Effects of Diet Macronutrient Composition on Weight Loss during Caloric Restriction and Subsequent Weight Regain during Refeeding in Aging Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice and Experimental Design
2.2. Caloric Restriction and Refeeding
2.3. Metabolic Measurements
2.4. Body Mass and Composition
2.5. Serum Leptin and Ghrelin
2.6. Statistical Analysis
3. Results
3.1. Body Mass
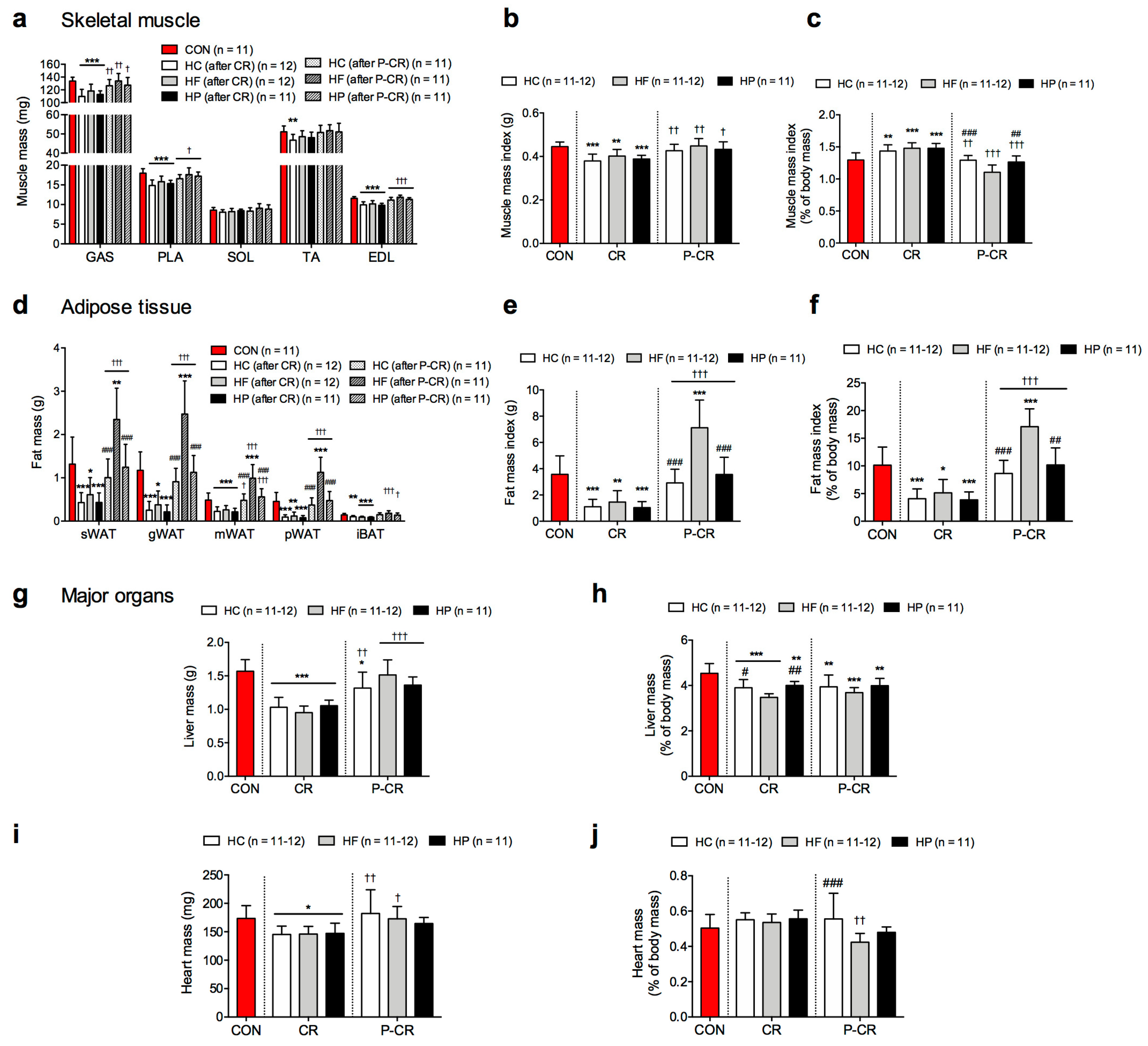
3.2. Body Composition
3.3. Serum Ghrelin and Leptin
3.4. Energy Metabolism
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, G.A.; Bouchard, C. The biology of human overfeeding: A systematic review. Obes. Rev. 2020, 21, e13040. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.D.; Farooqi, I.S.; Friedman, J.M.; Klein, S.; Loos, R.J.F.; Mangelsdorf, D.J.; O’rahilly, S.; Ravussin, E.; Redman, L.M.; Ryan, D.H.; et al. The energy balance model of obesity: Beyond calories in, calories out. Am. J. Clin. Nutr. 2022, 115, 1243–1254. [Google Scholar] [CrossRef] [PubMed]
- Kraft, T.S.; Venkataraman, V.V.; Wallace, I.J.; Crittenden, A.N.; Holowka, N.B.; Stieglitz, J.; Harris, J.; Raichlen, D.A.; Wood, B.; Gurven, M.; et al. The energetics of uniquely human subsistence strategies. Science 2021, 374, eabf0130. [Google Scholar] [CrossRef] [PubMed]
- Pontzer, H.; Durazo-Arvizu, R.; Dugas, L.R.; Plange-Rhule, J.; Bovet, P.; Forrester, T.E.; Lambert, E.V.; Cooper, R.S.; Schoeller, D.A.; Luke, A.; et al. Constrained Total Energy Expenditure and Metabolic Adaptation to Physical Activity in Adult Humans. Curr. Biol. 2016, 26, 410–417. [Google Scholar] [CrossRef]
- Flack, K.D.; Hays, H.M.; Moreland, J.; Long, D.E. Exercise for Weight Loss: Further Evaluating Energy Compensation with Exercise. Med. Sci. Sports Exerc. 2020, 52, 2466–2475. [Google Scholar] [CrossRef] [PubMed]
- Pontzer, H.; Raichlen, D.A.; Wood, B.M.; Mabulla, A.Z.P.; Racette, S.B.; Marlowe, F.W. Hunter-Gatherer Energetics and Human Obesity. PLoS ONE 2012, 7, e40503. [Google Scholar]
- Rubino, D.; Abrahamsson, N.; Davies, M.; Hesse, D.; Greenway, F.L.; Jensen, C.; Lingvay, I.; Mosenzon, O.; Rosenstock, J.; Rubio, M.A.; et al. Effect of Continued Weekly Subcutaneous Semaglutide vs Placebo on Weight Loss Maintenance in Adults With Overweight or Obesity: The STEP 4 Randomized Clinical Trial. JAMA 2021, 325, 1414. [Google Scholar] [CrossRef]
- Jastreboff, A.M.; Aronne, L.J.; Ahmad, N.N.; Wharton, S.; Connery, L.; Alves, B.; Kiyosue, A.; Zhang, S.; Liu, B.; Bunck, M.C.; et al. Tirzepatide Once Weekly for the Treatment of Obesity. N. Engl. J. Med. 2022, 387, 205–216. [Google Scholar] [CrossRef]
