Heptanoate Improves Compensatory Mechanism of Glucose Homeostasis in Mitochondrial Long-Chain Fatty Acid Oxidation Defect
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Experiment
2.2. Glucose Extraction, Derivatization and Gas Chromatography-Mass Spectrometry (GC-MS)
2.3. Free Glycerol Concentration
2.4. Metabolite Extraction, Derivatization and GC-MS for TCA Cycle Analysis
2.5. Metabolic Intermediates Concentration
2.6. GC-MS Data Processing and Isotope Correction
2.7. Label Incorporation
2.8. Computational Modelling of 13C Glucose Time Courses
2.9. Quantification and Statistical Analysis
3. Results
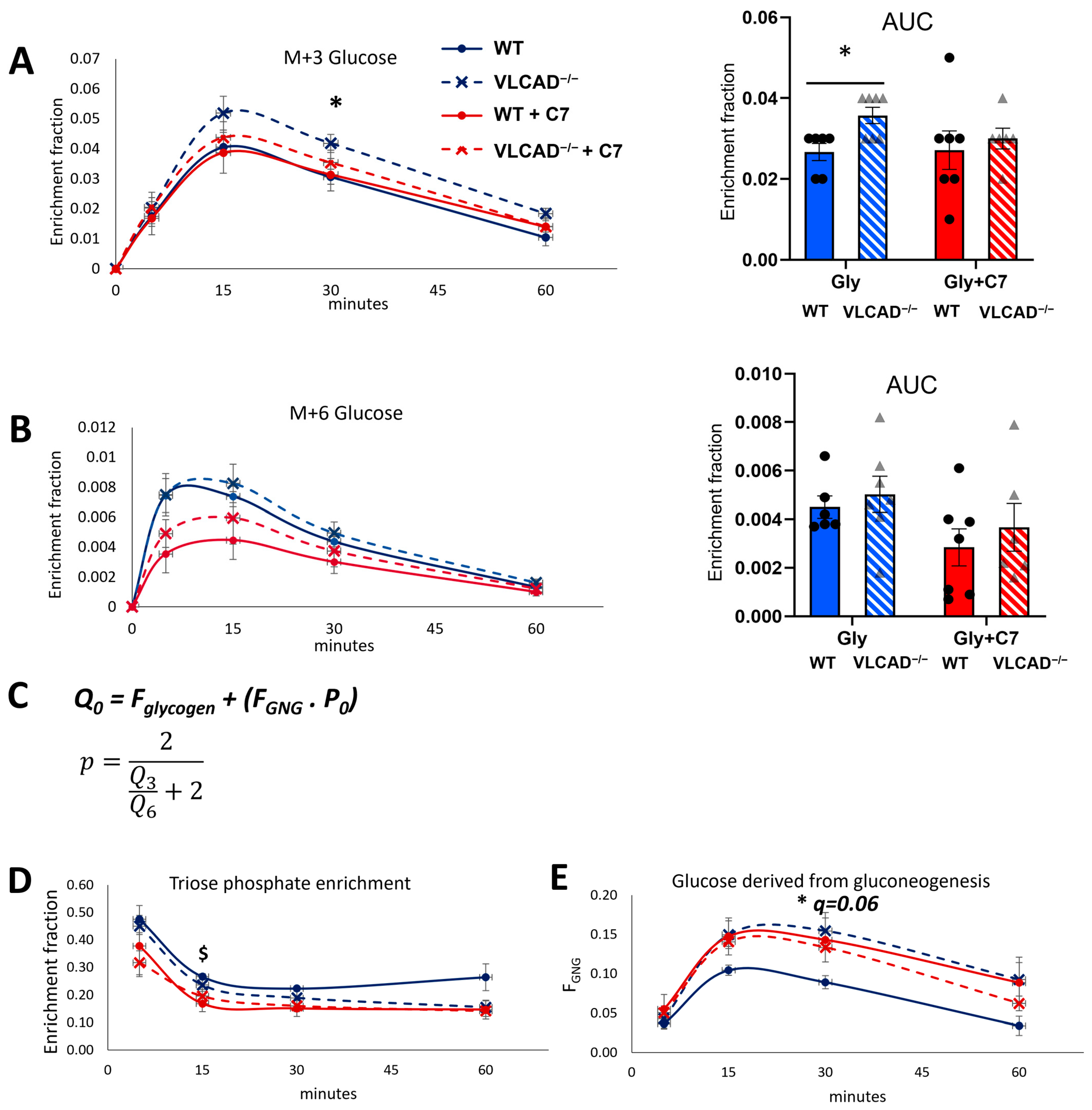
3.1. 13C3-Glycerol Bolus to Evaluate Gluconeogenesis
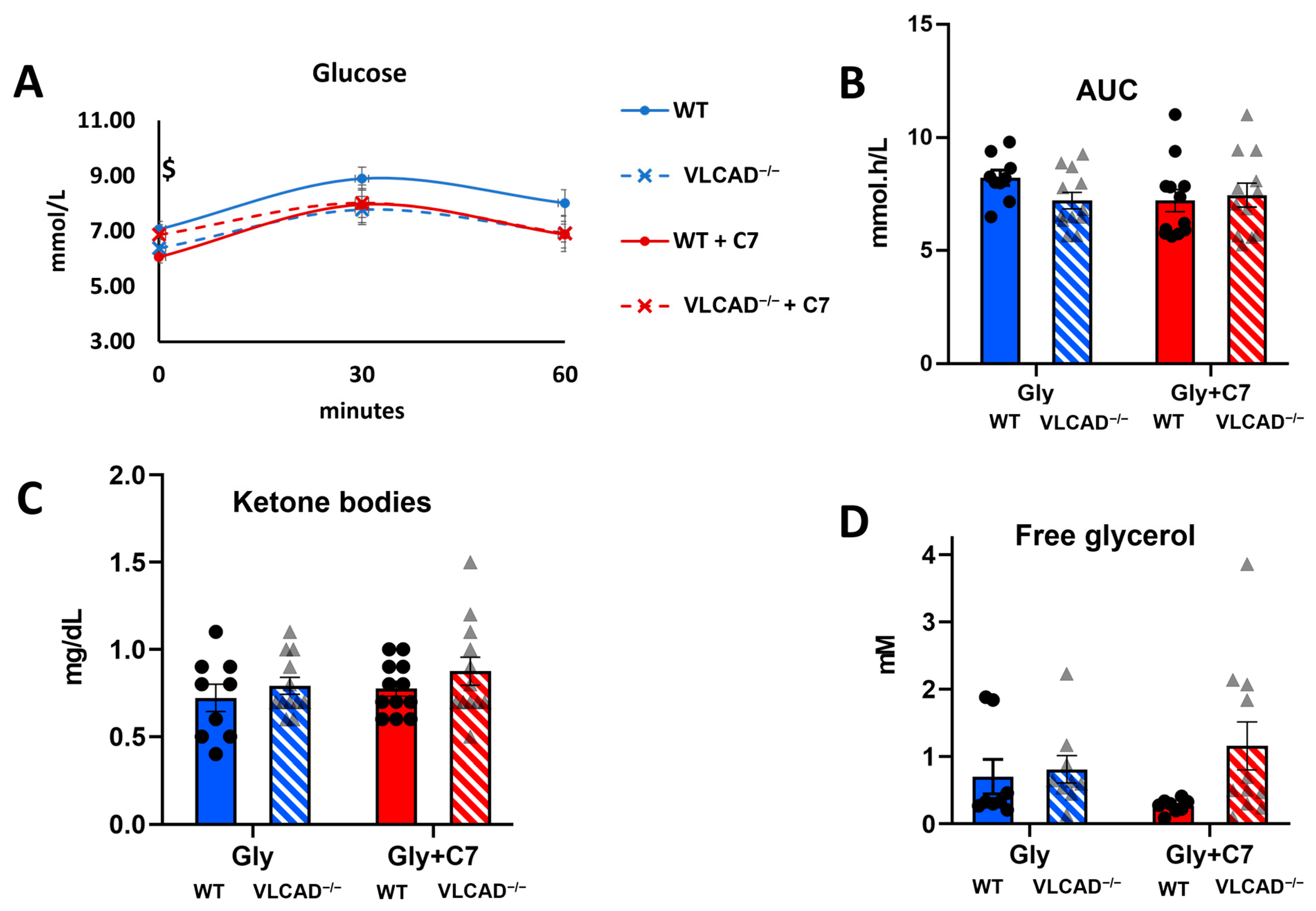
3.2. Loss of ACADVL Gene Does Not Affect Glucose Homeostasis
3.3. The Effect of Heptanoate on the Genotype-Specific Contribution of Glycerol to Gluconeogenesis
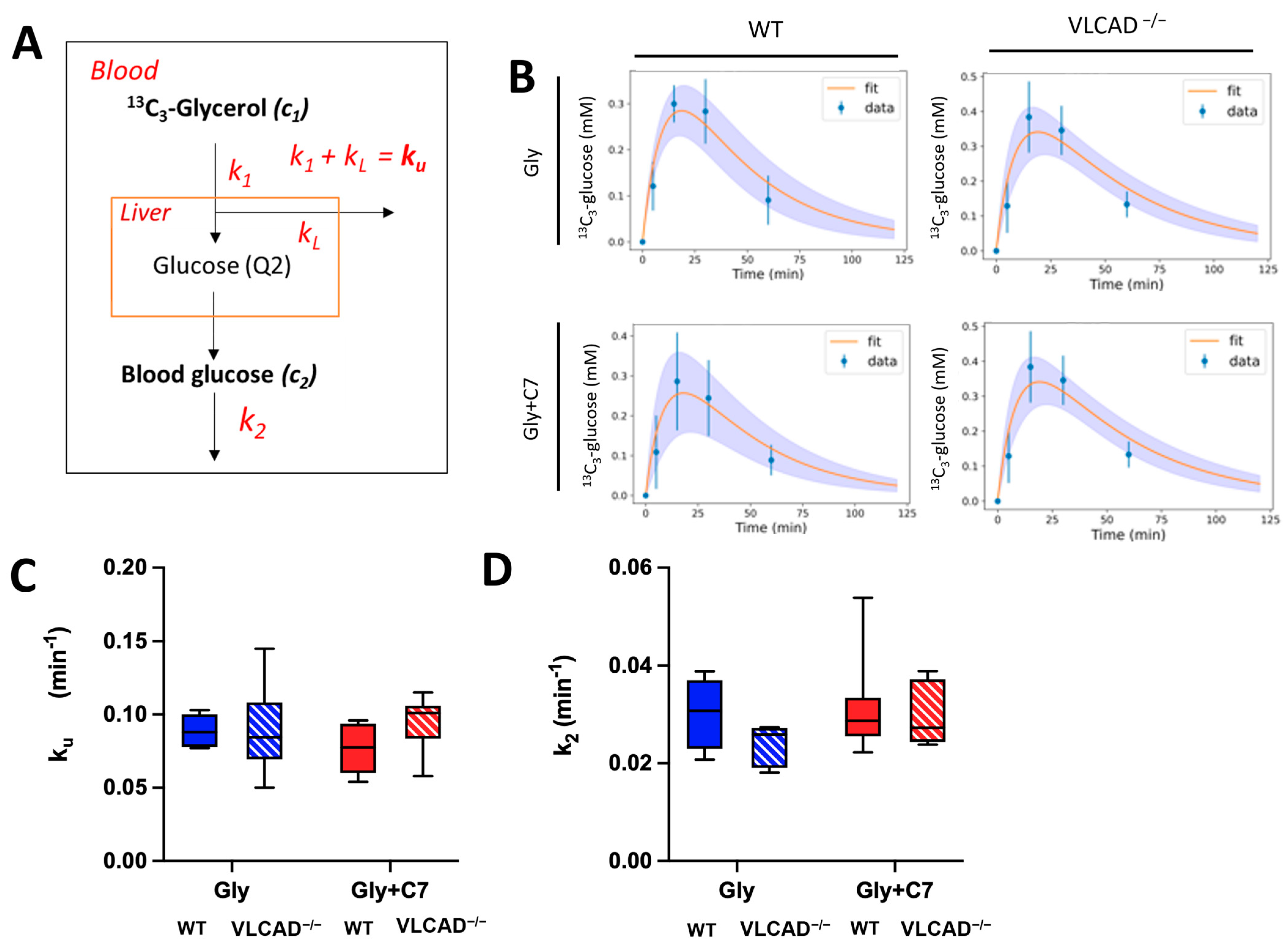
3.4. Blood Glucose Turnover Is Not Affected in VLCAD−/− Mice
