Recent Advances in Folates and Autoantibodies against Folate Receptors in Early Pregnancy and Miscarriage
Abstract
1. Introduction
2. Folate Transport and Metabolism
2.1. Folate Transporters
2.2. Folate Uptake and Mechanism of FOCM
3. Classical Viewpoints of FRAbs and Recent Advancements
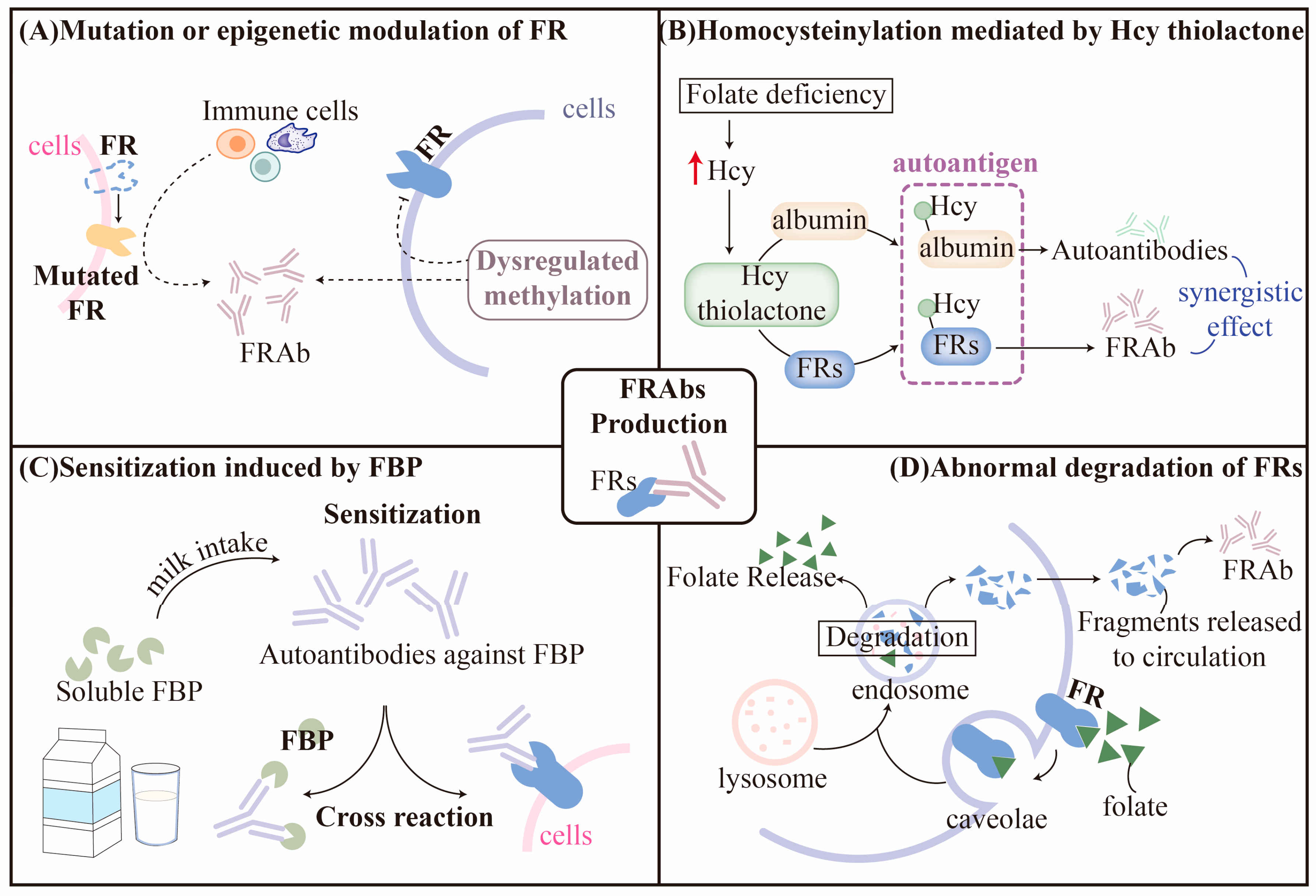
3.1. Hypothesis for Production of FRAbs
3.1.1. Mutation or Epigenetic Modulation of FRs
3.1.2. Homocysteinylation of FRs
3.1.3. Sensitization Induced by Folate-Binding Protein (FBP)
3.1.4. Abnormal Degradation of FRs
3.2. Classification and Characteristics of FRAbs
4. Association between Folate or FRAbs and Miscarriage
4.1. Folate Deficiency and Miscarriage
4.2. Expressional Profile of FRs at Maternal-Fetal Interface
4.3. FRAbs and Miscarriage
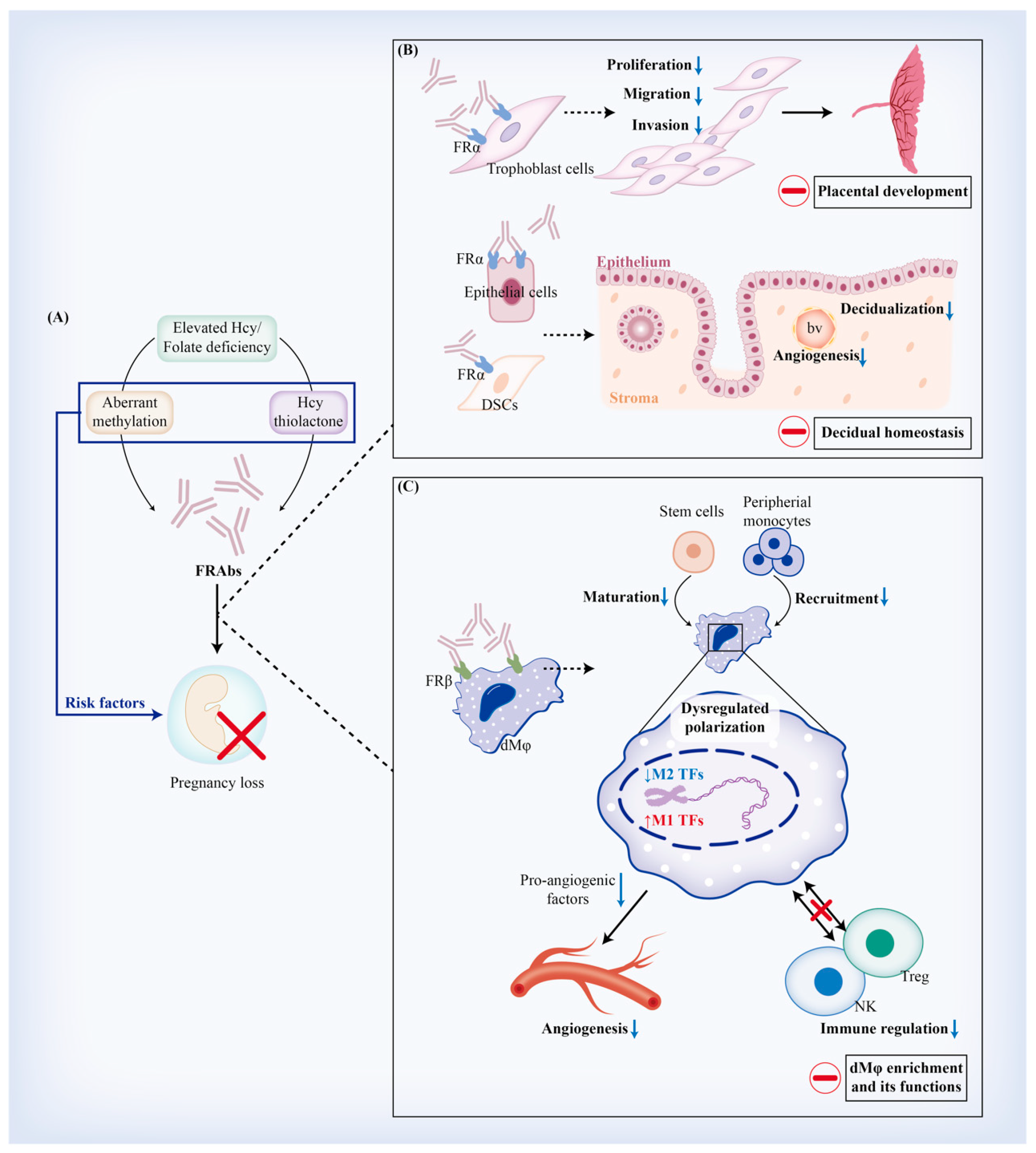
4.4. Implications of FRAb Production-Related Risk Factors in Miscarriage
5. Regulatory Effects of FRAbs against FRα (FRαAb) on Trophoblast Cells and Decidua
5.1. FRαAb and Trophoblastic Biofunctions
5.1.1. Viability and Proliferation of Trophoblast Cells
5.1.2. Placental Development
5.2. FRαAb and Decidual Homeostasis
5.2.1. Decidualization
5.2.2. Decidual Apoptosis and Autophagy
6. Regulatory Effects of FRAbs against FRβ (FRβAb) on Regulating Decidual Macrophages (dMφ)
6.1. FRβAb and Origin of dMφ
6.2. FRβAb and dMφ Polarization
6.3. FRβAb and dMφ-Mediated Angiogenesis
6.4. FRβAb and dMφ-Mediated Immune Regulation
7. Summary and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Yang, C.; Zhang, J.; Liao, M.; Yang, Y.; Wang, Y.; Yuan, Y.; Ouyang, L. Folate-mediated one-carbon metabolism: A targeting strategy in cancer therapy. Drug Discov. Today 2021, 26, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Scaglione, F.; Panzavolta, G. Folate, folic acid and 5-methyltetrahydrofolate are not the same thing. Xenobiotica 2014, 44, 480–488. [Google Scholar] [CrossRef]
- Lee, W.D.; Pirona, A.C.; Sarvin, B.; Stern, A.; Nevo-Dinur, K.; Besser, E.; Sarvin, N.; Lagziel, S.; Mukha, D.; Raz, S.; et al. Tumor Reliance on Cytosolic versus Mitochondrial One-Carbon Flux Depends on Folate Availability. Cell Metab. 2021, 33, 190–198.e6. [Google Scholar] [CrossRef] [PubMed]
- Shang, M.; Yang, H.; Yang, R.; Chen, T.; Fu, Y.; Li, Y.; Fang, X.; Zhang, K.; Zhang, J.; Li, H.; et al. The folate cycle enzyme MTHFD2 induces cancer immune evasion through PD-L1 up-regulation. Nat. Commun. 2021, 12, 1940. [Google Scholar] [CrossRef]
- Paulos, C.M.; Turk, M.J.; Breur, G.J.; Low, P.S. Folate receptor-mediated targeting of therapeutic and imaging agents to activated macrophages in rheumatoid arthritis. Adv. Drug Deliv. Rev. 2004, 56, 1205–1217. [Google Scholar] [CrossRef]
- Forges, T.; Monnier-Barbarino, P.; Alberto, J.M.; Guéant-Rodriguez, R.M.; Daval, J.L.; Guéant, J.L. Impact of folate and homocysteine metabolism on human reproductive health. Hum. Reprod. Update 2007, 13, 225–238. [Google Scholar] [CrossRef]
- Samaniego, R.; Palacios, B.S.; Domiguez-Soto, Á.; Vidal, C.; Salas, A.; Matsuyama, T.; Sánchez-Torres, C.; de la Torre, I.; Miranda-Carús, M.E.; Sánchez-Mateos, P.; et al. Macrophage uptake and accumulation of folates are polarization-dependent in vitro and in vivo and are regulated by activin A. J. Leukoc. Biol. 2014, 95, 797–808. [Google Scholar] [CrossRef]
- Lanska, D.J. Chapter 30: Historical aspects of the major neurological vitamin deficiency disorders: The water-soluble B vitamins. Handb. Clin. Neurol. 2010, 95, 445–476. [Google Scholar] [CrossRef] [PubMed]
- Mahomed, K. Folate supplementation in pregnancy. Cochrane Database Syst. Rev. 2000, Cd000183. [Google Scholar] [CrossRef]
- Burdge, G.C.; Lillycrop, K.A. Folic acid supplementation in pregnancy: Are there devils in the detail? Br. J. Nutr. 2012, 108, 1924–1930. [Google Scholar] [CrossRef]
- Bailey, R.L.; Dodd, K.W.; Gahche, J.J.; Dwyer, J.T.; McDowell, M.A.; Yetley, E.A.; Sempos, C.A.; Burt, V.L.; Radimer, K.L.; Picciano, M.F. Total folate and folic acid intake from foods and dietary supplements in the United States: 2003–2006. Am. J. Clin. Nutr. 2010, 91, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, X.; Peng, X.; Zhang, S.; Wang, X.; Zhu, C. Folic Acid and Risk of Preterm Birth: A Meta-Analysis. Front. Neurosci. 2019, 13, 1284. [Google Scholar] [CrossRef]
- Olapeju, B.; Saifuddin, A.; Wang, G.; Ji, Y.; Hong, X.; Raghavan, R.; Summers, A.; Keiser, A.; Ji, H.; Zuckerman, B.; et al. Maternal postpartum plasma folate status and preterm birth in a high-risk US population. Public Health Nutr. 2019, 22, 1281–1291. [Google Scholar] [CrossRef] [PubMed]
- Owen, M.D.; Baker, B.C.; Scott, E.M.; Forbes, K. Interaction between Metformin, Folate and Vitamin B(12) and the Potential Impact on Fetal Growth and Long-Term Metabolic Health in Diabetic Pregnancies. Int. J. Mol. Sci. 2021, 22, 5759. [Google Scholar] [CrossRef]
- Balogun, O.O.; da Silva Lopes, K.; Ota, E.; Takemoto, Y.; Rumbold, A.; Takegata, M.; Mori, R. Vitamin supplementation for preventing miscarriage. Cochrane Database Syst. Rev. 2016, 2016, Cd004073. [Google Scholar] [CrossRef]
- Gaskins, A.J.; Rich-Edwards, J.W.; Hauser, R.; Williams, P.L.; Gillman, M.W.; Ginsburg, E.S.; Missmer, S.A.; Chavarro, J.E. Maternal prepregnancy folate intake and risk of spontaneous abortion and stillbirth. Obstet. Gynecol. 2014, 124, 23–31. [Google Scholar] [CrossRef]
- Cetin, I.; Berti, C.; Calabrese, S. Role of micronutrients in the periconceptional period. Hum. Reprod. Update 2010, 16, 80–95. [Google Scholar] [CrossRef] [PubMed]
- Rah, H.; Chung, K.W.; Ko, K.H.; Kim, E.S.; Kim, J.O.; Sakong, J.H.; Kim, J.H.; Lee, W.S.; Kim, N.K. miR-27a and miR-449b polymorphisms associated with a risk of idiopathic recurrent pregnancy loss. PLoS ONE 2017, 12, e0177160. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Chen, H.; Jiang, Y.; Wang, Y.; Wang, D.; Li, M.; Dou, Y.; Sun, X.; Huang, G.; et al. Association of Maternal Folate and Vitamin B(12) in Early Pregnancy With Gestational Diabetes Mellitus: A Prospective Cohort Study. Diabetes Care 2021, 44, 217–223. [Google Scholar] [CrossRef]
- Jankovic-Karasoulos, T.; Furness, D.L.; Leemaqz, S.Y.; Dekker, G.A.; Grzeskowiak, L.E.; Grieger, J.A.; Andraweera, P.H.; McCullough, D.; McAninch, D.; McCowan, L.M.; et al. Maternal folate, one-carbon metabolism and pregnancy outcomes. Matern. Child Nutr. 2021, 17, e13064. [Google Scholar] [CrossRef]
- Krabbendam, I.; Dekker, G.A. Pregnancy outcome in patients with a history of recurrent spontaneous miscarriages and documented thrombophilias. Gynecol. Obstet. Investig. 2004, 57, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Ramaekers, V.T.; Blau, N. Cerebral folate deficiency. Dev. Med. Child Neurol. 2004, 46, 843–851. [Google Scholar] [CrossRef]
- Sequeira, J.M.; Desai, A.; Berrocal-Zaragoza, M.I.; Murphy, M.M.; Fernandez-Ballart, J.D.; Quadros, E.V. Exposure to Folate Receptor Alpha Antibodies during Gestation and Weaning Leads to Severe Behavioral Deficits in Rats: A Pilot Study. PLoS ONE 2016, 11, e0152249. [Google Scholar] [CrossRef]
- Frye, R.E.; Sequeira, J.M.; Quadros, E.V.; James, S.J.; Rossignol, D.A. Cerebral folate receptor autoantibodies in autism spectrum disorder. Mol. Psychiatry 2013, 18, 369–381. [Google Scholar] [CrossRef]
