- Godin, G.; Shephard, R.J. A simple method to assess exercise behavior in the community. Can. J. Appl. Sport Sci. 1985, 10, 141–146. [Google Scholar]
- de Lauzon, B.; Romon, M.; Deschamps, V.; Lafay, L.; Borys, J.M.; Karlsson, J.; Ducimetiere, P.; Charles, M.A.; Grp, F.S.; Fleurbaix Laventie Ville Sante Study, G.; et al. The three-factor eating questionnaire-R18 is able to distinguish among different eating patterns in a general population. J. Nutr. 2004, 134, 2372–2380. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, J.; Persson, L.O.; Sjöström, L.; Sullivan, M. Psychometric properties and factor structure of the Three-Factor Eating Questionnaire (TFEQ) in obese men and women. Results from the Swedish Obese Subjects (SOS) study. Int. J. Obes. 2000, 24, 1715–1725. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.; Robertson, M.D.; Morgan, L.M. Impact of restraint and disinhibition on PYY plasma levels and subjective feelings of appetite. Appetite 2010, 55, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Bryant, M.; Truesdale, K.P.; Dye, L. Modest changes in dietary intake across the menstrual cycle: Implications for food intake research. Br. J. Nutr. 2006, 96, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Brennan, I.M.; Feltrin, K.L.; Nair, N.S.; Hausken, T.; Little, T.J.; Gentilcore, D.; Wishart, J.M.; Jones, K.L.; Horowitz, M.; Feinle-Bisset, C. Effects of the phases of the menstrual cycle on gastric emptying, glycemia, plasma GLP-1 and insulin, and energy intake in healthy lean women. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, G602–G610. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, C.C.; Wiseman, S.B.; Copeland, J.L.; Bomhof, M.R. Influence of postexercise fasting on hunger and satiety in adults. Appl. Physiol. Nutr. Metab. 2020, 45, 1022–1030. [Google Scholar] [CrossRef]
- Flint, A.; Raben, A.; Blundell, J.E.; Astrup, A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 38–48. [Google Scholar] [CrossRef]
- Oliveira, C.L.P.; Boulé, N.G.; Berg, A.; Sharma, A.M.; Elliott, S.A.; Siervo, M.; Ghosh, S.; Prado, C.M. Consumption of a High-Protein Meal Replacement Leads to Higher Fat Oxidation, Suppression of Hunger, and Improved Metabolic Profile After an Exercise Session. Nutrients 2021, 13, 155. [Google Scholar] [CrossRef]
- Gilbert, J.A.; Gasteyger, C.; Raben, A.; Meier, D.H.; Astrup, A.; Sjödin, A. The Effect of Tesofensine on Appetite Sensations. Obesity 2012, 20, 553–561. [Google Scholar] [CrossRef]
- Sloth, B.; Due, A.; Larsen, T.M.; Holst, J.J.; Heding, A.; Astrup, A. The effect of a high-MUFA, low-glycaemic index diet and a low-fat diet on appetite and glucose metabolism during a 6-month weight maintenance period. Br. J. Nutr. 2008, 101, 1846–1858. [Google Scholar] [CrossRef]
- Chaput, J.-P.; Gilbert, J.-A.; Gregersen, N.T.; Pedersen, S.D.; Sjödin, A.M. Comparison of 150-mm versus 100-mm visual analogue scales in free living adult subjects. Appetite 2010, 54, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge Academic: New York, NY, USA, 1988. [Google Scholar]
- Rumbold, P.; Shaw, E.; James, L.; Stevenson, E. Milk consumption following exercise reduces subsequent energy intake in female recreational exercisers. Nutrients 2015, 7, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.A.; Green, B.P.; James, L.J.; Stevenson, E.J.; Rumbold, P.L. The Effect of a Dairy-Based Recovery Beverage on Post-Exercise Appetite and Energy Intake in Active Females. Nutrients 2016, 8, 355. [Google Scholar] [CrossRef]
