Abstract
This study aims to evaluate the prebiotic potential of polysaccharides derived from Stellariae Radix (SRPs) and explore their influence on the gut microbiota composition in mice. Lactobacillus acidophilus and Bifidobacterium longum were cultivated in an MRS medium, while their growth kinetics, clumping behavior, sugar utilization, pH variation, growth density, and probiotic index were meticulously monitored. Additionally, the impact of crude Stellariae Radix polysaccharides (CSRP) on the richness and diversity of gut microbiota in mice was assessed via 16S rDNA sequencing. The results demonstrated the remarkable ability of CSRPs to stimulate the proliferation of Lactobacillus acidophilus and Bifidobacterium longum. Moreover, the oral administration of CSRPs to mice led to a noticeable increase in beneficial bacterial populations and a concurrent decrease in detrimental bacterial populations within the intestinal flora. These findings provided an initial validation of CSRPs as a promising agent in maintaining the equilibrium of gut microbiota in mice, thereby offering a substantial theoretical foundation for developing Stellariae Radix as a prebiotic ingredient in various applications, including food, healthcare products, and animal feed. Furthermore, this study presented novel insights for the exploration and utilization of Stellariae Radix resources.
1. Introduction
Lactobacillus acidophilus and Bifidobacterium longum are representative probiotics found in the intestines, and they offer numerous benefits for human health [1]. Probiotics produce beneficial substances for the intestines, such as short-chain fatty acids (SCFAs), lactic acid, and other acidic metabolites, through prebiotics. These substances play a crucial role in maintaining intestinal health [2]. Prebiotics are food components that selectively ferment and alter the composition and activity of beneficial bacteria in the intestines. They mainly consist of non-digestible oligosaccharides and oligose [3]. Many studies have demonstrated that regulating the gut microbiota can reduce the risk of chronic metabolic diseases, including type 2 diabetes, obesity, and non-alcoholic fatty liver disease [4]. The gut microbiota prevents the infection of external pathogens through direct mechanisms (such as competition for nutrients and niches) and indirect mechanisms (such as enhancing the host’s defense) [5]. Therefore, maintaining and regulating the balance of the intestinal microecology is of great significance in disease prevention and promoting overall host health.
Polysaccharides are complex carbohydrates with various biological activities [6]. Plant polysaccharides are typically not directly or fully absorbed by the intestine. Instead, they are broken down into SCFAs and other beneficial metabolites through interactions with intestinal microbes and are then absorbed by the intestine [7]. In recent years, numerous studies have suggested that non-digestible polysaccharides may serve as potential prebiotics for the gut microbiota, leading to changes in microbial structure and composition [8]. For instance, Lycium barbarum polysaccharides have been found to promote the growth of Lactobacillus acidophilus and increase the proportion of intestinal probiotics, such as Akkermansia, Lactobacillus acidophilus, and Prevotellaceae, in mice [9]. Similarly, Dendrobium huoshanense polysaccharides have been shown to stimulate the production of cytokines, induce the proliferation and differentiation of immune cells in the small intestine, increase the relative abundance of Lactobacillus, Prevotella, and Porphyromonas in the mouse colon, and reduce the abundance of Helicobacter and Chlorotriazole [10]. Therefore, further research is needed to understand the role of polysaccharides in the gut microbiota and their impact on overall body health.
The traditional Chinese medicine Stellariae Radix is derived from the dried root of Stellaria dichotoma L. var. lanceolata Bge. [11]. It is characterized by its cold and sweet properties and is known for its efficacy in treating infantile malnutrition and bone tuberculosis [12]. With the advancement of modern medical research, the anti-inflammatory, anti-allergic, and anti-cancer effects of Stellariae Radix have been continuously discovered, enhancing its medicinal value. The effective components and pharmacological effects of Stellariae Radix, as a traditional Chinese medicine, have been extensively reported [13,14,15]. However, there has been no research conducted on the impact of Stellariae Radix polysaccharides (SRPs) on the intestinal microflora.
In this study, Lactobacillus acidophilus and Bifidobacterium longum were cultured in an MRS medium to monitor the dynamic changes in the growth curve, clump count, pH, growth density, and probiotic index of these two probiotics. Additionally, the effects of crude Stellariae Radix polysaccharides (CSRPs) on the abundance and diversity of gut microbiota in mice were analyzed using 16S rDNA. These findings provide a theoretical basis for the diversified development of food, health products, feed, and other related fields. Furthermore, they offer new insights for the development and utilization of Stellariae Radix resources.
2. Materials and Methods
2.1. Materials
Stellariae Radix was purchased from Ningxia, China. Lactobacillus acidophilus (CGMCC1.3013) and Bifidobacterium longum (CGMCC1.1878) were purchased from the China General Microbiological Culture Collection Center. The bacterial strains were inoculated in a broth (MRS) medium and activated under anaerobic conditions (37 °C, 12 h).
2.2. Preparation Process of CSRPs
Stellariae Radix medicinal materials were crushed through a 40-mesh sieve and degreased by supercritical CO2 extraction (a pressure of 35 MPa, an extraction temperature of 47 °C, a CO2 flow rate of 50 L/h, and extraction for 3 h). Then, ultrasonic-assisted extraction was used after concentration and fractional alcohol precipitation, and CSRPs were obtained by the Sevag deproteinization method [16].