- Boyles, A.L.; Ballard, J.L.; Gorman, E.B.; McConnaughey, D.R.; Cabrera, R.M.; Wilcox, A.J.; Lie, R.T.; Finnell, R.H. Association between inhibited binding of folic acid to folate receptor alpha in maternal serum and folate-related birth defects in Norway. Hum. Reprod. 2011, 26, 2232–2238. [Google Scholar] [CrossRef] [PubMed]
- da Costa, M.; Sequeira, J.M.; Rothenberg, S.P.; Weedon, J. Antibodies to folate receptors impair embryogenesis and fetal development in the rat. Birth Defects Res. Part A Clin. Mol. Teratol. 2003, 67, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Berrocal-Zaragoza, M.I.; Fernandez-Ballart, J.D.; Murphy, M.M.; Cavallé-Busquets, P.; Sequeira, J.M.; Quadros, E.V. Association between blocking folate receptor autoantibodies and subfertility. Fertil. Steril. 2009, 91, 1518–1521. [Google Scholar] [CrossRef][Green Version]
- Zhao, R.; Diop-Bove, N.; Visentin, M.; Goldman, I.D. Mechanisms of membrane transport of folates into cells and across epithelia. Annu. Rev. Nutr. 2011, 31, 177–201. [Google Scholar] [CrossRef]
- Carter, M.F.; Powell, T.L.; Li, C.; Myatt, L.; Dudley, D.; Nathanielsz, P.; Jansson, T. Fetal serum folate concentrations and placental folate transport in obese women. Am. J. Obstet. Gynecol. 2011, 205, e17–e25. [Google Scholar] [CrossRef]
- Keating, E.; Lemos, C.; Azevedo, I.; Martel, F. Comparison of folic acid uptake characteristics by human placental choriocarcinoma cells at acidic and physiological pH. Can. J. Physiol. Pharmacol. 2006, 84, 247–255. [Google Scholar] [CrossRef]
- Hou, Z.; Matherly, L.H. Biology of the major facilitative folate transporters SLC19A1 and SLC46A1. Curr. Top. Membr. 2014, 73, 175–204. [Google Scholar] [CrossRef]
- Newstead, S. Structural basis for recognition and transport of folic acid in mammalian cells. Curr. Opin. Struct. Biol. 2022, 74, 102353. [Google Scholar] [CrossRef]
- Hooijberg, J.H.; Peters, G.J.; Assaraf, Y.G.; Kathmann, I.; Priest, D.G.; Bunni, M.A.; Veerman, A.J.P.; Scheffer, G.L.; Kaspers, G.J.L.; Jansen, G. The role of multidrug resistance proteins MRP1, MRP2 and MRP3 in cellular folate homeostasis. Biochem. Pharmacol. 2003, 65, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Yeboah, D.; Kalabis, G.M.; Sun, M.; Ou, R.C.; Matthews, S.G.; Gibb, W. Expression and localisation of breast cancer resistance protein (BCRP) in human fetal membranes and decidua and the influence of labour at term. Reprod. Fertil. Dev. 2008, 20, 328–334. [Google Scholar] [CrossRef]
- Desai, A.; Sequeira, J.M.; Quadros, E.V. The metabolic basis for developmental disorders due to defective folate transport. Biochimie 2016, 126, 31–42. [Google Scholar] [CrossRef]
- Ducker, G.S.; Rabinowitz, J.D. One-Carbon Metabolism in Health and Disease. Cell Metab. 2017, 25, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Hasan, T.; Arora, R.; Bansal, A.K.; Bhattacharya, R.; Sharma, G.S.; Singh, L.R. Disturbed homocysteine metabolism is associated with cancer. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; Field, M.S.; Stover, P.J. Cell cycle regulation of folate-mediated one-carbon metabolism. Wiley Interdiscip. Rev. Syst. Biol. Med. 2018, 10, e1426. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Navneet, S.; Wang, J.; Roon, P.; Chen, W.; Xian, M.; Smith, S.B. Analysis of MTHFR, CBS, Glutathione, Taurine, and Hydrogen Sulfide Levels in Retinas of Hyperhomocysteinemic Mice. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1954–1963. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Berisa, M.; Schwörer, S.; Qin, W.; Cross, J.R.; Thompson, C.B. Transsulfuration Activity Can Support Cell Growth upon Extracellular Cysteine Limitation. Cell Metab. 2019, 30, 865–876.e5. [Google Scholar] [CrossRef]
- Lou, H.; Ling, G.S.; Cao, X. Autoantibodies in systemic lupus erythematosus: From immunopathology to therapeutic target. J. Autoimmun. 2022, 132, 102861. [Google Scholar] [CrossRef]
- McHugh, N.J.; Tansley, S.L. Autoantibodies in myositis. Nat. Rev. Rheumatol. 2018, 14, 290–302. [Google Scholar] [CrossRef]
- Hyland, K.; Shoffner, J.; Heales, S.J. Cerebral folate deficiency. J. Inherit. Metab. Dis. 2010, 33, 563–570. [Google Scholar] [CrossRef]
- Zhang, C.; Deng, X.; Wen, Y.; He, F.; Yin, F.; Peng, J. First case report of cerebral folate deficiency caused by a novel mutation of FOLR1 gene in a Chinese patient. BMC Med. Genet. 2020, 21, 235. [Google Scholar] [CrossRef]
- Ramaekers, V.T.; Segers, K.; Sequeira, J.M.; Koenig, M.; Van Maldergem, L.; Bours, V.; Kornak, U.; Quadros, E.V. Genetic assessment and folate receptor autoantibodies in infantile-onset cerebral folate deficiency (CFD) syndrome. Mol. Genet. Metab. 2018, 124, 87–93. [Google Scholar] [CrossRef]
- Han, X.; Cao, X.; Cabrera, R.M.; Pimienta Ramirez, P.A.; Zhang, C.; Ramaekers, V.T.; Finnell, R.H.; Lei, Y. KDM6B Variants May Contribute to the Pathophysiology of Human Cerebral Folate Deficiency. Biology 2022, 12, 74. [Google Scholar] [CrossRef]
- Li, J.; Hardy, K.; Olshansky, M.; Barugahare, A.; Gearing, L.J.; Prier, J.E.; Sng, X.Y.X.; Nguyen, M.L.T.; Piovesan, D.; Russ, B.E.; et al. KDM6B-dependent chromatin remodeling underpins effective virus-specific CD8(+) T cell differentiation. Cell Rep. 2021, 34, 108839. [Google Scholar] [CrossRef] [PubMed]
- Issa, N.; Bjeije, H.; Wilson, E.R.; Krishnan, A.; Dunuwille, W.M.B.; Parsons, T.M.; Zhang, C.R.; Han, W.; Young, A.L.; Ren, Z.; et al. KDM6B protects T-ALL cells from NOTCH1-induced oncogenic stress. Leukemia 2023, 37, 728–740. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.; Wang, C.; Fessler, J.; DeTomaso, D.; Avila-Pacheco, J.; Kaminski, J.; Zaghouani, S.; Christian, E.; Thakore, P.; Schellhaass, B.; et al. Metabolic modeling of single Th17 cells reveals regulators of autoimmunity. Cell 2021, 184, 4168–4185.e21. [Google Scholar] [CrossRef] [PubMed]
- Bliek, J.B.; Rothenberg, S.P.; Steegers-Theunissen, R.P. Maternal folate receptor autoantibodies and cleft lip and/or palate. Int. J. Gynaecol. Obstet. 2006, 93, 142–143. [Google Scholar] [CrossRef] [PubMed]
- Bergen, N.E.; Jaddoe, V.W.; Timmermans, S.; Hofman, A.; Lindemans, J.; Russcher, H.; Raat, H.; Steegers-Theunissen, R.P.; Steegers, E.A. Homocysteine and folate concentrations in early pregnancy and the risk of adverse pregnancy outcomes: The Generation R Study. BJOG Int. J. Obstet. Gynaecol. 2012, 119, 739–751. [Google Scholar] [CrossRef] [PubMed]
- Dhobale, M.; Chavan, P.; Kulkarni, A.; Mehendale, S.; Pisal, H.; Joshi, S. Reduced folate, increased vitamin B(12) and homocysteine concentrations in women delivering preterm. Ann. Nutr. Metab. 2012, 61, 7–14. [Google Scholar] [CrossRef] [PubMed]
- López-Alarcón, M.; Vital-Reyes, V.S.; Montalvo-Velarde, I.; Hinojosa-Cruz, J.C.; Puellotamara, E. Interactions between markers of endothelial damage (homocysteine and asymmetric dimethylarginine) and antioxidants and B-vitamins in preeclamptic women. Ginecol. Obstet. Mex. 2015, 83, 329–339. [Google Scholar]