- Clayton, D.J.; Stensel, D.J.; Watson, P.; James, L.J. The effect of post-exercise drink macronutrient content on appetite and energy intake. Appetite 2014, 82, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Monteyne, A.; Martin, A.; Jackson, L.; Corrigan, N.; Stringer, E.; Newey, J.; Rumbold, P.L.; Stevenson, E.J.; James, L.J. Whey protein consumption after resistance exercise reduces energy intake at a post-exercise meal. Eur. J. Nutr. 2016, 57, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.T.; Andersen, S.V.; Astrup, A.; Blundell, J.; Sjodin, A. Is reducing appetite beneficial for body weight management in the context of overweight and obesity? A systematic review and meta-analysis from clinical trials assessing body weight management after exposure to satiety enhancing and/or hunger reducing products. Obes. Rev. 2019, 20, 983–997. [Google Scholar] [CrossRef] [PubMed]
- Chambers, L.; McCrickerd, K.; Yeomans, M.R. Optimising foods for satiety. Trends Food Sci. Technol. 2015, 41, 149–160. [Google Scholar] [CrossRef]
- Kellow, N.J.; Coughlan, M.T.; Reid, C.M. Metabolic benefits of dietary prebiotics in human subjects: A systematic review of randomised controlled trials. Br. J. Nutr. 2014, 111, 1147–1161. [Google Scholar] [CrossRef]
- Karalus, M.; Clark, M.; Greaves, K.A.; Thomas, W.; Vickers, Z.; Kuyama, M.; Slavin, J. Fermentable fibers do not affect satiety or food intake by women who do not practice restrained eating. J. Acad. Nutr. Diet. 2012, 112, 1356–1362. [Google Scholar] [CrossRef]
- van der Beek, C.M.; Canfora, E.E.; Kip, A.M.; Gorissen, S.H.M.; Olde Damink, S.W.M.; van Eijk, H.M.; Holst, J.J.; Blaak, E.E.; Dejong, C.H.C.; Lenaerts, K. The prebiotic inulin improves substrate metabolism and promotes short-chain fatty acid production in overweight to obese men. Metabolism 2018, 87, 25–35. [Google Scholar] [CrossRef]
- Lee, I.; Shi, L.; Webb, D.L.; Hellstrom, P.M.; Riserus, U.; Landberg, R. Effects of whole-grain rye porridge with added inulin and wheat gluten on appetite, gut fermentation and postprandial glucose metabolism: A randomised, cross-over, breakfast study. Br. J. Nutr. 2016, 116, 2139–2149. [Google Scholar] [CrossRef] [PubMed]
- Peters, H.P.; Boers, H.M.; Haddeman, E.; Melnikov, S.M.; Qvyjt, F. No effect of added beta-glucan or of fructooligosaccharide on appetite or energy intake. Am. J. Clin. Nutr. 2009, 89, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Hess, J.R.; Birkett, A.M.; Thomas, W.; Slavin, J.L. Effects of short-chain fructooligosaccharides on satiety responses in healthy men and women. Appetite 2011, 56, 128–134. [Google Scholar] [CrossRef]
- Harrold, J.A.; Hughes, G.M.; O’Shiel, K.; Quinn, E.; Boyland, E.J.; Williams, N.J.; Halford, J.C. Acute effects of a herb extract formulation and inulin fibre on appetite, energy intake and food choice. Appetite 2013, 62, 84–90. [Google Scholar] [CrossRef]
- NabizadehAsl, L.; Sendur, S.N.; Ozer, B.; Lay, I.; Erbas, T.; Buyuktuncer, Z. Acute and short-term effects of Lactobacillus paracasei subsp. paracasei 431 and inulin intake on appetite control and dietary intake: A two-phases randomized, double blind, placebo-controlled study. Appetite 2022, 169, 105855. [Google Scholar] [CrossRef] [PubMed]
- Jurimae, J.; Ramson, R.; Maestu, J.; Purge, P.; Jurimae, T.; Arciero, P.J.; von Duvillard, S.P. Plasma visfatin and ghrelin response to prolonged sculling in competitive male rowers. Med. Sci. Sports Exerc. 2009, 41, 137–143. [Google Scholar] [CrossRef]
- Martins, C.; Morgan, L.M.; Bloom, S.R.; Robertson, M.D. Effects of exercise on gut peptides, energy intake and appetite. J. Endocrinol. 2007, 193, 251–258. [Google Scholar] [CrossRef]
- Laan, D.J.; Leidy, H.J.; Lim, E.; Campbell, W.W. Effects and reproducibility of aerobic and resistance exercise on appetite and energy intake in young, physically active adults. Appl. Physiol. Nutr. Metab. 2010, 35, 842–847. [Google Scholar] [CrossRef]
- Shorten, A.L.; Wallman, K.E.; Guelfi, K.J. Acute effect of environmental temperature during exercise on subsequent energy intake in active men. Am. J. Clin. Nutr. 2009, 90, 1215–1221. [Google Scholar] [CrossRef]
