1. Introduction
Diabetes mellitus is one of the leading causes of morbidity and mortality globally, and the burden of this disease has not been adequately addressed by the development of therapeutics. Type 2 diabetes mellitus (T2DM) is a chronic metabolic disorder characterized by hyperglycemia. It is caused by insulin resistance, which is impaired insulin action in target tissues, and insufficient secretion of insulin from pancreatic β-cells [
1]. The pro-longed synergy of hyperglycemia with other metabolic abnormalities in patients with diabetes can lead to organ damage and, ultimately, disabling and life-threatening consequences [
2]. In the practical approach to managing patients, biguanides, sulfonylureas, and thiazolidinediones (TZDs) are usually utilized with the risks of heart failure, hypo-glycemia, renal impairment, and weight gain [
3]. Consequently, research institutions and pharmaceutical companies throughout the world have worked on developing new, and safer anti-T2DM targets to produce better-tolerated antidiabetic medications.
Apart from being vital sources of energy, free fatty acids (FAs) also function as signaling molecules that control insulin secretion and sensitivity, inflammation, body weight, and various other metabolic processes [
1]. Especially, the beneficial effects of omega-3 fatty acids (ω-3 FAs) have been thoroughly documented due to their physiological function in stimulating insulin and gut hormone secretion, improving insulin sensitivity, anti-inflammatory effects, increasing glucose uptake, preventing metabolic disorder, and enabling homeostasis of healthy fat tissue [
2]. The medium- to long-chain free fatty acids, including linoleic acid (LA), eicosatrienoic acid, and docosahexaenoic acid (DHA), are known as endogenous ligands of G-protein-coupled receptor 40 (GPR40) and G-protein-coupled receptor 120 (GPR120). GPR40, also known as free fatty acid receptor 1 (FFA1), is a GPR that is primarily expressed in β-cells of the pancreas and enteroendocrine L-cells and functions to modulate glucagon-like peptide 1 (GLP-1) and insulin secretion when binding with its ligands. It has been shown that endogenous ligands or synthetic agonists control the release of insulin in a glucose-dependent way, contrary to insulin, sulfonylureas, and other widely used drugs, having minimal risk of hypoglycemia or other side effects [
3,
4,
5]. Along with GPR40 expressed in pancreatic β-cells and enteroendocrine L-cells, GPR120, known as free fatty acid receptor 4 (FFA4), is also highly expressed in mature adipocytes and macrophages [
6,
7,
8]. In addition to stimulating GLP-1 secretion from enteroendocrine cells, GPR120 expression in adipocytes and macrophages is reported to enhance glucose uptake and promote insulin sensitivity [
9]. A recent study further revealed that the activation of GPR120 in mice induced brown adipose tissue (BAT) activity and promoted white fat browning [
10]. Peroxisome proliferator-activated receptor (PPAR)γ agonists, especially the representative compounds, thiazolidinediones (TZDs), alleviate hyperglycemia by effectively increasing glucose uptake and insulin sensitivity and improving insulin resistance [
11,
12,
13]. However, strong PPARγ agonists such as TZDs have limited clinical use due to side effects such as weight gain [
14], edema [
15], bone damage [
16], and heart failure [
17,
18,
19]. Recently, Paschoal et al. suggested that the combined therapy of low-dose rosiglitazone with GPR120 agonist compound A might improve safety by reducing side effects and increasing the metabolic benefit on glucose uptake and insulin sensitivity [
20].
In drug discovery, natural products are abundant sources of chemical entities with structural diversity. Natural products have been used to treat diabetes for decades [
21]. Thus, the aim of this study was to identify a GPR40 and GPR120 dual agonist from our in-house natural compound library with little to no activation of PPARγ.
Herein, a ligand-based virtual screening approach followed by docking screening was used to select potential GPR40 and GPR120 dual agonists (
Figure S1, Supporting Information). We identified crocetin as a dual agonist of GPR40 and GPR120. Crocetin, a 20-carbon dicarboxylic acid, is a natural carotenoid and commercially available constituent of
Crocus sativus Linne (saffron) [
22].