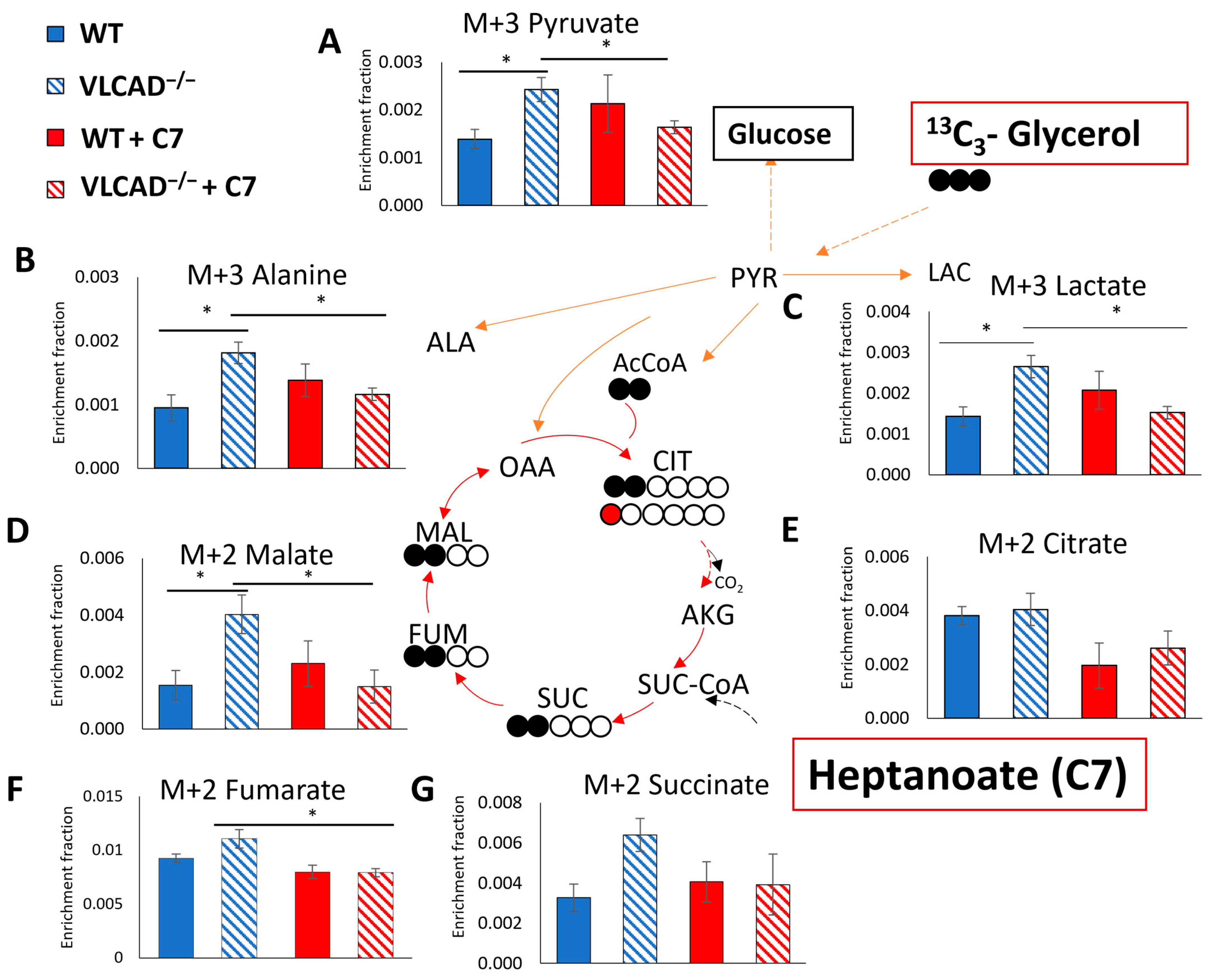
3.5. Effect of C7 on 13C Incorporation Central Carbon Metabolism Is Genotype Dependent
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goodpaster, B.H.; Sparks, L.M. Metabolic Flexibility in Health and Disease. Cell Metab. 2017, 25, 1027–1036. [Google Scholar] [CrossRef]
- Muoio, D.M. Metabolic Inflexibility: When Mitochondrial Indecision Leads to Metabolic Gridlock. Cell 2014, 159, 1253–1262. [Google Scholar] [CrossRef]
- Tucci, S.; Alatibi, K.I.; Wehbe, Z. Altered Metabolic Flexibility in Inherited Metabolic Diseases of Mitochondrial Fatty Acid Metabolism. Int. J. Mol. Sci. 2021, 22, 3799. [Google Scholar] [CrossRef] [PubMed]
- Saudubray, J.-M.; Garcia-Cazorla, À. Inborn Errors of Metabolism Overview: Pathophysiology, Manifestations, Evaluation, and Management. Pediatr. Clin. N. Am. 2018, 65, 179–208. [Google Scholar] [CrossRef]