2.3. Evaluation of Prebiotic Activity In Vitro
The growth and acid production capacity of Lactobacillus acidophilus and Bifidobacterium longum were determined to evaluate the prebiotic activity of CSRPs [17]. MRS carbohydrate-free broth (Qingdao Top Biotech Co., Ltd., Qingdao, China) was used as the basal medium. In order to cultivate Bifidobacterium longum, 5 g/L cysteine was added to the MRS medium as the reducing agent. The existing literature shows that the two probiotics have an optimal growth concentration of 2.5% (w/v) inulin; thus, 2.5% (w/v) Glc (negative control without inulin) and 2.5% (w/v) inulin are selected as the control groups [9]. Then, 2.5% (w/v) inulin (MRS-L) and 2.5% (w/v) Glc with different concentrations of CSRPs (2.5%, 5%, 10%, and 15% (w/v)) were added to the MRS broth medium as carbohydrate sources, and MRS-L was used as the control group. The broth medium was inoculated with 5% (v/v) (1−2 × 109 CFU/mL) activated Lactobacillus acidophilus or Bifidobacterium longum. And the culture medium was incubated under anaerobic conditions for 48 h (37 °C, 120 rpm), and random sampling was performed every 3 h to determine the OD value of the culture medium. In addition, the logistic model in Origin Pro 2019b software [18] was used, and the iterative algorithm used was the Levernberg–Marquardt optimization algorithm. And the algorithm (expression (1)) was as follows, and the fitting growth curves of two bacteria were obtained.
y = A2 + (A1 − A2)/(1 + (X/X0)^p)
In the formula, A1, A2, X0, and p are parameters; A1 is the deviation degree between the real curve and the model; A2 is the growth maximum predicted by the model; X0 is the inflection point time, representing the maximum growth rate at this time; and p is the growth rate coefficient, and the curve has a maximum slope at the crossing point (X0, A2), indicating the maximum growth rate.
The growth number of two probiotic strains was determined via the plate counting method, expressed as log CFU/mL. The control groups are 2.5% (w/v) Glc and 2.5% (w/v) inulin. The broths were inoculated with 5% (v/v) (10 × 109 CFU/mL) of stationary-phase Lactobacillus acidophilus and Bifidobacterium longum. The cultures were then incubated at 37 °C for 24 h with agitation (120 rpm) under anaerobic conditions. After incubation, serial dilutions (10−1–10−8) were performed using sterile normal saline and plated on an MRS agar at 37 °C for 48 h under anaerobic conditions. The results were recorded as CFU/mL of culture. The acid production ability and growth of the two probiotic strains with pH and OD values were determined, and the probiotic values (PIs) were calculated.
2.4. Evaluation of Prebiotic Activity In Vivo
Clean-grade ICR male mice (8 weeks old; weight: 20.0 ± 2.0 g) were purchased from the Experimental Animal Center of Ningxia Medical University (Ningxia, China) (Certificate No. SCXK Ningxia 2020-0001). The animal housing and feeding conditions were as follows: temperature of 20–26 °C, relative humidity of 40–70%, alternating light and dark for 12 h, free drinking water, and feeding with standard blocontrol maintenance feed.
The mice were randomly divided into four groups (6 in each group), respectively, the control group (CK), the low-dose group (L), the middle-dose group (M), and the high-dose group (H), and were allowed to acclimate for 1 week before the experiment. The control group (intragastric administration of normal saline), L group (CSRPs: 1 g/kg), M group (CSRPs: 2 g/kg), and H group (CSRPs: 4 g/kg), underwent an intragastric administration of 0.1 mL/10 g continuously for 14 days. After 24 h from the last administration, the blood was taken from the eyeballs of the mice, and fresh feces were collected.
2.5. Composition and Diversity of Gut Microbiota
The 16S rDNA amplicon sequencing technology was used to study microbial composition and structure in the mice’s intestine. Total microbial genomic DNA was extracted from the samples using the E.Z.N.A.® soil DNA kit (Omega Bio-tek, Norcross, GA, USA) according to the manufacturer’s instructions. The quality and concentration of DNA were determined with 1.0% agarose gel electropHoresis and a NanoDrop® ND-2000 spectropHotometer (Thermo Scientific Inc., Waltham, MA, USA). The hypervariable region, V3-V4, of the bacterial 16S rRNA gene was amplified with the primer pairs 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) by an ABI GeneAmp® 9700 PCR thermocycler (ABI, Los Angeles, CA, USA). All samples were amplified in triplicate after mixing PCR products of the same sample, and the PCR product was extracted from a 2% agarose gel and purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA) according to the manufacturer’s instructions and quantified using a Quantus™ Fluorometer (Promega, Madison, WI, USA). An Illumina Pair-End library was constructed, and the sub-library was expanded to perform pairing. Online measurements on the Illumina MiSeq platform were carried out.
Using UPARSE 7.1, the sequences were clustered into operational taxonomic units (OTUs) with 97% consistency. Bacterial annotation of OTU sequences was carried out, and a bacterial annotation analysis was carried out using the Mothur method and the SSUrRNA database of SILVA132 to obtain taxonomic information and the composition of the bacterial colonies of the samples to be tested. Alpha diversity indices for evaluating gut microbial community richness were calculated. A beta diversity analysis was used to investigate the structural variations in the microbial communities using the UniFrac distance.