- Ge, L.; Sadeghirad, B.; Ball, G.D.C.; da Costa, B.R.; Hitchcock, C.L.; Svendrovski, A.; Kiflen, R.; Quadri, K.; Kwon, H.Y.; Karamouzian, M.; et al. Comparison of dietary macronutrient patterns of 14 popular named dietary programmes for weight and cardiovascular risk factor reduction in adults: Systematic review and network meta-analysis of randomised trials. BMJ 2020, 369, m696. [Google Scholar] [CrossRef]
- Hall, K.D.; Guo, J. Obesity Energetics: Body Weight Regulation and the Effects of Diet Composition. Gastroenterology 2017, 152, 1718–1727.e3. [Google Scholar] [CrossRef]
- Minderis, P.; Fokin, A.; Dirmontas, M.; Kvedaras, M.; Ratkevicius, A. Caloric Restriction per se Rather Than Dietary Macronutrient Distribution Plays a Primary Role in Metabolic Health and Body Composition Improvements in Obese Mice. Nutrients 2021, 13, 3004. [Google Scholar] [PubMed]
- Jacquet, P.; Schutz, Y.; Montani, J.P.; Dulloo, A. How dieting might make some fatter: Modeling weight cycling toward obesity from a perspective of body composition autoregulation. Int. J. Obes. 2020, 44, 1243–1253. [Google Scholar]
- Kleinert, M.; Clemmensen, C.; Hofmann, S.M.; Moore, M.C.; Renner, S.; Woods, S.C.; Huypens, P.; Beckers, J.; de Angelis, M.H.; Schürmann, A.; et al. Animal models of obesity and diabetes mellitus. Nat. Rev. Endocrinol. 2018, 14, 140–162. [Google Scholar] [PubMed]
- Zamboni, M.; Mazzali, G. Obesity in the elderly: An emerging health issue. Int. J. Obes. 2012, 36, 1151–1152. [Google Scholar]
- Kvedaras, M.; Minderis, P.; Krusnauskas, R.; Ratkevicius, A. Effects of ten-week 30% caloric restriction on metabolic health and skeletal muscles of adult and old C57BL/6J mice. Mech. Ageing Dev. 2020, 190, 111320. [Google Scholar] [PubMed]
- Astrup, A.; Raben, A.; Geiker, N. The role of higher protein diets in weight control and obesity-related comorbidities. Int. J. Obes. 2015, 39, 721–726. [Google Scholar]
- Zhong, W.; Wang, H.; Yang, Y.; Zhang, Y.; Lai, H.; Cheng, Y.; Yu, H.; Feng, N.; Huang, R.; Liu, S.; et al. High-protein diet prevents fat mass increase after dieting by counteracting Lactobacillus-enhanced lipid absorption. Nat. Metab. 2022, 4, 1713–1731. [Google Scholar]
- Ebbeling, C.B.; Feldman, H.A.; Klein, G.L.; Wong, J.M.W.; Bielak, L.; Steltz, S.K.; Luoto, P.K.; Wolfe, R.R.; Wong, W.W.; Ludwig, D.S. Effects of a low carbohydrate diet on energy expenditure during weight loss maintenance: Randomized trial. BMJ 2018, 363, k4583. [Google Scholar]
- Hengist, A.; Sciarrillo, C.M.; Guo, J.; Walter, M.; Hall, K.D. Discordance between gut-derived appetite hormones and energy intake in humans. medRxiv 2023. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P. Men and mice: Relating their ages. Life Sci. 2016, 152, 244–248. [Google Scholar]