- Hoek, J.; Schoenmakers, S.; Ringelberg, B.; Reijnders, I.F.; Willemsen, S.P.; De Rijke, Y.B.; Mulders, A.; Steegers-Theunissen, R.P.M. Periconceptional maternal and paternal homocysteine levels and early utero-placental (vascular) growth trajectories: The Rotterdam periconception cohort. Placenta 2021, 115, 45–52. [Google Scholar] [CrossRef]
- Kjaergaard, A.D.; Wu, Y.; Ming, W.-K.; Wang, Z.; Kjaergaard, M.N.; Ellervik, C. Homocysteine and female fertility, pregnancy loss and offspring birthweight: A two-sample Mendelian randomization study. Eur. J. Clin. Nutr. 2022, 76, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Creus, M.; Deulofeu, R.; Peñarrubia, J.; Carmona, F.; Balasch, J. Plasma homocysteine and vitamin B12 serum levels, red blood cell folate concentrations, C677T methylenetetrahydrofolate reductase gene mutation and risk of recurrent miscarriage: A case-control study in Spain. Clin. Chem. Lab. Med. 2013, 51, 693–699. [Google Scholar] [CrossRef]
- Jakubowski, H. Homocysteine-thiolactone and S-nitroso-homocysteine mediate incorporation of homocysteine into protein in humans. Clin. Chem. Lab. Med. 2003, 41, 1462–1466. [Google Scholar] [CrossRef] [PubMed]
- Jakubowski, H.; Głowacki, R. Chemical biology of homocysteine thiolactone and related metabolites. Adv. Clin. Chem. 2011, 55, 81–103. [Google Scholar] [CrossRef]
- Jakubowski, H. Pathophysiological consequences of homocysteine excess. J. Nutr. 2006, 136, 1741s–1749s. [Google Scholar] [CrossRef]
- Jakubowski, H. Molecular basis of homocysteine toxicity in humans. Cell. Mol. Life Sci. 2004, 61, 470–487. [Google Scholar] [CrossRef]
- Wu, S.; Gao, X.; Yang, S.; Meng, M.; Yang, X.; Ge, B. The role of endoplasmic reticulum stress in endothelial dysfunction induced by homocysteine thiolactone. Fundam. Clin. Pharmacol. 2015, 29, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.C.; Kim, S.H.; Jeong, J.H.; Park, T.G. Folate receptor-mediated gene delivery using folate-poly(ethylene glycol)-poly(L-lysine) conjugate. Macromol. Biosci. 2005, 5, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Taparia, S.; Gelineau-van Waes, J.; Rosenquist, T.H.; Finnell, R.H. Importance of folate-homocysteine homeostasis during early embryonic development. Clin. Chem. Lab. Med. 2007, 45, 1717–1727. [Google Scholar] [CrossRef]
- Denny, K.J.; Kelly, C.F.; Kumar, V.; Witham, K.L.; Cabrera, R.M.; Finnell, R.H.; Taylor, S.M.; Jeanes, A.; Woodruff, T.M. Autoantibodies against homocysteinylated protein in a mouse model of folate deficiency-induced neural tube defects. Birth Defects Res. Part A Clin. Mol. Teratol. 2016, 106, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.S.; Khan, R.A.; Xiao, S.; Hansen, D.K.; Stabler, S.P.; Kusumanchi, P.; Jayaram, H.N.; Antony, A.C. Evidence Favoring a Positive Feedback Loop for Physiologic Auto Upregulation of hnRNP-E1 during Prolonged Folate Deficiency in Human Placental Cells. J. Nutr. 2017, 147, 482–498. [Google Scholar] [CrossRef]
- Padjas, A.; Undas, A.; Swadzba, J.; Musiał, J. Antibodies to N-homocysteinylated albumin in patients with systemic lupus erythematosus. Pol. Arch. Med. Wewn. 2007, 117, 20–25. [Google Scholar] [CrossRef]
- Lerner, A.; Agmon-Levin, N.; Shapira, Y.; Gilburd, B.; Reuter, S.; Lavi, I.; Shoenfeld, Y. The thrombophilic network of autoantibodies in celiac disease. BMC Med. 2013, 11, 89. [Google Scholar] [CrossRef]
- Van Hoozen, C.M.; Ling, E.H.; Halsted, C.H. Folate binding protein: Molecular characterization and transcript distribution in pig liver, kidney and jejunum. Biochem. J. 1996, 319 Pt 3, 725–729. [Google Scholar] [CrossRef]
- Nygren-Babol, L.; Jägerstad, M. Folate-binding protein in milk: A review of biochemistry, physiology, and analytical methods. Crit. Rev. Food Sci. Nutr. 2012, 52, 410–425. [Google Scholar] [CrossRef] [PubMed]
- Holm, J.; Hansen, S.I.; Nichols, C.W.; Høier-Madsen, M.; Helkjaer, P.E. Characterization of the folate receptor in human molar placenta. Biosci. Rep. 1996, 16, 379–389. [Google Scholar] [CrossRef]
- Ramaekers, V.T.; Sequeira, J.M.; Quadros, E.V. The basis for folinic acid treatment in neuro-psychiatric disorders. Biochimie 2016, 126, 79–90. [Google Scholar] [CrossRef]
- Bobrowski-Khoury, N.; Ramaekers, V.T.; Sequeira, J.M.; Quadros, E.V. Folate Receptor Alpha Autoantibodies in Autism Spectrum Disorders: Diagnosis, Treatment and Prevention. J. Pers. Med. 2021, 11, 710. [Google Scholar] [CrossRef]
- Ramaekers, V.T.; Thöny, B.; Sequeira, J.M.; Ansseau, M.; Philippe, P.; Boemer, F.; Bours, V.; Quadros, E.V. Folinic acid treatment for schizophrenia associated with folate receptor autoantibodies. Mol. Genet. Metab. 2014, 113, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Santi, D.; Crépieux, P.; Reiter, E.; Spaggiari, G.; Brigante, G.; Casarini, L.; Rochira, V.; Simoni, M. Follicle-Stimulating Hormone (FSH) Action on Spermatogenesis: A Focus on Physiological and Therapeutic Roles. J. Clin. Med. 2020, 9, 1014. [Google Scholar] [CrossRef] [PubMed]
- Sabharanjak, S.; Mayor, S. Folate receptor endocytosis and trafficking. Adv. Drug Deliv. Rev. 2004, 56, 1099–1109. [Google Scholar] [CrossRef]
- Schwartz, R.S. Autoimmune folate deficiency and the rise and fall of “horror autotoxicus”. New Engl. J. Med. 2005, 352, 1948–1950. [Google Scholar] [CrossRef]
- Burbelo, P.D.; Iadarola, M.J.; Keller, J.M.; Warner, B.M. Autoantibodies Targeting Intracellular and Extracellular Proteins in Autoimmunity. Front. Immunol. 2021, 12, 548469. [Google Scholar] [CrossRef] [PubMed]
- Mizutori, Y.; Chen, C.R.; Latrofa, F.; McLachlan, S.M.; Rapoport, B. Evidence that shed thyrotropin receptor A subunits drive affinity maturation of autoantibodies causing Graves’ disease. J. Clin. Endocrinol. Metab. 2009, 94, 927–935. [Google Scholar] [CrossRef]
- Jian Wang, B.Y.; Liang, W.; Cui, Y.X.; Ge, Y.F. Follicle-stimulating hormone autoantibody is involved in idiopathic spermatogenic dysfunction. Asian J. Androl. 2008, 10, 915–921. [Google Scholar] [CrossRef]
- Knutson, K.L.; Krco, C.J.; Erskine, C.L.; Goodman, K.; Kelemen, L.E.; Wettstein, P.J.; Low, P.S.; Hartmann, L.C.; Kalli, K.R. T-cell immunity to the folate receptor alpha is prevalent in women with breast or ovarian cancer. J. Clin. Oncol. 2006, 24, 4254–4261. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, L.; Lei, Y.; Yang, N.; Cabrera, R.M.; Finnell, R.H.; Ren, A. Gene variants in the folate pathway are associated with increased levels of folate receptor autoantibodies. Birth Defects Res. 2018, 110, 973–981. [Google Scholar] [CrossRef]
- Cai, C.; Yin, Z.; Liu, A.; Wang, H.; Zeng, S.; Wang, Z.; Qiu, H.; Li, S.; Zhou, J.; Wang, M. Identifying Rare Genetic Variants of Immune Mediators as Risk Factors for Autism Spectrum Disorder. Genes 2022, 13, 1098. [Google Scholar] [CrossRef] [PubMed]
- Triggianese, P.; Perricone, C.; De Martino, E.; D’Antonio, A.; Chimenti, M.S.; Conigliaro, P.; Ferrigno, S.; Giambini, I.; Greco, E.; De Carolis, C. Human Leukocyte Antigen (HLA) Typing Study Identifies Maternal DQ2 Susceptibility Alleles among Infertile Women: Potential Associations with Autoimmunity and Micronutrients. Nutrients 2021, 13, 3270. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Furusho, Y.; Li, H.; Hasui, K.; Matsukita, S.; Sueyoshi, K.; Yanagi, M.; Hatae, M.; Takao, S.; Matsuyama, T. Production of a High-affinity Monoclonal Antibody Reactive with Folate Receptors Alpha and Beta. Monoclon. Antibodies Immunodiagn. Immunother. 2015, 34, 181–190. [Google Scholar] [CrossRef]