2.6. Statistical Analysis
Data are presented as the mean ± SD, and the statistical analysis was performed using Origin 2019 software (OriginLab Corporation, Northampton, MA, USA). A one-way analysis of variance (ANOVA) plus the post hoc Duncan’s test (SPSS software, version 22.0) were used to evaluate the statistical significance. The Wilcox rank sum test and Tukey test were used to analyze the differences in bacterial diversity between the groups. A bioinformatic analysis of the gut microbiota was carried out using the Majorbio Cloud platform (https://cloud.majorbio.com, accessed on 30 September 2021).
3. Results
3.1. Composition of Polysaccharide from Stellariae Radix
The SRP consists of Gal, Glc, Xyl, Fru, Man, and Rha, with molar percentages of 61.86%, 32.51%, 4.77%, 0.39%, 0.28%, and 0.19%, respectively. It has a molecular weight of 31,309 Da, and the sugar ring structure of pyran sugar contains α-configuration glycosidic bonds and β-configuration glycosidic bonds [19].
3.2. Evaluation of Prebiotic Activity In Vitro
3.2.1. Fitting of Growth Curve
The growth curves of the two probiotic strains are plotted in Figure 1, and the model fitting had determination coefficient (R2) values greater than 0.9, indicating a good fitting effect. Figure 1a shows the growth curve of Lactobacillus acidophilus with different carbon (2.5% (w/v) inulin (MRS-L), 2.5% (w/v) Glc, and different concentrations of CSRP (2.5%, 5%, 10%, and 15% (w/v))) sources. A lag phase occurred from 0 to 3 h, followed by a log phase from 3 to 12 h. After 12 h, the growth rate slowed down, and from 15 to 48 h, a stationary phase was observed. Figure 1b depicts the growth curve of Bifidobacterium longum with different carbon (2.5% (w/v) inulin (MRS-L), 2.5% (w/v) Glc, and different concentrations of CSRP (2.5%, 5%, 10%, and 15% (w/v))) sources. In general, an extended lag phase was observed from 3 to 9 h, followed by a log period from 9 to 18 h. After 18 h, the growth rate decreased, and from 21 to 48 h, a stationary phase was observed.
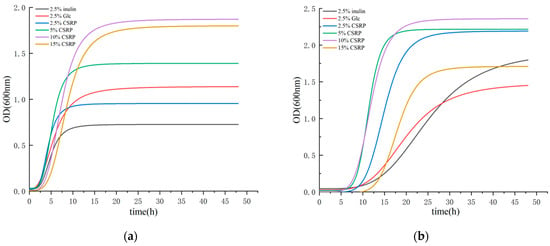
Figure 1.
(a) Growth fitting curves of Lactobacillus acidophilus; (b) growth fitting curves of Bifidobacterium longum. (The concentration 10% CSRPs is the optimal concentration for Lactobacillus acidophilus and Bifidobacterium longum growth).
By referring to Table 1, it can be seen that both probiotic strains exhibited rapid growth in the medium with different carbon (2.5% (w/v) inulin (MRS-L), 2.5% (w/v) Glc, and different concentrations of CSRP (2.5%, 5%, 10%, and 15% (w/v))) sources. In the medium with different carbon sources for Lactobacillus acidophilus, the addition of the 2.5% and 5% CSRP groups resulted in a faster entry into the logarithmic growth phase compared to the control group (2.5% inulin and 2.5% Glc) (CSRP group X0 < control group X0). However, the addition of the 10% and 15% CSRP groups entered the log phase later than the control group, and the former also entered the stationary phase earlier. Similarly, Bifidobacterium longum in different carbon-source mediums with different concentrations of CSRP groups entered the log phase faster than the control group (2.5% inulin and 2.5% Glc) (CSRP group X0 < control group X0). Furthermore, the maximum growth number of both probiotic strains in each CSRP concentration group was greater than the control group (the CSRP group’s p-value was greater than that of the control group) after entering the stable phase. These results indicate that the CSRP groups, at each concentration, effectively enhance the metabolic rate of the two beneficial strains and promote their proliferation compared to the control group (2.5% inulin and 2.5% Glc).

Table 1.
Nonlinear fitting parameters of growth kinetics equation.
3.2.2. Effects of CSRPs on Growth of Lactobacillus Acidophilus and Bifidobacterium Longum
The effects of CSRPs on the growth of Lactobacillus acidophilus and Bifidobacterium longum were evaluated by measuring the total number of viable bacteria (log10 CFU/mL). According to Table 2, the maximum growth of Lactobacillus acidophilus and Bifidobacterium longum was observed at a concentration of 10% CSRPs, while the growth showed a downward trend at the highest concentration.

Table 2.
Effects of CSRPs on the growth of Lactobacillus acidophilus and Bifidobacterium longum.
3.2.3. Effects of CSRPs on Acid Production of Two Probiotics
Polysaccharides serve as a carbon source that can be decomposed and utilized by probiotics. During this process, the probiotics produce short-chain fatty acids and other substances, leading to a decrease in pH. To evaluate the growth of probiotics, the changes in pH were monitored. In Figure 2, the pH changes of Lactobacillus acidophilus (Figure 2a) and Bifidobacterium longum (Figure 2b) are shown when CSRPs were used as a carbon source, replacing glucose in the MRS medium.