- Larson-Meyer, D.E.; Palm, S.; Bansal, A.; Austin, K.J.; Hart, A.M.; Alexander, B.M. Influence of running and walking on hormonal regulators of appetite in women. J. Obes. 2012, 2012, 730409. [Google Scholar] [CrossRef]
- Cani, P.D.; Joly, E.; Horsmans, Y.; Delzenne, N.M. Oligofructose promotes satiety in healthy human: A pilot study. Eur. J. Clin. Nutr. 2006, 60, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Parnell, J.A.; Reimer, R.A. Weight loss during oligofructose supplementation is associated with decreased ghrelin and increased peptide YY in overweight and obese adults. Am. J. Clin. Nutr. 2009, 89, 1751–1759. [Google Scholar] [CrossRef] [PubMed]
- Thackray, A.E.; Stensel, D.J. The impact of acute exercise on appetite control: Current insights and future perspectives. Appetite 2023, 186, 106557. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.S.; Viardot, A.; Psichas, A.; Morrison, D.J.; Murphy, K.G.; Zac-Varghese, S.E.; MacDougall, K.; Preston, T.; Tedford, C.; Finlayson, G.S.; et al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut 2015, 64, 1744–1754. [Google Scholar] [CrossRef]
- Frampton, J.; Serrano-Contreras, J.I.; Garcia-Perez, I.; Franco-Becker, G.; Penhaligan, J.; Tan, A.S.Y.; de Oliveira, A.C.C.; Milner, A.J.; Murphy, K.G.; Frost, G.; et al. The metabolic interplay between dietary carbohydrate and exercise and its role in acute appetite regulation in males: A randomized controlled study. J. Physiol. 2023, 601, 3461–3480. [Google Scholar] [CrossRef]
- Rahat-Rozenbloom, S.; Fernandes, J.; Cheng, J.; Wolever, T.M. Acute increases in serum colonic short-chain fatty acids elicited by inulin do not increase GLP-1 or PYY responses but may reduce ghrelin in lean and overweight humans. Eur. J. Clin. Nutr. 2016, 71, 953–958. [Google Scholar] [CrossRef]
- Malkova, D.; Polyviou, T.; Rizou, E.; Gerasimidis, K.; Chambers, E.S.; Preston, T.; Tedford, M.C.; Frost, G.; Morrison, D.J. Moderate intensity exercise training combined with inulin-propionate ester supplementation increases whole body resting fat oxidation in overweight women. Metabolism 2020, 104, 154043. [Google Scholar] [CrossRef]
- Bonnema, A.L.; Kolberg, L.W.; Thomas, W.; Slavin, J.L. Gastrointestinal tolerance of chicory inulin products. J. Am. Diet. Assoc. 2010, 110, 865–868. [Google Scholar] [CrossRef]
- Bruhwyler, J.; Carreer, F.; Demanet, E.; Jacobs, H. Digestive tolerance of inulin-type fructans: A double-blind, placebo-controlled, cross-over, dose-ranging, randomized study in healthy volunteers. Int. J. Food Sci. Nutr. 2009, 60, 165–175. [Google Scholar] [CrossRef]
- Pedersen, C.; Lefevre, S.; Peters, V.; Patterson, M.; Ghatei, M.A.; Morgan, L.M.; Frost, G.S. Gut hormone release and appetite regulation in healthy non-obese participants following oligofructose intake. A dose-escalation study. Appetite 2013, 66, 44–53. [Google Scholar] [CrossRef]
- Dewulf, E.M.; Cani, P.D.; Claus, S.P.; Fuentes, S.; Puylaert, P.G.; Neyrinck, A.M.; Bindels, L.B.; de Vos, W.M.; Gibson, G.R.; Thissen, J.P.; et al. Insight into the prebiotic concept: Lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women. Gut 2013, 62, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.J.S.; Snipe, R.M.J.; Kitic, C.M.; Gibson, P.R. Systematic review: Exercise-induced gastrointestinal syndrome-implications for health and intestinal disease. Aliment. Pharmacol. Ther. 2017, 46, 246–265. [Google Scholar] [CrossRef] [PubMed]
- Lis, D.M. Exit Gluten-Free and Enter Low FODMAPs: A Novel Dietary Strategy to Reduce Gastrointestinal Symptoms in Athletes. Sports Med. 2019, 49, 87–97. [Google Scholar] [CrossRef]
- Garriguet, D. Accounting for misreporting when comparing energy intake across time in Canada. Health Rep. 2018, 29, 3–12. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).