- Cabrera, R.M.; Shaw, G.M.; Ballard, J.L.; Carmichael, S.L.; Yang, W.; Lammer, E.J.; Finnell, R.H. Autoantibodies to folate receptor during pregnancy and neural tube defect risk. J. Reprod. Immunol. 2008, 79, 85–92. [Google Scholar] [CrossRef]
- Frye, R.E.; Delhey, L.; Slattery, J.; Tippett, M.; Wynne, R.; Rose, S.; Kahler, S.G.; Bennuri, S.C.; Melnyk, S.; Sequeira, J.M.; et al. Blocking and Binding Folate Receptor Alpha Autoantibodies Identify Novel Autism Spectrum Disorder Subgroups. Front. Neurosci. 2016, 10, 80. [Google Scholar] [CrossRef]
- Frye, R.E.; Wynne, R.; Rose, S.; Slattery, J.; Delhey, L.; Tippett, M.; Kahler, S.G.; Bennuri, S.C.; Melnyk, S.; Sequeira, J.M.; et al. Thyroid dysfunction in children with autism spectrum disorder is associated with folate receptor α autoimmune disorder. J. Neuroendocrinol. 2017, 29, 3. [Google Scholar] [CrossRef]
- Sequeira, J.M.; Ramaekers, V.T.; Quadros, E.V. The diagnostic utility of folate receptor autoantibodies in blood. Clin. Chem. Lab. Med. 2013, 51, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Fei, Y.; Li, J.; Shi, Y.; Yang, X. A Novel Review of Homocysteine and Pregnancy Complications. BioMed Res. Int. 2021, 2021, 6652231. [Google Scholar] [CrossRef]
- Yin, X.; Gao, R.; Geng, Y.; Chen, X.; Liu, X.; Mu, X.; Ding, Y.; Wang, Y.; He, J. Autophagy regulates abnormal placentation induced by folate deficiency in mice. Mol. Hum. Reprod. 2019, 25, 305–319. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gao, R.; Liu, X.; Chen, X.; Liao, X.; Geng, Y.; Ding, Y.; Wang, Y.; He, J. Folate Deficiency Could Restrain Decidual Angiogenesis in Pregnant Mice. Nutrients 2015, 7, 6425–6445. [Google Scholar] [CrossRef]
- Geng, Y.; Gao, R.; Chen, X.; Liu, X.; Liao, X.; Li, Y.; Liu, S.; Ding, Y.; Wang, Y.; He, J. Folate deficiency impairs decidualization and alters methylation patterns of the genome in mice. Mol. Hum. Reprod. 2015, 21, 844–856. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Ma, X.; Rusie, A.; Hemingway, J.; Ostmann, A.B.; Chung, D.; Das, S.K. Epigenetic changes through DNA methylation contribute to uterine stromal cell decidualization. Endocrinology 2012, 153, 6078–6090. [Google Scholar] [CrossRef]
- Zetterberg, H. Methylenetetrahydrofolate reductase and transcobalamin genetic polymorphisms in human spontaneous abortion: Biological and clinical implications. Reprod. Biol. Endocrinol. 2004, 2, 7. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, N.K.; Choi, Y.K.; Kang, M.S.; Choi, D.H.; Cha, S.H.; An, M.O.; Lee, S.; Jeung, M.; Ko, J.J.; Oh, D. Influence of combined methylenetetrahydrofolate reductase (MTHFR) and thymidylate synthase enhancer region (TSER) polymorphisms to plasma homocysteine levels in Korean patients with recurrent spontaneous abortion. Thromb. Res. 2006, 117, 653–658. [Google Scholar] [CrossRef]
- Cavallé-Busquets, P.; Inglès-Puig, M.; Fernandez-Ballart, J.D.; Haro-Barceló, J.; Rojas-Gómez, A.; Ramos-Rodriguez, C.; Ballesteros, M.; Meyer, K.; Ueland, P.M.; Murphy, M.M. Moderately elevated first trimester fasting plasma total homocysteine is associated with increased probability of miscarriage. The Reus-Tarragona Birth Cohort Study. Biochimie 2020, 173, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Laanpere, M.; Altmäe, S.; Kaart, T.; Stavreus-Evers, A.; Nilsson, T.K.; Salumets, A. Folate-metabolizing gene variants and pregnancy outcome of IVF. Reprod. Biomed. Online 2011, 22, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Rah, H.; Choi, Y.S.; Jeon, Y.J.; Choi, Y.; Cha, S.H.; Choi, D.H.; Ko, J.J.; Shim, S.H.; Kim, N.K. Solute Carrier Family 19, member 1 (SLC19A1) polymorphisms (-43T>C, 80G>A, and 696C>T), and haplotypes in idiopathic recurrent spontaneous abortion in a Korean population. Reprod. Sci. 2012, 19, 513–519. [Google Scholar] [CrossRef]
- Mohtaram, S.; Sheikhha, M.H.; Honarvar, N.; Sazegari, A.; Maraghechi, N.; Feizollahi, Z.; Ghasemi, N. An Association Study of the SLC19A1 Gene Polymorphisms/Haplotypes with Idiopathic Recurrent Pregnancy Loss in an Iranian Population. Genet. Test. Mol. Biomark. 2016, 20, 235–240. [Google Scholar] [CrossRef]
- Guerra-Shinohara, E.M.; Pereira, P.M.; Kubota, A.M.; Silva, T.A.; Reis, J.L.; Miyashita, G.S.; D’Almeida, V.; Allen, R.H.; Stabler, S.P. Increased MMA concentration and body mass index are associated with spontaneous abortion in Brazilian women: A pilot study. Clin. Chim. Acta 2010, 411, 423–427. [Google Scholar] [CrossRef]
- Crişan, T.O.; Trifa, A.; Farcaş, M.; Militaru, M.; Netea, M.; Pop, I.; Popp, R. The MTHFD1 c.1958 G>A polymorphism and recurrent spontaneous abortions. J. Matern. Fetal Neonatal. Med. 2011, 24, 189–192. [Google Scholar] [CrossRef]
- Madjunkova, S.; Volk, M.; Peterlin, B.; Plaseska-Karanfilska, D. Detection of thrombophilic mutations related to spontaneous abortions by a multiplex SNaPshot method. Genet. Test. Mol. Biomark. 2012, 16, 259–264. [Google Scholar] [CrossRef]
- Palomares, A.R.; Ruiz-Galdon, M.; Liu, K.; Reyes-Engel, A.; Rodriguez-Wallberg, K.A. Profiling the Influence of Gene Variants Related to Folate-Mediated One-Carbon Metabolism on the Outcome of In Vitro Fertilization (IVF) with Donor Oocytes in Recipients Receiving Folic Acid Fortification. Int. J. Mol. Sci. 2022, 23, 11298. [Google Scholar] [CrossRef]
- Jauniaux, E.; Johns, J.; Gulbis, B.; Spasic-Boskovic, O.; Burton, G.J. Transfer of folic acid inside the first-trimester gestational sac and the effect of maternal smoking. Am. J. Obstet. Gynecol. 2007, 197, 58.E1–58.E6. [Google Scholar] [CrossRef]
- Yasuda, S.; Hasui, S.; Yamamoto, C.; Yoshioka, C.; Kobayashi, M.; Itagaki, S.; Hirano, T.; Iseki, K. Placental folate transport during pregnancy. Biosci. Biotechnol. Biochem. 2008, 72, 2277–2284. [Google Scholar] [CrossRef]
- Rosario, F.J.; Powell, T.L.; Jansson, T. Mechanistic target of rapamycin (mTOR) regulates trophoblast folate uptake by modulating the cell surface expression of FR-α and the RFC. Sci. Rep. 2016, 6, 31705. [Google Scholar] [CrossRef]
- Weitman, S.D.; Weinberg, A.G.; Coney, L.R.; Zurawski, V.R.; Jennings, D.S.; Kamen, B.A. Cellular localization of the folate receptor: Potential role in drug toxicity and folate homeostasis. Cancer Res. 1992, 52, 6708–6711. [Google Scholar]
- Prasad, P.D.; Ramamoorthy, S.; Moe, A.J.; Smith, C.H.; Leibach, F.H.; Ganapathy, V. Selective expression of the high-affinity isoform of the folate receptor (FR-alpha) in the human placental syncytiotrophoblast and choriocarcinoma cells. Biochim. Biophys. Acta 1994, 1223, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Cherukad, J.; Wainwright, V.; Watson, E.D. Spatial and temporal expression of folate-related transporters and metabolic enzymes during mouse placental development. Placenta 2012, 33, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.; Sequeira, J.M.; Quadros, E.V. Prevention of behavioral deficits in rats exposed to folate receptor antibodies: Implication in autism. Mol. Psychiatry 2017, 22, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Ross, J.F.; Wang, X.; Ratnam, M. Identification of a novel folate receptor, a truncated receptor, and receptor type beta in hematopoietic cells: cDNA cloning, expression, immunoreactivity, and tissue specificity. Biochemistry 1994, 33, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.W.; Kim, H.J.; Bae, S.M.; Kim, Y.J.; Shin, J.C.; Chun, H.J.; Rhie, J.W.; Kim, J.; Kim, H.; Ahn, W.S. Time-course transcriptional profiling of human amniotic fluid-derived stem cells using microarray. Cancer Res. Treat. 2010, 42, 82–94. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lu, R.J.; Shirvani, P.; Holick, M.F. A Novel Immunomodulatory Mechanism by Which Vitamin D Influences Folate Receptor 3 Expression to Reduce COVID-19 Severity. Anticancer Res. 2022, 42, 5043–5048. [Google Scholar] [CrossRef]