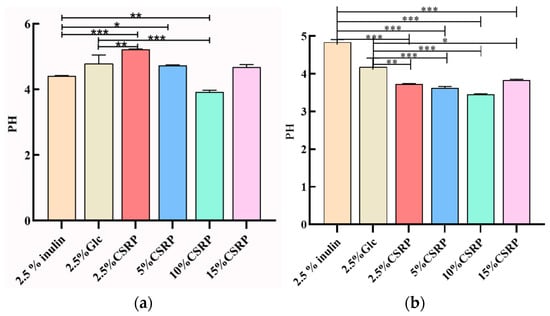
Figure 2.
(a) Effects of different concentrations of CSRPs on acid production of Lactobacillus acidophilus; (b) effects of different concentrations of CSRPs on acid production of Bifidobacterium longum. (* p < 0.05, ** p < 0.01, *** p < 0.001). (10% CSRPs can promote acid production by probiotics).
For Lactobacillus acidophilus, the pH of the 10% CSRP group was significantly lower than that of the control group (2.5% inulin and 2.5% Glc). Similarly, for Bifidobacterium longum, the pH of the different concentrations of CSRP groups was significantly lower than that of the control group (2.5% inulin and 2.5% Glc). These results indicate that CSRPs can promote the growth of probiotics by reducing the pH of the medium and consuming the carbon source.
3.2.4. Effects of Different Concentrations of CSRPs on Growth Density of two Probiotic Strains
The growth of probiotics can also be assessed by measuring the optical density (OD) values of the culture medium, as they reflect the growth rate of the strains. In Figure 3a,b, the OD values of the culture medium for both Lactobacillus acidophilus and Bifidobacterium longum are recorded. It was observed that with the increase in CSRP concentrations, the OD values gradually increased, reaching their maximum value in the 10% CSRP group. This indicates that higher concentrations of CSRPs promote the growth of probiotics, resulting in higher OD values in the culture medium.
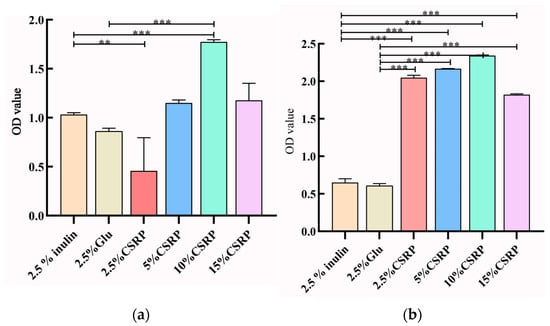
Figure 3.
(a) Effects of different concentrations of CSRPs on growth density of Lactobacillus acidophilus; (b) effects of different concentrations of CSRPs on growth density of Bifidobacterium longum. ** p < 0.01, *** p < 0.001). (10% CSRP can promote the growth of probiotics).
3.2.5. Probiotic Index Calculation of Different Concentrations of CSRPs on Two Probiotic Strains
As shown in the Table 3, the results of the two probiotic strains showed that the addition of 10% CSRPs to the medium had the best effect on promoting the probiotic strains’ growth.

Table 3.
Effects of CSRPs on PI values of probiotics.
3.3. Evaluation of Prebiotic Activity In Vivo
Effects of CSRP Intake on the Composition of Cecal Gut Microbiotas
A high-throughput analysis of the 16S rDNA V3-V4 region of the mice feces revealed a total of 1,844,690 sequences and 477 operational taxonomic units (OUTs). The data were stored in NCBI sequence reading files with the login number SRP463499. To assess the richness and diversity of the microbial community, the Chao1 index and Ace index were used to analyze richness, while the Shannon index and Simpson index were used to describe diversity and evenness. Coverage was used to represent the coverage of the microbial community [20]. The results, shown in Table 4, indicated that as the concentration of CSRPs increased, the index values initially increased and then decreased. Among the groups, the M group exhibited the highest richness, while the H group had the highest diversity.

Table 4.
α-diversity index of gut microbiota in each group.
Additionally, a principal component analysis (PCA) (Figure 4A) and the unweighted UNIFRAC distance were employed to calculate a Principal Coordinate Analysis (PCoA) (Figure 4B) to evaluate the community composition. The results demonstrated a significant separation between the groups, indicating a significant difference in gut microbiota with or without the intake of CSRPs. The analysis of the gut microbiota composition at the phylum level revealed that the dominant microbial phyla were Bacteroidota, Firmicutes, Actinobacteriota, Desulfobacterota, and Campilobacterota (Figure 4C). At the genus level, the dominant flora included Muribaculaceae, Alloprevotella, Dubosiella, Bacteroides, Lactobacillus, Lachnospiraceae, Faecalibaculum, Bifidobacterium, Eubacterium, and others. With increasing concentrations of CSRPs, the relative abundance of beneficial bacteria such as Dubosiella, Lactobacillus, Faecalibaculum, and Bifidobacterium increased, while the growth of Bacteroides and Enterorhabdus was inhibited (Figure 4D).