- Marsden, D.; Bedrosian, C.L.; Vockley, J. Impact of newborn screening on the reported incidence and clinical outcomes associated with medium- and long-chain fatty acid oxidation disorders. Genet. Med. 2021, 23, 816–829. [Google Scholar] [CrossRef] [PubMed]
- Bleeker, J.C.; Kok, I.L.; Ferdinandusse, S.; van der Pol, W.L.; Cuppen, I.; Bosch, A.M.; Langeveld, M.; Derks TG, J.; Williams, M.; de Vries, M.; et al. Impact of newborn screening for very-long-chain acyl-CoA dehydrogenase deficiency on genetic, enzymatic, and clinical outcomes. J. Inherit. Metab. Dis. 2019, 42, 414–423. [Google Scholar] [CrossRef]
- Han, H.-S.; Kang, G.; Kim, J.S.; Choi, B.H.; Koo, S.-H. Regulation of glucose metabolism from a liver-centric perspective. Exp. Mol. Med. 2016, 48, e218. [Google Scholar] [CrossRef] [PubMed]
- Camp, K.M.; Lloyd-Puryear, M.A.; Huntington, K.L. Nutritional treatment for inborn errors of metabolism: Indications, regulations, and availability of medical foods and dietary supplements using phenylketonuria as an example. Mol. Genet. Metab. 2012, 107, 3–9. [Google Scholar] [CrossRef]