In recent years, studies have demonstrated that saffron (
Crocus sativus L.) is a natural product with promising glycemic control effects. Saffron and its components have been demonstrated to have hypolipidemic, antidiabetic, and antihypertensive properties in in vitro, in vivo, and clinical trials investigations [
23]. The major saffron compounds are crocin, crocetin, picrocrocin, and safranal. Among them, the carotenoid components in saffron, crocin and crocetin, have been extensively researched for their potential to decrease cholesterol and prevent diabetes. Crocins are a unique class of remarkably hydrophilic apocarotenoids, comprising the primary components of saffron. Within saffron, crocetin, the aglycone derived from crocins, naturally occurs and is biosynthetically produced through the enzymatic cleavage of crocins within biological systems [
24]. Several studies have shown that crocetin possesses significant pharmacological effects in various diseases, including neuroprotective, cardioprotective, hepatoprotective, antiviral, antinociceptive, antidepressant, anticancer, antidiabetic, and memory enhancing properties [
22]. However, the antidiabetic activity of crocetin has not been studied as extensively as crocin. In vitro studies suggest that 10 μM of crocetin attenuates palmitate-induced insulin resistance in 3T3-L1 adipocytes via phosphorylation of insulin receptor substrate-1 (IRS-1) serine 307 [
25,
26]. In vivo studies reveal that 5 μM of crocetin (5 μM) treatment therapy reduced proliferative damage to diabetic endothelial progenitor cells (EPCs) and increased caspase-3 activity, LDH release, and cell death [
27]. Crocetin decreased the expression levels of IL-6, TNF-α, and IL-1β, increased the levels of antioxidant enzymes such as SOD, GSH-Px, GSH, and CAT, and increased body weight in rats with STZ-induced gestational diabetes mellitus (GDM). It also suppressed the levels of intercellular adhesion molecule-1 (ICAM-1), COX-2, and PGE2 [
28]. Clinical trials involving patients who consumed saffron capsules have demonstrated significant alleviation of sleep problems, anxiety, and mild-to-moderate comorbid depression-anxiety (CDA). Additionally, other studies have reported that daily saffron consumption can enhance sleep quality in diabetic patients and exert a positive impact on their anxiety levels [
22]. However, the effects of crocetin on glucose uptake and insulin and GLP-1 secretion, as well as the antidiabetic mechanism of crocetin, remain unclear.
In this study, we have successfully identified saffron’s antidiabetic component at the molecular level, highlighting crocetin as a GPR40/120 agonist. This pioneering discovery positions crocetin as a promising therapeutic candidate for type 2 diabetes, marking the first unveiling of such potential. Furthermore, we characterized crocetin’s mechanism of action and assessed its antidiabetic properties in various cell types, including mouse pancreatic beta cells (MIN6), mouse intestinal neuroendocrine cells (STC-1), and embryonic mouse fibroblast (3T3-L1) adipocytes, shedding light on its potential role in diabetes management.
2. Materials and Methods
2.1. Cell Culture and Differentiation
Mouse 3T3-L1 pre-adipocytes (Zen-Bio, Inc., Durham, NC, USA) were cultured in high glucose Dulbecco’s modified Eagle’s medium (DMEM; LM 001-07, Welgene Biotech Co., Ltd., Gyeongsan-si, Republic of Korea) supplemented with 10% bovine calf serum (BCS; Thermo Fisher Scientific Korea Ltd., Seoul, Republic of Korea) and 1% 100× antibiotic–antimycotic solution (Welgene Biotech Co., Ltd., Gyeongsan-si, Republic of Korea) at 37 °C in 5% CO2. Cell differentiation was initiated by adding DMEM supplemented with 10% fetal bovine serum (FBS; Welgene Biotech Co., Ltd., Gyeongsan-si, Republic of Korea), 1% 100× antibiotic–antimycotic solution, 0.5 mM isobutylmethylxanthine (IBMX; Merk KGaA, Darmstadt, Germany), 1 µM dexamethasone (Sigma-Aldrich, Saint Louis, MI, USA), and 5 µg/mL insulin (Merck KgaA, Darmstadt, Germany) when 3T3-L1 cells reached 100% confluence. Subsequently, the cells were incubated for 48 h at 37 °C in 5% CO2. Then, the culture medium was changed to DMEM supplemented with 10% FBS, 1% 100× antibiotic–antimycotic solution, and 5 μg/mL insulin (differentiation medium II). After an additional 48 h of incubation, the cells were cultured in DMEM containing only 10% FBS and 1% 100× antibiotic–antimycotic solution, with medium changes every 48 h. Cells were maintained at 37 °C in 5% CO2. Chinese hamster ovary (CHO)-K1 cells (#CCL-61, ATCC, Manassas, VA, USA) were maintained in DMEM containing 10% FBS and 1% 100× antibiotic–antimycotic solution at 37 °C in 5% CO2. The MIN6 cells (#C0018008, AddexBio Korea, Seoul, Republic of Korea) were cultured in DMEM containing 15% heat-inactivated FBS (10082147, Gibco, Grand Island, NY, USA), 1% 100× antibiotic–antimycotic solution, and 55 μM β-mercaptoethanol (21985023, Gibco, Grand Island, NY, USA) at 37 °C in 5% CO2. The STC-1 cells (#CRL-3254, ATCC, Manassas, VA, USA) were cultured in DMEM containing 10% heat-inactivated FBS and 1% 100× antibiotic–antimycotic solution. All of the reagents are reported as their final concentrations in solution.