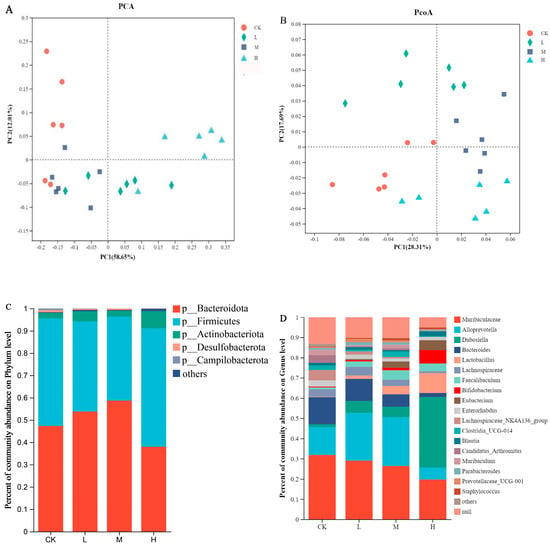
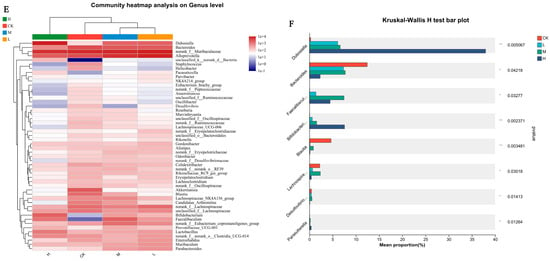
Figure 4.
Effects of CSRPs on gut microbiota in mice. Control group was intragastrically administered with normal saline, L group was intragastrically administered with low-dose CSRP, M group was intragastrically administered with medium-dose CSRP, and H group was intragastrically administered with high-dose CSRP. (A) Principal component analysis (PCA); (B) calculating principal component analysis based on unweighted UNIFRAC distance (PCoA); (C) bacteria relative abundance at phylum level (%); (D) bacteria relative abundance at genus level (%); (E) bacterial clustering heat map; (F) significance test of difference between groups, * p < 0.05, ** p < 0.01.
A cluster analysis revealed high composition similarity at the genus level within each group, with some genera showing high similarity and others displaying significant differences. As the concentrations of CSRPs increased, the differences between the groups became more pronounced (Figure 4E). A significant difference test analysis of gut microbiota at the genus level was conducted between the groups, and the results indicated significant differences in the bacterial composition of gut microbiota at the genus level in mice receiving different intragastric doses. This included Dubosiella, Bacteroides, Faecalibaculum, Bifidobacterium, Trichospirillum, Eubacterium, Segmented filamentous bacteria, Faecalibacterium, and Enterorhabdus (one-way ANOVA) (Figure 4F).
4. Discussion
Physicochemical changes in prebiotics can have a significant impact on the composition, digestive function, and immune response of the gastrointestinal microbiota. Prebiotics are selectively utilized by beneficial microorganisms in the host and have various health benefits. They can enhance resistance to pathogens, regulate immune function, improve mineral absorption, promote intestinal function, affect metabolism, and contribute to satiety [21]. Probiotics, on the other hand, play a role in immune regulation, the production of organic acids and antimicrobial compounds, interactions with resident microbiota, interactions with the host, improvement in intestinal barrier integrity, and enzyme formation [2]. Stellariae Radix is a traditional Chinese medicine, but its polysaccharides have been rarely studied. Our research has demonstrated that Stellariae Radix contains abundant water-soluble polysaccharides and possesses free radical scavenging effects [16]. In this study, we found that CSRPs can promote the growth of Lactobacillus acidophilus and Bifidobacterium longum in an MRS medium. This was evident from the significant increase in the sugar consumption and clump count of the two probiotics, as well as the reduction in pH in the sugar-free MRS medium. The PI values also indicated that the addition of 10% crude polysaccharides in the medium had the most pronounced effect on promoting the growth of probiotics. Therefore, our in vitro evaluation of prebiotic activity showed that CSRPs exhibited stronger prebiotic activity than inulin in promoting the growth of these two probiotics. This provides a theoretical basis for the potential development of Stellariae Radix as a prebiotic drug in the fields of food, healthcare products, and animal feed.
The gut microbiota plays a crucial role in human health and is associated with various diseases. The treatment and prevention of many intestinal-related diseases using probiotics and prebiotics involve modulating the microbiota and/or its functions, such as increasing the proportion of probiotics in the intestine and regulating the intestinal microenvironment [22]. In the intestine, Firmicutes and Bacteroidetes are the dominant bacterial phyla, and their proportion changes have been studied in relation to diseases such as obesity. Obesity is characterized by an increase in the proportion of Firmicutes and Bacteroidetes in the gut microbiota [23]. In our study, the results of the alpha diversity analysis showed that the low-dose and medium-dose groups increased the diversity and richness of the gut microbiota compared to the control group, while the high-dose group reduced them. At the phylum level, the low-dose and medium-dose groups reduced the abundance of Firmicutes and increased the abundance of Bacteroidetes, thereby reducing the Firmicutes/Bacteroidetes ratio. This suggests that low and medium doses of CSRPs in mice may have a preventive and therapeutic effect on obesity by modulating the intestinal environment. Firmicutes and Bacteroidetes represent both beneficial and potentially harmful bacteria, and their relative abundance lies between the optimal levels [24]. Therefore, the reduction in the diversity and richness of the gut microbiota observed in the high-dose group may be due to a decrease in the abundance or even the elimination of certain harmful bacteria.