- Chang, A.Y.; Skirbekk, V.F.; Tyrovolas, S.; Kassebaum, N.J.; Dieleman, J.L. Measuring population ageing: An analysis of the Global Burden of Disease Study 2017. Lancet Public Health 2019, 4, e159–e167. [Google Scholar] [PubMed]
- Lapinskas, E.; Krusnauskas, R.; Cekanauskaite, A.; Ratkevicius, A. Effect of fasting on body composition and proteolysis gene expression in skeletal muscles and liver of BEH+/+ and BEL mice. Growth Factors 2020, 38, 259–268. [Google Scholar] [CrossRef]
- Defour, M.; Hooiveld, G.J.E.J.; Van Weeghel, M.; Kersten, S. Probing metabolic memory in the hepatic response to fasting. Physiol. Genom. 2020, 52, 602–617. [Google Scholar]
- Mitchell, S.E.; Tang, Z.; Kerbois, C.; Delville, C.; Konstantopedos, P.; Bruel, A.; Derous, D.; Green, C.; Aspden, R.M.; Goodyear, S.R.; et al. The effects of graded levels of calorie restriction: I. impact of short term calorie and protein restriction on body composition in the C57BL/6 mouse. Oncotarget 2015, 6, 15902–15930. [Google Scholar]
- Huang, N.; Mao, E.W.; Hou, N.N.; Liu, Y.P.; Han, F.; Sun, X.D. Novel insight into perirenal adipose tissue: A neglected adipose depot linking cardiovascular and chronic kidney disease. World J. Diabetes 2020, 11, 115–125. [Google Scholar] [CrossRef]
- Wang, L.; Pan, Y.; Ye, X.; Zhu, Y.; Lian, Y.; Zhang, H.; Xu, M.; Liu, M.; Ruan, X. Perirenal fat thickness and liver fat fraction are independent predictors of MetS in adults with overweight and obesity suspected with NAFLD: A retrospective study. Diabetol. Metab. Syndr. 2023, 15, 56. [Google Scholar] [CrossRef]
- Chia, C.W.; Egan, J.M.; Ferrucci, L. Age-Related Changes in Glucose Metabolism, Hyperglycemia, and Cardiovascular Risk. Circ. Res. 2018, 123, 886–904. [Google Scholar] [PubMed]
- Ludwig, D.S.; Aronne, L.J.; Astrup, A.; de Cabo, R.; Cantley, L.C.; Friedman, M.I.; Heymsfield, S.B.; Johnson, J.D.; King, J.C.; Krauss, R.M.; et al. The carbohydrate-insulin model: A physiological perspective on the obesity pandemic. Am. J. Clin. Nutr. 2021, 114, 1873–1885. [Google Scholar] [PubMed]
- Speakman, J.R.; Hall, K.D. Carbohydrates, insulin, and obesity. Science 2021, 372, 577–578. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, D.S.; Willett, W.C.; Volek, J.S.; Neuhouser, M.L. Dietary fat: From foe to friend? Science 2018, 362, 764–770. [Google Scholar] [CrossRef]
- Minderis, P.; Fokin, A.; Dirmontas, M.; Ratkevicius, A. Hypocaloric Low-Carbohydrate and Low-Fat Diets with Fixed Protein Lead to Similar Health Outcomes in Obese Mice. Obesity 2020, 28, 1494–1502. [Google Scholar] [CrossRef]