2.2. SRE and CRE Reporter Luciferase Assay
The CHO cells were plated in 96-well white plates (Corning® Costar®, Sigma-Aldrich, Saint Louis, MI, USA) at a concentration of 1.5 × 104 cells per well in DMEM containing 10% FBS and 1% 100× antibiotic–antimycotic solution and incubated for 24 h at 37 °C in a 5% CO2 environment. The SRE (pGL4.33[luc2P/SRE/Hygro]) and CRE (pGL4.33[luc2P/CRE/Hygro]) plasmids were acquired from Promega (Madison, WI, USA) and cotransfected into CHO cells with GPR40 and GPR120 using the lipofetamine™ 3000 (L3000015, ThermoFisher, Waltham, MA, USA) as the transfection reagent. Following a 3 h incubation, the transfected cells were treated with the compounds of interest, while control cells were treated with 0.1% DMSO in culture medium and incubated for an additional 24 h. Luciferase activity was quantified using the Bright-Glo™ Assay System (Cat. #E2620; Promega, Madison, WI, USA). All individual in vitro assays for determining the average efficiencies were repeated more than three times with triplicate wells for each treatment.
2.3. PPARg Transactivation Activity
Briefly, CHO cells were plated in 96-well plates at a density of 1.5 × 104 cells per well in DMEM containing 10% FBS and 1% 100× antibiotic–antimycotic solution. After a 24 h incubation, the cells were cotransfected with pPPRE-TK-Luc (addgene, #1015) and pCMV6-hPPARG-GFP (RG201538, OriGene Technologies, Inc., Rockville, MD, US) using Lipofetamine 3000 (Cat. # L3000015, Invitrogen, Waltham, MA, US). Following a 3 h transfection, the cells were treated with the compounds of interest and incubated for an additional 24 h. Control cells were treated with 0.2% DMSO in culture medium. Luciferase activity was assessed using the Bright-Glo™ Assay System (Cat. #E2620; Promega, Madison, WI, USA). Each individual in vitro assay for determining the average efficiencies was performed three times with triplicate wells for each treatment.
2.4. Insulin Secretion Assay
Briefly, MIN6 cells were seeded in 96-well plates (5 × 105 cells/well) and incubated overnight to achieve 90% confluence. 1M glucose stock was used to prepare fresh 3 mM glucose KRBB buffer or 17 mM glucose KRBB buffer. Then, the culture medium was aspirated, and the cells were washed twice with 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES)-balanced Krebs/Ringer bicarbonate buffer (KRBB) containing 0.5% bovine serum albumin (BSA, 9048-46-8, Sigma-Aldrich, St. Louis, MI, USA). Then, the cells were starved at 37 °C for 2 h in 3 mM glucose KRBB buffer. After starvation, the culture medium was changed via the indicated compounds at 37 °C for 2 h in KRBB buffer containing 3 mM or 17 mM glucose. The supernatant was collected after centrifugation and stored at −80 °C until insulin assay. Insulin levels were determined using a mouse high-range insulin enzyme-linked immunosorbent assay (ELISA) kit (80-INSMSH-E01, ALPCO, Windham, NH, USA). All individual in vitro assays for determining the average efficiencies were performed more than three times using triplicate wells for each treatment.
2.5. GLP-1 Secretion Assay
In brief, STC-1 cells were plated in 96-well poly-D-lysine coated plates (354640, Corning®, New York, NY, USA) at a concentration of 1 × 105 cells per well in the culture medium. The plate was subsequently incubated for 48 h at 37 °C in a humidified 5% CO2. Following incubation, cells were gently washed with 200 μL prewarmed Hank’s balanced salt buffer (HBSS, SH30268.02, Hyclone, Logan, UT, USA) with 0.1% BSA to remove background secretions. Subsequently, the cells were subjected to a 3 h period of serum starvation in HBSS with 0.1% BSA. The treatment compounds were diluted in DMEM with 0.1% BSA supplemented with 5 μM the dipeptidyl peptidase 4 (DPP-4) inhibitor, KR-62436 (761414-79-3, Sigma-Aldrich), and incubated for 1 h at 37 °C. The cell supernatant was collected following centrifugation and stored at −80 °C until analysis. The levels of secreted GLP-1 were quantified using the mouse/human/rat GLP-1/glucagon-like peptide 1 ELISA kit (LS-F412, LSBIO, Seattle, WA, USA).