In the analysis of bacterial composition at the genus level, the hypothesis was confirmed. With an increase in the concentration of crude polysaccharides, the relative abundance of Dubosiella, Faecalibaculum, Bifidobacterium, and Lactobacillus significantly increased, while harmful bacteria such as Enterorhabdus decreased significantly. It has been reported that Dubosiella, Bifidobacterium, and Lactobacillus are positively correlated with preventing and improving obesity and related diseases [25]. Zhu et al. found that Dubosiella can improve abnormal indexes in obese mice induced by a high-fat diet, reduce LDL-C, TG, and body weight, improve the digestion and absorption ability of glucose in mice, and reduce the expression levels of lipid metabolism genes such as CD36, FASN, and PPARγ in the liver, thereby alleviating the disorder of lipid metabolism caused by obesity [24]. Additionally, Dubosiella can increase the abundance of beneficial microorganisms (Bifidobacterium and Lactobacillus) in obese mice, improve the disorder of intestinal flora structure caused by a high-fat diet, improve intestinal metabolism and immunity, and reduce the occurrence of bacterial infections and other diseases. Numerous studies have also found that the content of Dubosiella increases during the treatment of obesity [26,27,28,29,30,31,32]. It has been proven to improve the metabolism of the body, normalize blood lipid metabolism, and increase the content of short-chain fatty acids (SCFAs) such as butyrate. Butyrate has the ability to promote the conversion of white adipose tissue (WAT) to brown adipose tissue (BAT). WAT stores energy, while BAT uses energy for heat production, resulting in increased energy consumption by the host and the degradation of fat by regulating the host’s TCA cycle. In this study, with the increase in CSRP concentration, the abundance of Lactobacillus, Bifidobacterium, and Dubosiella in the intestinal tract of the mice significantly increased. Therefore, this further supports the speculation that CSRPs can regulate the gut microbiota and potentially treat obesity.
Furthermore, certain intestinal microorganisms (Bacteroides, Prevotella, and Lactobacillus) play a crucial role in hydrolyzing polysaccharides into soluble cytokines [33]. The microbial fermentation of dietary carbohydrates, including prebiotics, promotes the production of SCFAs [34]. SCFAs are well-known signaling molecules. Firstly, SCFAs create an acidic environment in the colon, inhibiting the growth of pathogens. Secondly, SCFAs can regulate the growth and differentiation of intestinal cells and provide them with energy [35,36]. Studies have shown that Bacteroides can stimulate the production of propionic acid, which limits acetate synthesis and reduces SCFA synthesis, resulting in a negative correlation between SCFA content and the number of Bacteroides in the intestine [37]. In this study, compared to the control group, the treatment groups significantly reduced the relative abundance of Bacteroides in the intestine (p < 0.05) and inhibited its growth with increasing dosage. This indicates that crude polysaccharides can effectively inhibit the growth of Bacteroides, providing a theoretical basis for further studying the effects of SRPs on the SCFA content in the intestine.
5. Conclusions
In summary, this study provides a solid foundation for understanding the prebiotic effect of CSRPs. It demonstrates that CSRPs have the ability to enhance the growth of beneficial bacteria in mice, surpassing the prebiotic activity of inulin in vitro. Additionally, CSRPs have the potential to regulate the abundance and diversity of gut microbiota in mice. These findings suggest that a CSRP could be utilized as a prebiotic to modulate the gut microbiota, paving the way for the further exploration and utilization of Stellariae Radix as a valuable medicinal resource. This study provides a crucial theoretical basis for future research on CSRPs.
6. Patents
Peng, L., Wang, H., Song, L., Feng, L., Niu, P.L., Li, Z.K., Li, Y.Q., Li, H.S. & Wu, W., et al. A crude polysaccharide of Stellariae Radix with prebiotic activity, and its preparation method [P]. China: CN115677874A, 3 February 2023.
Author Contributions
H.W.: performed the study and analyzed the results. H.L.: data interpretation and manuscript revision. Z.L.: conceptualization and data interpretation. L.F.: performed the study and analyzed the results. L.P.: designed the study, contributed to the concept generation, and wrote and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Key Research and Development Program of Ningxia (No. 2021BEG02042).
Institutional Review Board Statement
The investigation complies with the Guide for Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 2011) and was approved by the Experimental Animal Ethics Committee of Ningxia Medical University (Production license number: SCXK (Ning) 2020-0001. Usage license number: SYXK(Ning) 2020-0001).