- Hu, S.; Wang, L.; Togo, J.; Yang, D.; Xu, Y.; Wu, Y.; Douglas, A.; Speakman, J.R. The carbohydrate-insulin model does not explain the impact of varying dietary macronutrients on the body weight and adiposity of mice. Mol. Metab. 2020, 32, 27–43. [Google Scholar] [CrossRef]
- Hu, S.; Wang, L.; Yang, D.; Li, L.; Togo, J.; Wu, Y.; Liu, Q.; Li, B.; Li, M.; Wang, G.; et al. Dietary Fat, but Not Protein or Carbohydrate, Regulates Energy Intake and Causes Adiposity in Mice. Cell Metab. 2018, 28, 415–431.e4. [Google Scholar] [CrossRef]
- Sims, E.D.; Jennings, W.J.; Empringham, B.; Fleming, A.; Portwine, C.; Johnston, D.L.; Zelcer, S.M.; Rassekh, S.R.; Burrow, S.; Thabane, L.; et al. Circulating leptin levels are associated with adiposity in survivors of childhood brain tumors. Sci. Rep. 2020, 10, 4711. [Google Scholar] [CrossRef] [PubMed]
- Raubenheimer, D.; Simpson, S.J. Protein Leverage: Theoretical Foundations and Ten Points of Clarification. Obesity 2019, 27, 1225–1238. [Google Scholar] [CrossRef]
- Sørensen, A.; Mayntz, D.; Raubenheimer, D.; Simpson, S.J. Protein-leverage in Mice: The Geometry of Macronutrient Balancing and Consequences for Fat Deposition. Obesity 2008, 16, 566–571. [Google Scholar] [CrossRef]
- Holst, J.J. Incretin hormones and the satiation signal. Int. J. Obes. 2013, 37, 1161–1168. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minderis, P.; Fokin, A.; Povilonis, T.; Kvedaras, M.; Ratkevicius, A. Effects of Diet Macronutrient Composition on Weight Loss during Caloric Restriction and Subsequent Weight Regain during Refeeding in Aging Mice. Nutrients 2023, 15, 4836. https://doi.org/10.3390/nu15224836
Minderis P, Fokin A, Povilonis T, Kvedaras M, Ratkevicius A. Effects of Diet Macronutrient Composition on Weight Loss during Caloric Restriction and Subsequent Weight Regain during Refeeding in Aging Mice. Nutrients. 2023; 15(22):4836. https://doi.org/10.3390/nu15224836
Chicago/Turabian StyleMinderis, Petras, Andrej Fokin, Tomas Povilonis, Mindaugas Kvedaras, and Aivaras Ratkevicius. 2023. "Effects of Diet Macronutrient Composition on Weight Loss during Caloric Restriction and Subsequent Weight Regain during Refeeding in Aging Mice" Nutrients 15, no. 22: 4836. https://doi.org/10.3390/nu15224836
APA StyleMinderis, P., Fokin, A., Povilonis, T., Kvedaras, M., & Ratkevicius, A. (2023). Effects of Diet Macronutrient Composition on Weight Loss during Caloric Restriction and Subsequent Weight Regain during Refeeding in Aging Mice. Nutrients, 15(22), 4836. https://doi.org/10.3390/nu15224836