2.6. Glucose Uptake Assay
Mature 3T3-L1 cells were initially subjected to a 16 h period of serum starvation in low-glucose DMEM (2323667, Gibco BRL, Thermo Fisher Scientific Korea Ltd.). Subsequently, they were either treated for an additional 2 h with crocetin or 30 min with 100 nM insulin in glucose-depleted DMEM (11966025Gibco BRL, Thermo Fisher Scientific Korea Ltd.). Following treatment, the cells were exposed to 100 µM 2-NBDG (Thermo Fisher Scientific Korea Ltd., Seoul, Republic of Korea) at 37 °C for 1 h. After incubation, the cells were gently washed with precooled PBS, and the fluorescence intensity was quantified (Ex/Em = 485/535 nm) using a fluorescence microplate reader (VictorTM X4, PerkinElmer, Waltham, MA, USA).
2.7. Western Blotting
The 3T3-L1 preadipocytes were seeded in six-well plates at a concentration of 1.5 × 105 cells/well in a final volume of 2 mL and differentiated as previously described. Then, the cells were incubated in low-glucose DMEM. After overnight incubation, the medium was changed to glucose-depleted DMEM. The cells were then treated with different concentrations of the compound in the absence or presence of 100 nM insulin and incubated for 1 h. The cells were harvested by RIPA buffer (Sigma-Aldrich, Saint Louis, MI, USA) containing a protease inhibitor cocktail (Roche Korea, Seoul, Republic of Korea), and proteins (20 µg) from each lysate were separated by 10% sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene difluoride (PVDF) membranes (Merk KgaA, Darmstadt, Germany), maintained at 200 mA for 120 min. The membranes were then blocked with 5% skim milk in 0.1% tris-buffered saline with 0.1% Tween® 20 Detergent (TBST) for 1 h at room temperature. The membranes were then probed overnight at 4 °C with primary antibodies in 0.1% BSA. Secondary antibodies were used at a concentration of 1:5000 for 1 h at room temperature. Immunoreactive bands were marked using EzWestLumi plus detection reagents (ATTO Corporation, Tokyo, Japan) and detected by a LuminoGraph II imaging system (ATTO Corporation, Tokyo, Japan). The antibodies used were anti-p-Akt (cat.# 4060, Cell Signaling Technology, Inc., Beverly, MA, USA), anti-t-Akt (cat.# 4691, Cell Signaling Technology, Inc., Beverly, MA, USA), and anti-beta-actin antibodies (cat.# GTX109639, GeneTex, Irvine, CA, USA).
2.8. Structure-Based In-House Library Search
From the in-house library composed with 1158 natural compounds, we conducted a similarity search on our hit compounds with the Canvas similarity and Clustering module in Schrödinger. Tanimoto similarity metric was used, and candidates were selected via similarity score and visual inspection.
2.9. Molecular Modeling
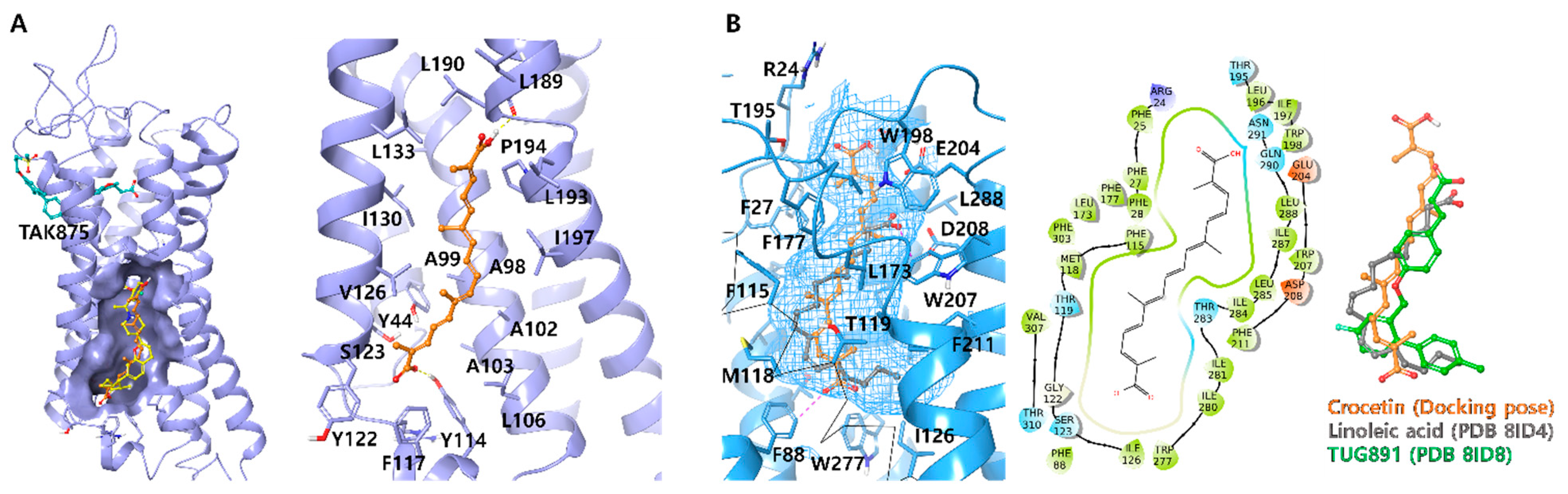