Data Availability Statement
The original contributions presented in the study are included in the article, and further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Altermann, E.; Russell, W.M.; Azcarate-Peril, M.A.; Barrangou, R.; Buck, B.L.; McAuliffe, O.; Souther, N.; Dobson, N.; Duong, T.; Callanan, M. Complete genome sequence of the probiotic lactic acidbacterium Lactobacillus acidophilus NCFM. Proc. Natl. Acad. Sci. USA 2005, 102, 3906–3912. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wu, Y.-J.; Sun, P.-L.; Zhang, F.-M.; Linhardt, R.-J.; Zhang, A.-Q. Chemically modified polysaccharides: Synthesis, characterization, structure activity relationships of action. Int. J. Biol. Macromol. 2019, 132, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lu, C.; Wu, C.; Xu, M.; Kou, X.; Kong, D.; Jing, G. Polysaccharide of Boschniakia rossica induces apoptosis on laryngeal carcinoma Hep2 cells. Gene 2014, 536, 203–206. [Google Scholar] [CrossRef]
- Yang, Y.; Ji, J.; Di, L.; Li, J.; Hu, L.; Qiao, H.; Wang, L.; Feng, Y. Resource, chemical structure and activity of natural polysaccharides against alcoholic liver damages. Carbohydr. Polym. 2020, 241, 116355. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.-Y.; Aweya, J.J.; Li, N.; We, H.; Sun, C.; Li, J.; Jiang, Y. Microbial catabolism of Porphyra haitanensis polysaccharides by human gut microbiota. Food Chem. 2019, 289, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Fang, Y.; Nishinari, K.; We, H.; Sun, C.; Li, J.; Jiang, Y. Physicochemical characteristics of polysaccharide conjugates prepared from fresh tea leaves and their improving impaired glucose tolerance. Carbohydr. Polym. 2014, 112, 77–84. [Google Scholar] [CrossRef]
- Chen, R.; Liu, B.; Wang, X.; Chen, K.; Zhang, K.; Zhang, L.; Fei, C.; Wang, C.; Liu, Y.; Xue, F. Effects of polysaccharide from Pueraria lobata on gut microbiota in mice. Int. J. Biol. Macromol. 2020, 158, 740–749. [Google Scholar] [CrossRef]
- Porter, N.T.; Martens, E.C. The Critical Roles of Polysaccharides in Gut Microbial Ecology and Physiology. Annu. Rev. Microbiol. 2017, 71, 349–369. [Google Scholar] [CrossRef]
- Zhu, W.; Zhou, S.; Liu, J.; McLean, R.; Chu, W. Prebiotic, immuno-stimulating and gut microbiota-modulating effects of Lycium barbarum polysaccharide. Biomed. Pharmacother. 2020, 121, 109591. [Google Scholar] [CrossRef]
- Xie, S.-Z.; Liu, B.; Ye, H.-Y.; Li, Q.-M.; Pan, L.-H.; Zha, X.-Q.; Liu, J.; Duan, J.; Luo, J.-P. Dendrobium huoshanense polysaccharide regionally regulates intestinal mucosal barrier function and intestinal microbiota in mice. Carbohydr. Polym. 2019, 206, 149–162. [Google Scholar] [CrossRef]
- Fang, W.P.; Zhang, Z.R. Flora Reipublicae Popularis Sinicae; Science Press: Beijing, China, 2004. [Google Scholar]
- China Pharmacopoeia; National Pharmacopoeia Committee: Beijing, China, 2020; pp. 330–331.
- Li, Z.K.; Wang, H.; Song, L.; Feng, L.; Li, Y.Q.; Yang, Y.; Peng, L. Origin Characteristics and Correlation Analysis of Stellarae Radix Based on Inorganic Elements and Effective Components. Chin. J. Mod. Appl. Pharm. 2023, 40, 894–902. [Google Scholar]
- Song, L.; Wang, H.; Li, Z.K.; Liu, D.H.; Peng, L. Optimization of Supercritical CO2 Extraction Process and Component Analysis of Extract from Stellariae Radix. Chin. J. Mod. Appl. Pharm. 2022, 39, 2498–2504. [Google Scholar]
- Li, Z.K.; Ma, L.; Bai, M.S.; Huang, Y.X.; Song, L.; Wang, H.; Feng, L.; Peng, L. Infrared fingerprint of Ningxia Stellariae Radix based on chemometrics and discrimination analysis of counterfeit products. Mod. Tradit. Chin. Med. Mater. Medica-World Sci. Technol. 2023, 25, 175–183. [Google Scholar]
- Wang, H.; Peng, L.; Song, L.; Feng, L.; Li, Z.K.; Li, Y.Q.; Gao, T. Optimization of Ultrasonic-assisted Extraction Process and Analysis of Antioxidant Activity of Polysaccharide from Stellariae Radix. Food Ind. Sci. Technol. 2023, 1–12. [Google Scholar] [CrossRef]
- Zhang, T.Y.; Zhang, X.Y.; Wang, D.P.; Xu, X.X. The Effect of Polysaccharide of Poriacocos on Key Metabolites of Bifidobacterium BB-12. Sci. Technol. Food Ind. 2022, 43, 24–33. [Google Scholar]
- Zwietering, M.; Jongenburger, I.; Rombouts, F.; Riet, K. Modeling of the bacterial growth curve. Appl. Environ. Microbiol. 1990, 56, 1875–1881. [Google Scholar] [CrossRef]
- Su, J.; Su, L.; Li, D.; Shuai, O.; Zhang, Y.; Liang, H.; Jiao, C.; Xu, Z.; Lai, Y.; Xie, Y. Antitumor Activity of Extract from the Sporoderm-Breaking Spore of Ganoderma lucidum: Restoration on Exhausted Cytotoxic T Cell with Gut Microbiota Remodeling. Front. Immunol. 2018, 9, 1765. [Google Scholar] [CrossRef]