- Li, L.; Wang, R.; He, S.; Shen, X.; Kong, F.; Li, S.; Zhao, H.; Lian, M.; Fang, J. The identification of induction chemo-sensitivity genes of laryngeal squamous cell carcinoma and their clinical utilization. Eur. Arch. Oto-Rhino-Laryngol. 2018, 275, 2773–2781. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Hirota, K.; Nagahama, K.; Ohkawa, K.; Takahashi, T.; Nomura, T.; Sakaguchi, S. Control of immune responses by antigen-specific regulatory T cells expressing the folate receptor. Immunity 2007, 27, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Kalekar, L.A.; Mueller, D.L. Relationship between CD4 Regulatory T Cells and Anergy In Vivo. J. Immunol. 2017, 198, 2527–2533. [Google Scholar] [CrossRef]
- Zarin, P.; Shwartz, Y.; Ortiz-Lopez, A.; Hanna, B.S.; Sassone-Corsi, M.; Hsu, Y.C.; Mathis, D.; Benoist, C. Treg cells require Izumo1R to regulate γδT cell-driven inflammation in the skin. Proc. Natl. Acad. Sci. USA 2023, 120, e2221255120. [Google Scholar] [CrossRef]
- Luhrs, C.A.; Slomiany, B.L. A human membrane-associated folate binding protein is anchored by a glycosyl-phosphatidylinositol tail*. J. Biol. Chem. 1989, 264, 21446–21449. [Google Scholar] [CrossRef]
- Elnakat, H.; Ratnam, M. Distribution, functionality and gene regulation of folate receptor isoforms: Implications in targeted therapy. Adv. Drug Deliv. Rev. 2004, 56, 1067–1084. [Google Scholar] [CrossRef]
- Condon, J.C.; Jeyasuria, P.; Faust, J.M.; Mendelson, C.R. Surfactant protein secreted by the maturing mouse fetal lung acts as a hormone that signals the initiation of parturition. Proc. Natl. Acad. Sci. USA 2004, 101, 4978–4983. [Google Scholar] [CrossRef]
- Ramaekers, V.T.; Rothenberg, S.P.; Sequeira, J.M.; Opladen, T.; Blau, N.; Quadros, E.V.; Selhub, J. Autoantibodies to folate receptors in the cerebral folate deficiency syndrome. New Engl. J. Med. 2005, 352, 1985–1991. [Google Scholar] [CrossRef]
- Solanky, N.; Requena Jimenez, A.; D’Souza, S.W.; Sibley, C.P.; Glazier, J.D. Expression of folate transporters in human placenta and implications for homocysteine metabolism. Placenta 2010, 31, 134–143. [Google Scholar] [CrossRef]
- Gilmore, J.C.; Hoque, M.T.; Dai, W.; Mohan, H.; Dunk, C.; Serghides, L.; Bendayan, R. Interaction between dolutegravir and folate transporters and receptor in human and rodent placenta. eBioMedicine 2022, 75, 103771. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Shen, J.; Streaker, E.D.; Lockwood, M.; Zhu, Z.; Low, P.S.; Dimitrov, D.S. A folate receptor beta-specific human monoclonal antibody recognizes activated macrophage of rheumatoid patients and mediates antibody-dependent cell-mediated cytotoxicity. Arthritis Res. Ther. 2011, 13, R59. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Hilgenbrink, A.R.; Matteson, E.L.; Lockwood, M.B.; Cheng, J.X.; Low, P.S. A functional folate receptor is induced during macrophage activation and can be used to target drugs to activated macrophages. Blood 2009, 113, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Tanaka, M.; Hasui, K.; Shirahama, H.; Kitajima, S.; Yonezawa, S.; Xu, B.; Matsuyama, T. Effect of an immunotoxin to folate receptor beta on bleomycin-induced experimental pulmonary fibrosis. Clin. Exp. Immunol. 2010, 161, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.R.; Appios, A.; Zhao, X.; Dutkiewicz, R.; Donde, M.; Lee, C.Y.C.; Naidu, P.; Lee, C.; Cerveira, J.; Liu, B.; et al. Phenotypic and functional characterization of first-trimester human placental macrophages, Hofbauer cells. J. Exp. Med. 2021, 218, e20200891. [Google Scholar] [CrossRef]
- Croxatto, D.; Micheletti, A.; Montaldo, E.; Orecchia, P.; Loiacono, F.; Canegallo, F.; Calzetti, F.; Fulcheri, E.; Munari, E.; Zamò, A.; et al. Group 3 innate lymphoid cells regulate neutrophil migration and function in human decidua. Mucosal Immunol. 2016, 9, 1372–1383. [Google Scholar] [CrossRef] [PubMed]
- Bert, S.; Ward, E.J.; Nadkarni, S. Neutrophils in pregnancy: New insights into innate and adaptive immune regulation. Immunology 2021, 164, 665–676. [Google Scholar] [CrossRef]
- Gimeno-Molina, B.; Muller, I.; Kropf, P.; Sykes, L. The Role of Neutrophils in Pregnancy, Term and Preterm Labour. Life 2022, 12, 1512. [Google Scholar] [CrossRef]
- Bianchi, E.; Doe, B.; Goulding, D.; Wright, G.J. Juno is the egg Izumo receptor and is essential for mammalian fertilization. Nature 2014, 508, 483–487. [Google Scholar] [CrossRef]
- Bobrowski-Khoury, N.; Sequeira, J.M.; Arning, E.; Bottiglieri, T.; Quadros, E.V. Absorption and Tissue Distribution of Folate Forms in Rats: Indications for Specific Folate Form Supplementation during Pregnancy. Nutrients 2022, 14, 2397. [Google Scholar] [CrossRef]
- Cuckle, H.S. Recurrence risk of neural tube defects following a miscarriage. Prenat. Diagn. 1983, 3, 287–289. [Google Scholar] [CrossRef] [PubMed]
- Chitayat, D.; Farrell, S.A.; Huang, T.; Meier, C.; Wyatt, P.R.; Summers, A.M. Double-positive maternal serum screening results for down syndrome and open neural tube defects: An indicator for fetal structural or chromosomal abnormalities and adverse obstetric outcomes. Am. J. Obstet. Gynecol. 2002, 187, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Shapira, I.; Sequeira, J.M.; Quadros, E.V. Folate receptor autoantibodies in pregnancy related complications. Birth Defects Res. Part A Clin. Mol. Teratol. 2015, 103, 1028–1030. [Google Scholar] [CrossRef] [PubMed]
- Ramaekers, V.T.; Sequeira, J.M.; Blau, N.; Quadros, E.V. A milk-free diet downregulates folate receptor autoimmunity in cerebral folate deficiency syndrome. Dev. Med. Child Neurol. 2008, 50, 346–352. [Google Scholar] [CrossRef]
- Rothenberg, S.P.; da Costa, M.P.; Sequeira, J.M.; Cracco, J.; Roberts, J.L.; Weedon, J.; Quadros, E.V. Autoantibodies against folate receptors in women with a pregnancy complicated by a neural-tube defect. New Engl. J. Med. 2004, 350, 134–142. [Google Scholar] [CrossRef]
- Steegers-Theunissen, R.P.; Smith, S.C.; Steegers, E.A.; Guilbert, L.J.; Baker, P.N. Folate affects apoptosis in human trophoblastic cells. BJOG Int. J. Obstet. Gynaecol. 2000, 107, 1513–1515. [Google Scholar] [CrossRef]
- Kamudhamas, A.; Pang, L.; Smith, S.D.; Sadovsky, Y.; Nelson, D.M. Homocysteine thiolactone induces apoptosis in cultured human trophoblasts: A mechanism for homocysteine-mediated placental dysfunction? Am. J. Obstet. Gynecol. 2004, 191, 563–571. [Google Scholar] [CrossRef]