Ligand docking was carried out using Schrödinger Maestro 2020-4 in Windows 10 system (Schrödinger Release 2020-4: Schrödinger, LLC, New York, NY, USA, 2020). Liga 19nds were prepared by using the 2D sketch module and Ligprep module. To facilitate docking, all possible ionization states of the ligands, including the neutral state, were generated under physiological pH conditions. The X-ray and Cryo-EM structures of human GPR40 (PDB ID: 5TZR and 5TZY) and GPR120 (PDB ID: 8ID4 and 8ID8) were obtained from the RCSB Protein Data Bank (
https://www.rcsb.org/ (accessed on 19 May 2023)) [
29] and were prepared using the protein preparation module. Prime was employed to replace any missing side chains, while extraneous water molecules were eliminated. For XP-docking, the ligand docking module employed XP-glide with default settings, and no constraints were applied. The co-crystallized ligand was used to define the centers of the grid boxes, with all grid boxes set to 25 Å. A total of 20 poses were generated for each ligand and exported for manual comparison.
2.10. Chemicals
The 1158 natural compounds were obtained from Biopurify Phytochemicals Ltd., Chengdu, China, without further purification.
2.11. Statistical Analysis
GraphPad Prism 7 (GraphPad Software, Inc., San Diego, CA, USA) was used to analyze the data and find significant differences. Single comparisons between the two experimental groups were performed using an unpaired Student’s t-test. Multiple comparisons were performed using a one-way ANOVA followed by a Tukey’s test. Data are presented as mean ± standard deviation (SD).
4. Discussion
In recent years, in vitro and in vivo studies and clinical trials have demonstrated that saffron is a natural product with promising glycemic control effects. Multiple studies have confirmed saffron’s beneficial effects on diabetic animals’ metabolic conditions such as hyperglycemia, dyslipidemia, and insulin resistance. However, in vitro studies on the antidiabetic potential of saffron are mainly focused on its chief ingredients, crocin. In this study, we have, for the first time, identified the molecular-level target responsible for the authentic active component in saffron which imparts its antidiabetic effect. Our findings clearly establish that crocetin, as opposed to crocin, acts as an agonist for GPR40 and GPR120, positioning it as a potential therapy option for type 2 diabetes.
GPR40 and GPR120 are highly expressed in pancreatic cells and enteroendocrine cells, participate in the potentiation of insulin by free fatty acids, and, most importantly, prevent hypoglycemic complications. Several synthetic compounds and traditional natural compounds have been reported as GPR40 or GPR120 agonists in the literature. However, no GPR40/120 ligands have been approved as antidiabetic drugs. Unfortunately, trials with the GPR40 agonist TAK875 were stopped in phase III, owing to the liver toxicity associated with its chemical structure. In contrast, crocetin is a plant-derived compound from saffron, which is treated as a natural healthcare product, owing to its safety. Thus, crocetin may find application in the treatment of T2DM as a dual agonist of GPR40 and GPR120.