- Peng, L.; Wang, H.; Song, L.; Feng, L.; Niu, P.L.; Li, Z.K.; Li, Y.Q.; Li, H.S.; Wu, W. A Crude Polysaccharide of Stellariae Radix with Prebiotic Activity, and Its Preparation. Method. Patent CN115677874A, 3 February 2023. [Google Scholar]
- Liu, B.; Liu, T.T.; Zhang, X.K.; Wang, T.; Zhang, C.X.; Li, M.; Zheng, X.Y.; Wang, D.P.; Zhang, D.Z.; Meng, X.J. Research progress of prebiotics and their applications. Food Med. 2021, 23, 485–492. [Google Scholar]
- Huang, L.; Ke, H.; Chen, G.H.; Zheng, Y. Preparation and quality analysis of Polygonatum polysaccharide prebiotic cake. Food Ind. 2020, 41, 90–94. [Google Scholar]
- Zhang, S.; Dang, Y. Roles of gut microbiota and metabolites in overweight and obesity of children. Front. Endocrinol. 2022, 13, 994930. [Google Scholar] [CrossRef]
- Seo, K.H.; Kim, D.H.; Jeong, D. Chardonnay grape seed flour supplemented diets alter intestinal microbiota in diet-induced obese mice. J. Food Biochem. 2017, 41, e12396. [Google Scholar] [CrossRef]
- Zhu, S.L.; Chen, Y.Q.; Jiang, X. Application of Dunaliella in Preventing and Improving Obesity and Related. Diseases. Patent CN111686133A, 22 September 2020. [Google Scholar]
- Zhou, X.; Ma, L.; Dong, L.; Li, D.; Chen, F.; Hu, X. Bamboo shoot dietary fiber alleviates gut microbiota dysbiosis and modulates liver fatty acid metabolism in mice with high-fat diet-induced obesity. Front. Nutr. 2023, 10, 1161698. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Daza, M.C.; Roquim, M.; Dudonné, S.; Pilon, G.; Levy, E.; Marette, A.; Roy, D.; Desjardins, Y. Berry Polyphenols and Fibers Modulate Distinct Microbial Metabolic Functions and Gut Microbiota Enterotype-Like Clustering in Obese Mice. Front. Microbiol. 2020, 11, 2032. [Google Scholar] [CrossRef]
- Gao, J.; Ma, L.; Yin, J.; Liu, G.; Ma, J.; Xia, S.; Gong, S.; Han, Q.; Li, T.; Chen, Y. Camellia (Camellia oleifera bel.) seed oil reprograms gut microbiota and alleviates lipid accumulation in high fat-fed mice through the mTOR pathway. Food Funct. 2022, 13, 4977–4992. [Google Scholar] [CrossRef]
- Yang, K.; Jian, S.; Wen, C.; Guo, D.; Liao, P.; Wen, J.; Kuang, T.; Han, S.; Liu, Q.; Deng, B. Gallnut Tannic Acid Exerts Anti-stress Effects on Stress-Induced Inflammatory Response, Dysbiotic Gut Microbiota, and Alterations of Serum Metabolic Profile in Beagle Dogs. Front. Nutr. 2022, 9, 847966. [Google Scholar] [CrossRef]
- Qiu, X.; Macchietto, M.G.; Liu, X.; Lu, Y.; Ma, Y.; Guo, H.; Saqui-Salces, M.; Bernlohr, D.A.; Chen, C.; Shen, S.; et al. Identification of gut microbiota and microbial metabolites regulated by an antimicrobial peptide lipocalin 2 in high fat diet-induced obesity. Int. J. Obes. 2021, 45, 143–154. [Google Scholar] [CrossRef]
- Zhu, Z.; Huang, R.; Huang, A.; Wang, J.; Liu, W.; Wu, S.; Chen, M.; Chen, M.; Xie, Y.; Jiao, C.; et al. Polysaccharide from Agrocybe cylindracea prevents diet-induced obesity through inhibiting inflammation mediated by gut microbiota and associated metabolites. Int. J. Biol. Macromol. 2022, 209 Pt A, 1430–1438. [Google Scholar] [CrossRef]
- Deng, M.; Zhang, S.; Dong, L.; Huang, F.; Jia, X.; Su, D.; Chi, J.; Muhammad, Z.; Ma, Q.; Zhao, D.; et al. Shatianyu (Citrus grandis L. Osbecontrol) Flavonoids and Dietary Fiber in Combination Are More Effective Than Individually in Alleviating High-Fat-Diet-Induced Hyperlipidemia in Mice by Altering Gut Microbiota. J. Agric. Food Chem. 2022, 70, 14654–14664. [Google Scholar] [CrossRef] [PubMed]
- Poeker, S.A.; Geirnaert, A.; Berchtold, L.; Greppi, A.; Krych, L.; Steinert, R.E.; de Wouters, T.; Lacroix, C. Understanding the prebiotic potential of different dietary fibers using an in vitro continuous adult fermentation model (PolyFermS). Sci. Rep. 2018, 8, 4318. [Google Scholar] [CrossRef]
- Yadav, M.K.; Kumari, I.; Singh, B.; Sharma, K.K.; Tiwari, S.K. Probiotics, prebiotics and synbiotics: Safe options for next-generation therapeutics. Appl. Microbiol. Biotechnol. 2022, 106, 505–521. [Google Scholar] [CrossRef]
- Cummings, J.H.; Pomare, E.W.; Branch, W.J.; Naylor, C.P.; Macfarlane, G.T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 1987, 28, 1221. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Cai, G.; Liu, T.; Liu, Z. Relationships Among Gut Microbiota, Ischemic Stroke and Its Risk Factors: Based on Research Evidence. Int. J. Gen. Med. 2022, 15, 2003–2023. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Wang, Y.; Zeng, L.; Dong, J.; Bi, Q.; Yang, X.; Che, Y.; He, S.; Yu, J. Polysaccharides from Polygonatum kingianum improve Glccose and lipid metabolism in rats fed a high fat diet. Biomed. Pharmacother. 2020, 125, 109910. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).