- Di Simone, N.; Riccardi, P.; Maggiano, N.; Piacentani, A.; D’Asta, M.; Capelli, A.; Caruso, A. Effect of folic acid on homocysteine-induced trophoblast apoptosis. Mol. Hum. Reprod. 2004, 10, 665–669. [Google Scholar] [CrossRef]
- Williams, P.J.; Bulmer, J.N.; Innes, B.A.; Broughton Pipkin, F. Possible roles for folic acid in the regulation of trophoblast invasion and placental development in normal early human pregnancy. Biol. Reprod. 2011, 84, 1148–1153. [Google Scholar] [CrossRef] [PubMed]
- Moussa, C.; Ross, N.; Jolette, P.; MacFarlane, A.J. Altered folate metabolism modifies cell proliferation and progesterone secretion in human placental choriocarcinoma JEG-3 cells. Br. J. Nutr. 2015, 114, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Fellus, I.; Gaudet, J.; MacFarlane, A.J.; Fontaine-Bisson, B.; Bainbridge, S.A. Effect of folic acid on human trophoblast health and function in vitro. Placenta 2016, 37, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Dhobale, M. Neurotrophic Factors and Maternal Nutrition During Pregnancy. Vitam. Horm. 2017, 104, 343–366. [Google Scholar] [CrossRef] [PubMed]
- Reijnders, I.F.; Mulders, A.; van der Windt, M.; Steegers, E.A.P.; Steegers-Theunissen, R.P.M. The impact of periconceptional maternal lifestyle on clinical features and biomarkers of placental development and function: A systematic review. Hum. Reprod. Update 2019, 25, 72–94. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, A.; Wang, K.; Zhou, Q.; Duan, T. Folate ameliorates dexamethasone-induced fetal and placental growth restriction potentially via improvement of trophoblast migration. Int. J. Clin. Exp. Pathol. 2015, 8, 3008–3014. [Google Scholar] [PubMed]
- Carletti, J.V.; Correia-Branco, A.; Silva, C.R.; Andrade, N.; Silva, L.O.P.; Martel, F. The effect of oxidative stress induced by tert-butylhydroperoxide under distinct folic acid conditions: An in vitro study using cultured human trophoblast-derived cells. Reprod. Toxicol. 2018, 77, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Rahat, B.; Mahajan, A.; Bagga, R.; Hamid, A.; Kaur, J. Epigenetic modifications at DMRs of placental genes are subjected to variations in normal gestation, pathological conditions and folate supplementation. Sci. Rep. 2017, 7, 40774. [Google Scholar] [CrossRef] [PubMed]
- Christofides, A.; Konstantinidou, E.; Jani, C.; Boussiotis, V.A. The role of peroxisome proliferator-activated receptors (PPAR) in immune responses. Metab. Clin. Exp. 2021, 114, 154338. [Google Scholar] [CrossRef]
- Meher, A.; Sundrani, D.; Joshi, S. Maternal nutrition influences angiogenesis in the placenta through peroxisome proliferator activated receptors: A novel hypothesis. Mol. Reprod. Dev. 2015, 82, 726–734. [Google Scholar] [CrossRef]
- Meher, A.P.; Joshi, A.A.; Joshi, S.R. Maternal micronutrients, omega-3 fatty acids, and placental PPARγ expression. Appl. Physiol. Nutr. Metab. 2014, 39, 793–800. [Google Scholar] [CrossRef]
- Ng, S.W.; Norwitz, G.A.; Pavlicev, M.; Tilburgs, T.; Simón, C.; Norwitz, E.R. Endometrial Decidualization: The Primary Driver of Pregnancy Health. Int. J. Mol. Sci. 2020, 21, 4092. [Google Scholar] [CrossRef] [PubMed]
- Gellersen, B.; Brosens, J.J. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr. Rev. 2014, 35, 851–905. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.F.; Zheng, L.W.; Yang, Z.Q.; Wang, Y.S.; Huang, J.C.; Liu, S.; Yue, Z.P.; Guo, B. Bmp2 regulates Serpinb6b expression via cAMP/PKA/Wnt4 pathway during uterine decidualization. J. Cell. Mol. Med. 2020, 24, 7023–7033. [Google Scholar] [CrossRef]
- Adiguzel, D.; Celik-Ozenci, C. FoxO1 is a cell-specific core transcription factor for endometrial remodeling and homeostasis during menstrual cycle and early pregnancy. Hum. Reprod. Update 2021, 27, 570–583. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.L.; Stoikos, C.; Findlay, J.K.; Salamonsen, L.A. TGF-beta superfamily expression and actions in the endometrium and placenta. Reproduction 2006, 132, 217–232. [Google Scholar] [CrossRef]
- Jin, X.; Cui, L.; Zhao, W.; Li, X.; Liu, L.; Li, Y.; Fu, Q.; Li, D.; Yu, M.; Du, M. Decidualization-derived cAMP regulates phenotypic and functional conversion of decidual NK cells from CD56(dim)CD16(-) NK cells. Cell. Mol. Immunol. 2021, 18, 1596–1598. [Google Scholar] [CrossRef]
- Kim, K.H.; Jelovac, D.; Armstrong, D.K.; Schwartz, B.; Weil, S.C.; Schweizer, C.; Alvarez, R.D. Phase 1b safety study of farletuzumab, carboplatin and pegylated liposomal doxorubicin in patients with platinum-sensitive epithelial ovarian cancer. Gynecol. Oncol. 2016, 140, 210–214. [Google Scholar] [CrossRef]
- Altwerger, G.; Bonazzoli, E.; Bellone, S.; Egawa-Takata, T.; Menderes, G.; Pettinella, F.; Bianchi, A.; Riccio, F.; Feinberg, J.; Zammataro, L.; et al. In Vitro and In Vivo Activity of IMGN853, an Antibody-Drug Conjugate Targeting Folate Receptor Alpha Linked to DM4, in Biologically Aggressive Endometrial Cancers. Mol. Cancer Ther. 2018, 17, 1003–1011. [Google Scholar] [CrossRef]
- Farran, B.; Pavitra, E.; Kasa, P.; Peela, S.; Rama Raju, G.S.; Nagaraju, G.P. Folate-targeted immunotherapies: Passive and active strategies for cancer. Cytokine Growth Factor Rev. 2019, 45, 45–52. [Google Scholar] [CrossRef]
- Nawaz, F.Z.; Kipreos, E.T. Emerging roles for folate receptor FOLR1 in signaling and cancer. Trends Endocrinol. Metab. 2022, 33, 159–174. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Shen, H.H.; Qin, X.Y.; Wang, C.J.; Hu, W.T.; Liu, S.P.; Wu, J.N.; Xie, F.; Xu, F.Y.; Zhao, S.M.; et al. IL-27 promotes decidualization via the STAT3-ESR/PGR regulatory axis. J. Reprod. Immunol. 2022, 151, 103623. [Google Scholar] [CrossRef]
- Zhao, M.; Chen, Y.H.; Dong, X.T.; Zhou, J.; Chen, X.; Wang, H.; Wu, S.X.; Xia, M.Z.; Zhang, C.; Xu, D.X. Folic acid protects against lipopolysaccharide-induced preterm delivery and intrauterine growth restriction through its anti-inflammatory effect in mice. PLoS ONE 2013, 8, e82713. [Google Scholar] [CrossRef] [PubMed]
- Qu, Q.C.; Shen, H.H.; Wang, C.J.; Zhang, X.Y.; Wu, J.N.; Lu, H.C.; Qiu, X.M.; Ding, J.Y.; Tan, X.F.; Liu, L.B.; et al. A positive COX-2/IL-1β loop promotes decidualization by upregulating CD82. Reproduction 2021, 162, 227–236. [Google Scholar] [CrossRef]
- St-Louis, I.; Singh, M.; Brasseur, K.; Leblanc, V.; Parent, S.; Asselin, E. Expression of COX-1 and COX-2 in the endometrium of cyclic, pregnant and in a model of pseudopregnant rats and their regulation by sex steroids. Reprod. Biol. Endocrinol. 2010, 8, 103. [Google Scholar] [CrossRef] [PubMed]
- Leno-Durán, E.; Ruiz-Magaña, M.J.; Muñoz-Fernández, R.; Requena, F.; Olivares, E.G.; Ruiz-Ruiz, C. Human decidual stromal cells secrete soluble pro-apoptotic factors during decidualization in a cAMP-dependent manner. Hum. Reprod. 2014, 29, 2269–2277. [Google Scholar] [CrossRef]