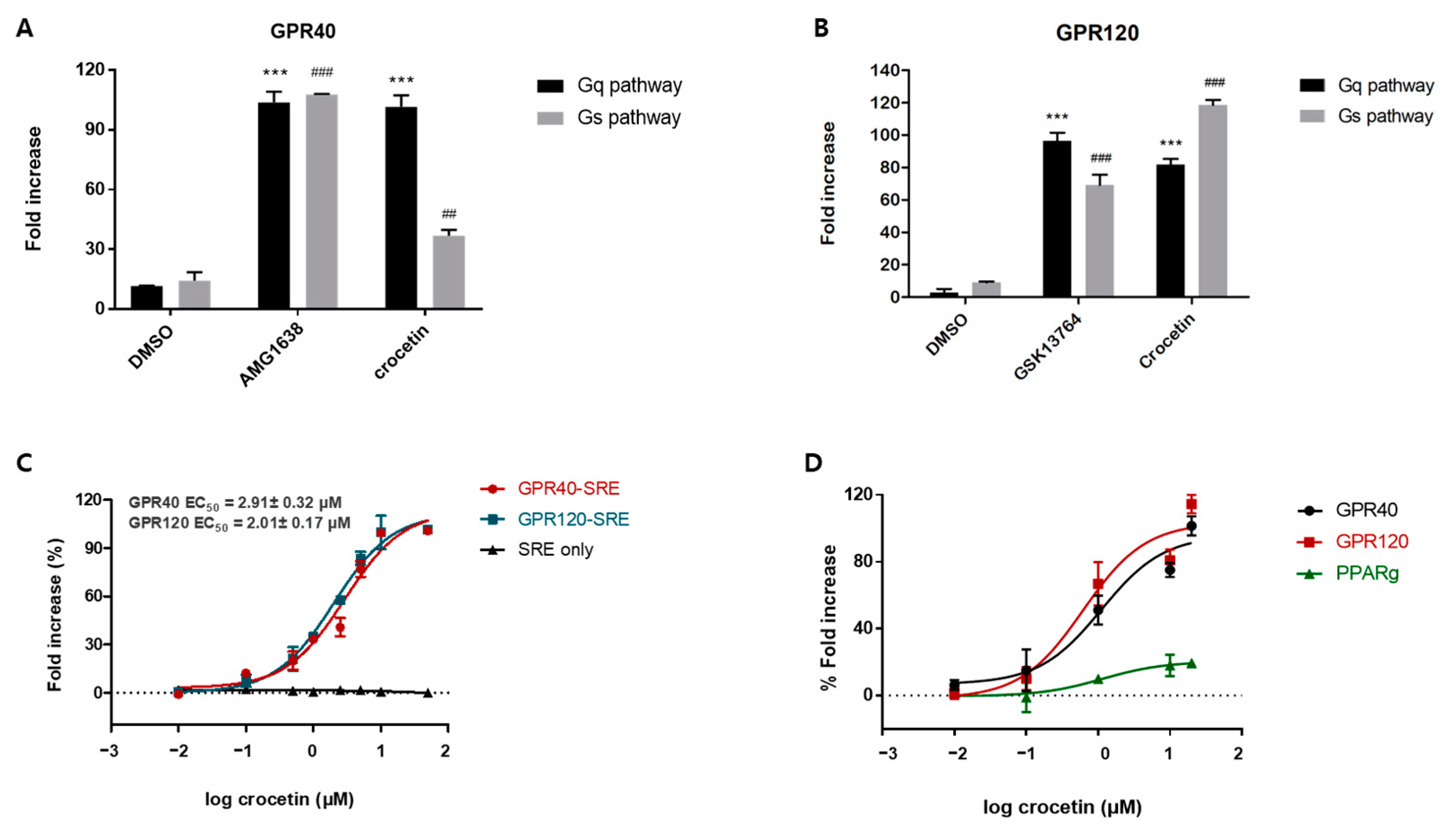
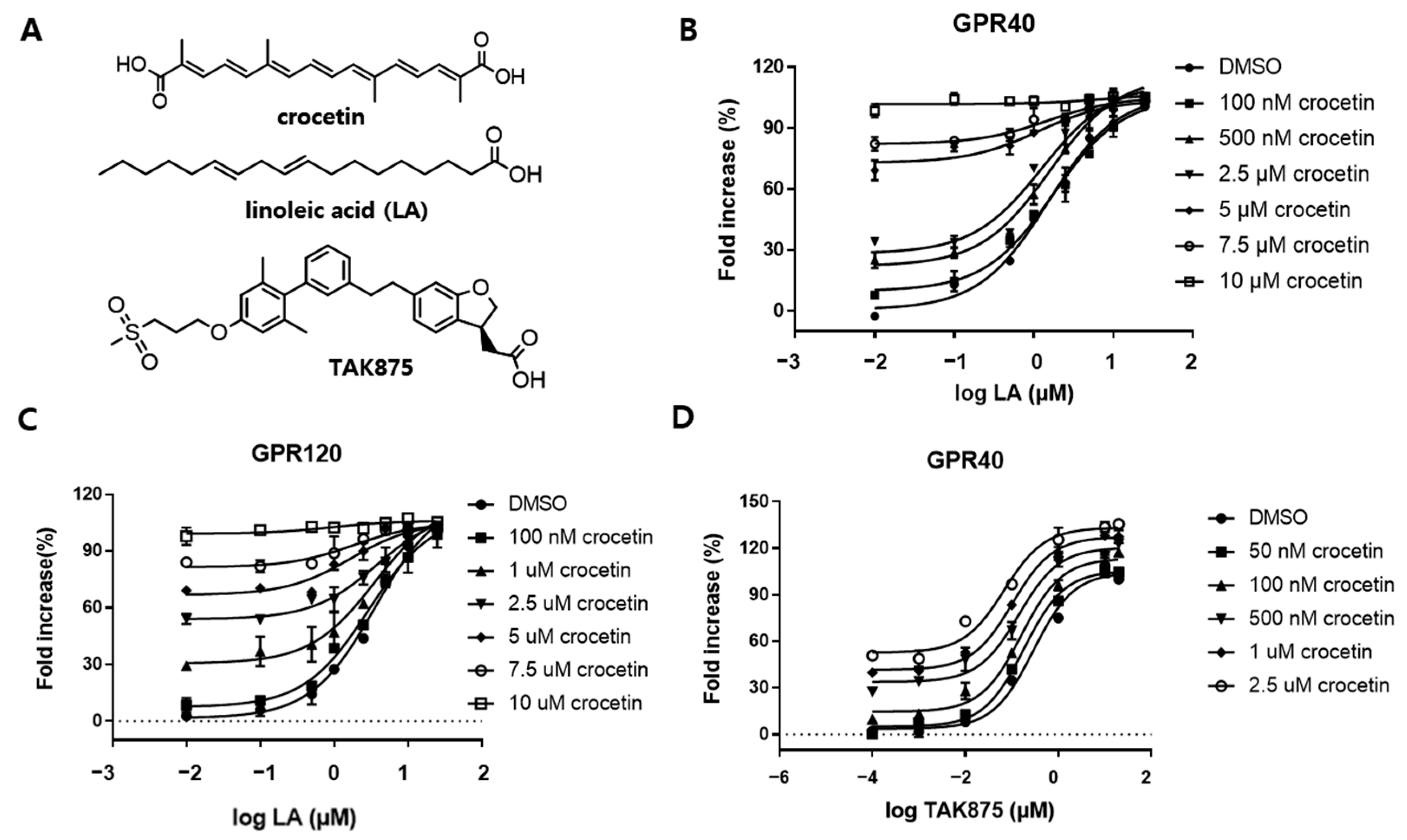
After conducting the ligand-based similarity search, docking, and cell-based reporter assay screening, crocetin was selected as the most active compound which exhibited an agonistic effect against GPR40 and GPR120 with an EC50 value of 2.91 ± 0.32 μM and 2.01 ± 0.17 μM, respectively. In the kinetic study, crocetin was identified as an orthosteric full agonist, which moderately activated both Gq and Gs pathways and revealed positive cooperation with the partial agonist TAK875 on GPR40. It was observed that both MIN6 and STC-1 cells displayed excellent insulin and GLP-1 secretion upon treatment with crocetin. This suggests that the dual agonist exhibited a powerful and dual mechanism of action for stimulating insulin secretion. Furthermore, as GLP-1 levels were also elevated in crocetin-treated enteroendocrine cells, this dual impact may arise during in vivo investigations when crocetin may directly affect insulin secretion or boost incretin secretion.
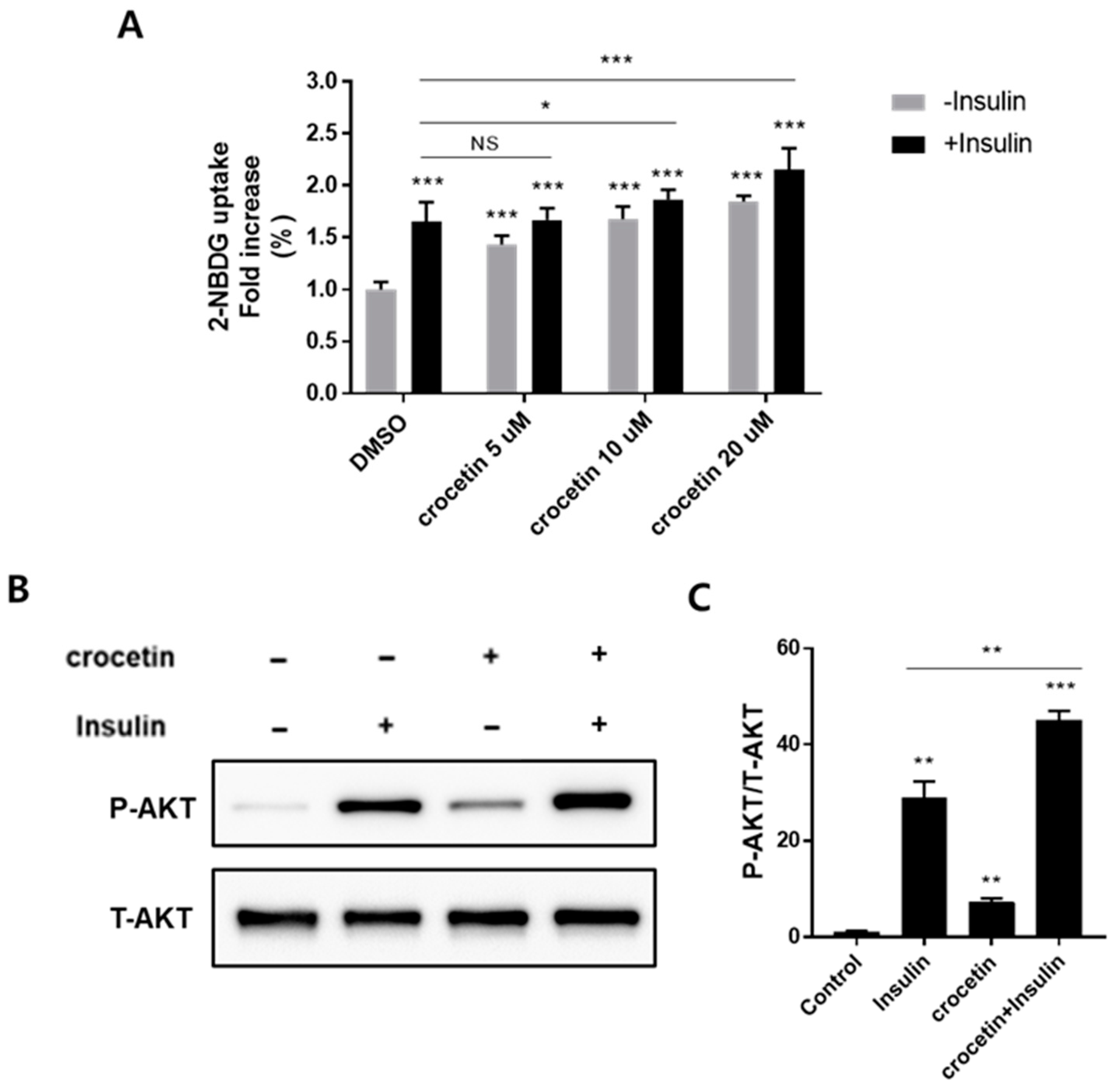
However, in comparison to adipocyte models, myocyte models have been used more extensively in vitro to study metabolic disease progression. In our case, we are working on the adipocyte models to explore the glucose uptake effect of crocetin, since GPR120 is likewise barely expressed in muscle but abundantly expressed in mature adipocytes [
9]. In mature 3T3-L1 adipocytes, activation of the Akt pathway is one of the main mechanisms of glucose absorption via the activation of the signaling cascade involving PI3-kinase through the activation of GPCRs. However, unlike other insulin-dependent pathways via the insulin receptor substrate (IRS) family or tyrosine phosphatases, the PI3-kinase activation through GPCRs is dependent on Gq protein recruitment in an insulin-independent manner. Thus, in the absence of insulin, crocetin still exhibited a significant effect on Akt phosphorylation, while in the presence of insulin, crocetin showed synergistic Akt phosphorylation-improving effects, suggesting that crocetin could increase insulin sensitivity and shows potential as an insulin-sensitizing agent to treat type 2 diabetes. Nonetheless, while our research has substantiated the GPCR-dependent nature of glucose uptake, the conclusive characterization of the specific involvement of GPR40 and GPR120 in the antidiabetic function necessitates the utilization of GPR40 and GPR120 antagonists or gene silencing techniques to furnish direct evidence.
Since the primary mechanism for glucose uptake into fat cells is glucose transporter 4 (GLUT4)’s translocation to the cell surface, analysis of the GLUT4 translocation was not conducted in this study. Additional studies are required to measure the basal- and crocetin-stimulated translocation of GLUT4 to the plasma membrane in adipocytes. Nonetheless, it is reasonable to anticipate that the influence of crocetin may become more evident in subsequent in vivo analysis. This expectation is based on the established role of insulin in regulating glucose metabolism as it facilitates the translocation of intracellular GLUT4 to the cell surface. Moreover, crocetin’s capacity to stimulate insulin secretion from pancreatic cells can further enhance glucose uptake in adipocytes, potentially showcasing its effects more prominently in in vivo studies.
In conclusion, our research has unveiled a novel insight into the molecular basis of saffron’s antidiabetic effect. At the molecular level, we have pinpointed the key component and its target receptors. Notably, we have established that crocetin, distinct from crocin, acts as a dual orthosteric full agonist of GPR40 and GPR120, effectively promoting insulin and GLP-1 secretion while enhancing cellular glucose homeostasis. This discovery positions crocetin as a potent and groundbreaking candidate for diabetic therapy. To advance its development for clinical applications, further investigations involving in vivo studies to assess plasma glucose and insulin levels are imperative.