- Chen, Q.; Gao, R.; Geng, Y.; Chen, X.; Liu, X.; Zhang, L.; Mu, X.; Ding, Y.; Wang, Y.; He, J. Decreased autophagy was implicated in the decreased apoptosis during decidualization in early pregnant mice. J. Mol. Histol. 2018, 49, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.G.; Li, Y.L.; Gao, R.F.; Geng, Y.Q.; Chen, X.M.; Liu, X.Q.; Ding, Y.B.; Mu, X.Y.; Wang, Y.X.; He, J.L. Folate deficiency decreases apoptosis of endometrium decidual cells in pregnant mice via the mitochondrial pathway. Nutrients 2015, 7, 1916–1932. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, R.; Zhang, L.; Geng, Y.; Chen, Q.; Chen, X.; Liu, X.; Mu, X.; Ding, Y.; Wang, Y.; et al. AMPK/mTOR downregulated autophagy enhances aberrant endometrial decidualization in folate-deficient pregnant mice. J. Cell. Physiol. 2021, 236, 7376–7389. [Google Scholar] [CrossRef]
- Samaniego, R.; Domínguez-Soto, Á.; Ratnam, M.; Matsuyama, T.; Sánchez-Mateos, P.; Corbí, Á.L.; Puig-Kröger, A. Folate Receptor β (FRβ) Expression in Tissue-Resident and Tumor-Associated Macrophages Associates with and Depends on the Expression of PU.1. Cells 2020, 9, 1445. [Google Scholar] [CrossRef]
- Reddy, J.A.; Haneline, L.S.; Srour, E.F.; Antony, A.C.; Clapp, D.W.; Low, P.S. Expression and functional characterization of the beta-isoform of the folate receptor on CD34(+) cells. Blood 1999, 93, 3940–3948. [Google Scholar] [CrossRef] [PubMed]
- Antony, A.C.; Briddell, R.A.; Brandt, J.E.; Straneva, J.E.; Verma, R.S.; Miller, M.E.; Kalasinski, L.A.; Hoffman, R. Megaloblastic hematopoiesis in vitro. Interaction of anti-folate receptor antibodies with hematopoietic progenitor cells leads to a proliferative response independent of megaloblastic changes. J. Clin. Investig. 1991, 87, 313–325. [Google Scholar] [CrossRef]
- Wei, C.; Yang, C.; Wang, S.; Shi, D.; Zhang, C.; Lin, X.; Liu, Q.; Dou, R.; Xiong, B. Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis. Mol. Cancer 2019, 18, 64. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Chen, Y.; Nagashimada, M.; Ni, Y.; Zhuge, F.; Chen, G.; Li, H.; Pan, T.; Yamashita, T.; Mukaida, N.; et al. CC chemokine ligand 3 deficiency ameliorates diet-induced steatohepatitis by regulating liver macrophage recruitment and M1/M2 status in mice. Metab. Clin. Exp. 2021, 125, 154914. [Google Scholar] [CrossRef]
- Mukaida, N.; Sasaki, S.I.; Baba, T. CCL4 Signaling in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1231, 23–32. [Google Scholar] [CrossRef]
- Puig-Kröger, A.; Sierra-Filardi, E.; Domínguez-Soto, A.; Samaniego, R.; Corcuera, M.T.; Gómez-Aguado, F.; Ratnam, M.; Sánchez-Mateos, P.; Corbí, A.L. Folate Receptor β Is Expressed by Tumor-Associated Macrophages and Constitutes a Marker for M2 Anti-inflammatory/Regulatory Macrophages. Cancer Res. 2009, 69, 9395–9403. [Google Scholar] [CrossRef]
- Han, W.; Zaynagetdinov, R.; Yull, F.E.; Polosukhin, V.V.; Gleaves, L.A.; Tanjore, H.; Young, L.R.; Peterson, T.E.; Manning, H.C.; Prince, L.S.; et al. Molecular imaging of folate receptor β-positive macrophages during acute lung inflammation. Am. J. Respir. Cell Mol. Biol. 2015, 53, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Warmink, K.; Siebelt, M.; Low, P.S.; Riemers, F.M.; Wang, B.; Plomp, S.G.M.; Tryfonidou, M.A.; van Weeren, P.R.; Weinans, H.; Korthagen, N.M. Folate Receptor Expression by Human Monocyte-Derived Macrophage Subtypes and Effects of Corticosteroids. Cartilage 2022, 13, 19476035221081469. [Google Scholar] [CrossRef]
- Jiemy, W.F.; van Sleen, Y.; van der Geest, K.S.; Ten Berge, H.A.; Abdulahad, W.H.; Sandovici, M.; Boots, A.M.; Heeringa, P.; Brouwer, E. Distinct macrophage phenotypes skewed by local granulocyte macrophage colony-stimulating factor (GM-CSF) and macrophage colony-stimulating factor (M-CSF) are associated with tissue destruction and intimal hyperplasia in giant cell arteritis. Clin. Transl. Immunol. 2020, 9, e1164. [Google Scholar] [CrossRef]
- Sierra-Filardi, E.; Puig-Kröger, A.; Blanco, F.J.; Nieto, C.; Bragado, R.; Palomero, M.I.; Bernabéu, C.; Vega, M.A.; Corbí, A.L. Activin A skews macrophage polarization by promoting a proinflammatory phenotype and inhibiting the acquisition of anti-inflammatory macrophage markers. Blood 2011, 117, 5092–5101. [Google Scholar] [CrossRef]
- Nagai, T.; Tanaka, M.; Tsuneyoshi, Y.; Matsushita, K.; Sunahara, N.; Matsuda, T.; Yoshida, H.; Komiya, S.; Onda, M.; Matsuyama, T. In vitro and in vivo efficacy of a recombinant immunotoxin against folate receptor beta on the activation and proliferation of rheumatoid arthritis synovial cells. Arthritis Rheum. 2006, 54, 3126–3134. [Google Scholar] [CrossRef] [PubMed]
- Nalio Ramos, R.; Missolo-Koussou, Y.; Gerber-Ferder, Y.; Bromley, C.P.; Bugatti, M.; Núñez, N.G.; Tosello Boari, J.; Richer, W.; Menger, L.; Denizeau, J.; et al. Tissue-resident FOLR2(+) macrophages associate with CD8(+) T cell infiltration in human breast cancer. Cell 2022, 185, 1189–1207.e25. [Google Scholar] [CrossRef] [PubMed]
- Tie, Y.; Zheng, H.; He, Z.; Yang, J.; Shao, B.; Liu, L.; Luo, M.; Yuan, X.; Liu, Y.; Zhang, X.; et al. Targeting folate receptor β positive tumor-associated macrophages in lung cancer with a folate-modified liposomal complex. Signal Transduct. Target. Ther. 2020, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Chen, M.; Lash, G.E. Role of osteopontin (OPN) in uterine spiral artery remodeling. Placenta 2022, 126, 70–75. [Google Scholar] [CrossRef]
- Sharma, A.; Seow, J.J.W.; Dutertre, C.A.; Pai, R.; Blériot, C.; Mishra, A.; Wong, R.M.M.; Singh, G.S.N.; Sudhagar, S.; Khalilnezhad, S.; et al. Onco-Fetal Reprogramming of Endothelial Cells Drives Immunosuppressive Macrophages in Hepatocellular Carcinoma. Cell 2020, 183, 377–394.e21. [Google Scholar] [CrossRef]
- Liu, X.; Xu, X.; Wu, Z.; Shan, Q.; Wang, Z.; Wu, Z.; Ding, X.; Huang, W.; Wang, Z. Integrated single-cell RNA-seq analysis identifies immune heterogeneity associated with KRAS/TP53 mutation status and tumor-sideness in colorectal cancers. Front. Immunol. 2022, 13, 961350. [Google Scholar] [CrossRef]
- Ohradanova-Repic, A.; Machacek, C.; Charvet, C.; Lager, F.; Le Roux, D.; Platzer, R.; Leksa, V.; Mitulovic, G.; Burkard, T.R.; Zlabinger, G.J.; et al. Extracellular Purine Metabolism Is the Switchboard of Immunosuppressive Macrophages and a Novel Target to Treat Diseases With Macrophage Imbalances. Front. Immunol. 2018, 9, 852. [Google Scholar] [CrossRef]
- Bayer, A.L.; Fraker, C.A. The Folate Cycle As a Cause of Natural Killer Cell Dysfunction and Viral Etiology in Type 1 Diabetes. Front. Endocrinol. 2017, 8, 315. [Google Scholar] [CrossRef]
- Yuan, R.; Li, S.; Geng, H.; Wang, X.; Guan, Q.; Li, X.; Ren, C.; Yuan, X. Reversing the polarization of tumor-associated macrophages inhibits tumor metastasis. Int. Immunopharmacol. 2017, 49, 30–37. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, A.; Lynn, R.C.; Poussin, M.; Eiva, M.A.; Shaw, L.C.; O’Connor, R.S.; Minutolo, N.G.; Casado-Medrano, V.; Lopez, G.; Matsuyama, T.; et al. CAR-T cell-mediated depletion of immunosuppressive tumor-associated macrophages promotes endogenous antitumor immunity and augments adoptive immunotherapy. Nat. Commun. 2021, 12, 877. [Google Scholar] [CrossRef]
- Wallace-Povirk, A.; Rubinsak, L.; Malysa, A.; Dzinic, S.H.; Ravindra, M.; Schneider, M.; Glassbrook, J.; O’Connor, C.; Hou, Z.; Kim, S.; et al. Targeted therapy of pyrrolo[2,3-d]pyrimidine antifolates in a syngeneic mouse model of high grade serous ovarian cancer and the impact on the tumor microenvironment. Sci. Rep. 2022, 12, 11346. [Google Scholar] [CrossRef]
- Scott, E.M.; Jacobus, E.J.; Lyons, B.; Frost, S.; Freedman, J.D.; Dyer, A.; Khalique, H.; Taverner, W.K.; Carr, A.; Champion, B.R.; et al. Bi- and tri-valent T cell engagers deplete tumour-associated macrophages in cancer patient samples. J. Immunother. Cancer 2019, 7, 320. [Google Scholar] [CrossRef]
- Sun, F.; Wang, S.; Du, M. Functional regulation of decidual macrophages during pregnancy. J. Reprod. Immunol. 2021, 143, 103264. [Google Scholar] [CrossRef] [PubMed]
- Orecchioni, M.; Ghosheh, Y.; Pramod, A.B.; Ley, K. Macrophage Polarization: Different Gene Signatures in M1(LPS+) vs. Classically and M2(LPS-) vs. Alternatively Activated Macrophages. Front. Immunol. 2019, 10, 1084. [Google Scholar] [CrossRef]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef] [PubMed]
- Boutilier, A.J.; Elsawa, S.F. Macrophage Polarization States in the Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 6995. [Google Scholar] [CrossRef]
- Zhang, Y.H.; He, M.; Wang, Y.; Liao, A.H. Modulators of the Balance between M1 and M2 Macrophages during Pregnancy. Front. Immunol. 2017, 8, 120. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Qu, X.; Zhang, M.; Zhang, L.; Yang, T.; Ma, M.; Jing, M.; Zhang, N.; Song, R.; Zhang, Y.; et al. Identification of a six-gene prognostic signature for bladder cancer associated macrophage. Front. Immunol. 2022, 13, 930352. [Google Scholar] [CrossRef]
- Bosmans, L.A.; van Tiel, C.M.; Aarts, S.; Willemsen, L.; Baardman, J.; van Os, B.W.; den Toom, M.; Beckers, L.; Ahern, D.J.; Levels, J.H.M.; et al. Myeloid CD40 deficiency reduces atherosclerosis by impairing macrophages’ transition into a pro-inflammatory state. Cardiovasc. Res. 2023, 119, 1146–1160. [Google Scholar] [CrossRef]
- Yao, Y.; Xu, X.H.; Jin, L. Macrophage Polarization in Physiological and Pathological Pregnancy. Front. Immunol. 2019, 10, 792. [Google Scholar] [CrossRef]
- Bezemer, R.E.; Schoots, M.H.; Timmer, A.; Scherjon, S.A.; Erwich, J.; van Goor, H.; Gordijn, S.J.; Prins, J.R. Altered Levels of Decidual Immune Cell Subsets in Fetal Growth Restriction, Stillbirth, and Placental Pathology. Front. Immunol. 2020, 11, 1898. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zhou, L.; Chen, J.; Lu, Y.; Cao, C.; Lv, S.; Wei, Z.; Wang, L.; Chen, J.; Hu, X.; et al. The Immune Atlas of Human Deciduas With Unexplained Recurrent Pregnancy Loss. Front. Immunol. 2021, 12, 689019. [Google Scholar] [CrossRef] [PubMed]
- Minami, K.; Hiwatashi, K.; Ueno, S.; Sakoda, M.; Iino, S.; Okumura, H.; Hashiguchi, M.; Kawasaki, Y.; Kurahara, H.; Mataki, Y.; et al. Prognostic significance of CD68, CD163 and Folate receptor-β positive macrophages in hepatocellular carcinoma. Exp. Ther. Med. 2018, 15, 4465–4476. [Google Scholar] [CrossRef]
- Moser, G.; Drewlo, S.; Huppertz, B.; Armant, D.R. Trophoblast retrieval and isolation from the cervix: Origins of cervical trophoblasts and their potential value for risk assessment of ongoing pregnancies. Hum. Reprod. Update 2018, 24, 484–496. [Google Scholar] [CrossRef]
- Frye, R.E.; Rossignol, D.A.; Scahill, L.; McDougle, C.J.; Huberman, H.; Quadros, E.V. Treatment of Folate Metabolism Abnormalities in Autism Spectrum Disorder. Semin. Pediatr. Neurol. 2020, 35, 100835. [Google Scholar] [CrossRef]
- Bobrowski-Khoury, N.; Sequeira, J.M.; Quadros, E.V. Brain Uptake of Folate Forms in the Presence of Folate Receptor Alpha Antibodies in Young Rats: Folate and Antibody Distribution. Nutrients 2023, 15, 1167. [Google Scholar] [CrossRef] [PubMed]

| Isoforms of FRs | GPI Anchor (Yes/No) | Species | Tissue | Localization of FRs Expression | References |
|---|---|---|---|---|---|
| FRα | Yes | Human | Placenta | syncytiotrophoblast (apical surface) | [29,84,104,105,106,107,108] |
| Decidua | decidual glandular epithelial cells, decidual stromal cells | ||||
| Mouse | Placenta | syncytiotrophoblasts, trophoblast giant cells, glycogen trophoblast cells and their progenitors | [109] | ||
| Rat | Placenta | syncytiotrophoblasts, cytotrophoblasts | [26,110] | ||
| Decidua | decidual cells | ||||
| FRβ | Yes | Human | Decidua | decidual monocytes/macrophages | [7,84,108] |
| FRγ | No | Human | (?) | secreted by neutrophils (?) | [111,112,113,114] |
| FRδ | Yes | Human/Mouse | Decidua (?) | decidual Treg cells (?) | [115,116,117] |
| Ref. | Year | Subjects | Methods | Findings/Conclusions |
|---|---|---|---|---|
| [26] | 2003 | Rat | Intraperitoneal injection of antiserum to FRs at GD8 |
|
| [27] | 2009 | Human | Case-control study included in subfertility women (n = 17) and healthy controls (n = 25) |
|
| [135] | 2015 | Human | Case report of a FRAbs-positive woman with recurrent pregnancy losses |
|
| [133] | 1983 | Human | Retrospective study included in 280 sibships | There exist pairwise relationships among the NTD, FRAbs and miscarriage
|
| [134] | 2002 | Human | Case-control study included in cases of positive maternal serum screening results for Down syndrome (n = 189) and matched controls (n = 945) | |
| [137] | 2004 | Human | Case-control study enrolled in women of NTD-pregnancy (n = 12) and controls (n = 24) | |
| [85] | 2008 | Human | Observational study collected maternal serum during 15–18th week of pregnancy | |
| [91,92] | 2015 | Mouse | Mouse fed with folate-free diet for 5 weeks before mating | Risk factor involving in FRAbs production were correlated with miscarriage
|
| Characteristics/Functions | Source of Macrophages | Details | References |
|---|---|---|---|
| Differentiation | Differentiate from hematopoietic stem cells |
| [171,172] |
| Recruitment | Recruit from peripheral monocytes |
| [127,173,174,175] |
| Polarization | Induced from monocytes by M-CSF |
| [176] |
| Lung macrophages/Monocyte-derived macrophages |
| [177,178] | |
| [7,178,179,180] | ||
| [126,181] | ||
| Pro-angiogenesis | TAMs (Breast/lung cancer) |
| [182,183] |
| HBCs |
| [127,184] | |
| Immune regulation | TAMs (Hepatocellular/colorectal tumor, melanoma) |
| [176,185,186] |
| Synovial macrophages |
| [187,188] | |
| Peritoneal macrophages |
| [189] | |
| TAMs |
| [190,191,192] | |
| HBCs |
| [127] | |
| TAMs (Breast cancer) |
| [182] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, X.-Y.; Ha, S.-Y.; Chen, L.; Zhang, T.; Li, M.-Q. Recent Advances in Folates and Autoantibodies against Folate Receptors in Early Pregnancy and Miscarriage. Nutrients 2023, 15, 4882. https://doi.org/10.3390/nu15234882
Qin X-Y, Ha S-Y, Chen L, Zhang T, Li M-Q. Recent Advances in Folates and Autoantibodies against Folate Receptors in Early Pregnancy and Miscarriage. Nutrients. 2023; 15(23):4882. https://doi.org/10.3390/nu15234882
Chicago/Turabian StyleQin, Xue-Yun, Si-Yao Ha, Lu Chen, Tao Zhang, and Ming-Qing Li. 2023. "Recent Advances in Folates and Autoantibodies against Folate Receptors in Early Pregnancy and Miscarriage" Nutrients 15, no. 23: 4882. https://doi.org/10.3390/nu15234882
APA StyleQin, X.-Y., Ha, S.-Y., Chen, L., Zhang, T., & Li, M.-Q. (2023). Recent Advances in Folates and Autoantibodies against Folate Receptors in Early Pregnancy and Miscarriage. Nutrients, 15(23), 4882. https://doi.org/10.3390/nu15234882