- Leslie, N.D.; Saenz-Ayala, S. Very Long-Chain Acyl-Coenzyme A Dehydrogenase Deficiency; University of Washington: Seattle, WA, USA, 2022. [Google Scholar]
- Zand, D.; Doan, J.; Yi, S.; Wang, J.; Ma, L.; Akinshola, E.; Chakder, S.; Meyer, J.; Pacanowski, M.; Johnson, L.L.; et al. Regulatory news: Dojolvi (triheptanoin) as a source of calories and fatty acids in long-chain fatty acid oxidation disorders: FDA approval summary. J. Inherit. Metab. Dis. 2021, 44, 515–517. [Google Scholar] [CrossRef]
- Roe, C.R.; Sweetman, L.; Roe, D.S.; David, F.; Brunengraber, H. Treatment of cardiomyopathy and rhabdomyolysis in long-chain fat oxidation disorders using an anaplerotic odd-chain triglyceride. J. Clin. Investig. 2002, 110, 259–269. [Google Scholar] [CrossRef]
- Jahoor, F.; Peters, E.J.; Wolfe, R.R. The relationship between gluconeogenic substrate supply and glucose production in humans. Am. J. Physiol.-Endocrinol. Metab. 1990, 258, E288–E296. [Google Scholar] [CrossRef] [PubMed]
- Vockley, J.; Marsden, D.; McCracken, E.; DeWard, S.; Barone, A.; Hsu, K.; Kakkis, E. Long-term major clinical outcomes in patients with long chain fatty acid oxidation disorders before and after transition to triheptanoin treatment—A retrospective chart review. Mol. Genet. Metab. 2015, 116, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Vockley, J.; Burton, B.; Berry, G.; Longo, N.; Phillips, J.; Sanchez-Valle, A.; Chapman, K.; Tanpaiboon, P.; Grunewald, S.; Murphy, E.; et al. Effects of triheptanoin (UX007) in patients with long-chain fatty acid oxidation disorders: Results from an open-label, long-term extension study. J. Inherit. Metab. Dis. 2021, 44, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Jang, C.; Chen, L.; Rabinowitz, J.D. Metabolomics and Isotope Tracing. Cell 2018, 173, 822–837. [Google Scholar] [CrossRef]
- Bodamer, O.A.F.; Halliday, D. Uses of stable isotopes in clinical diagnosis and research in the paediatric population. Arch. Dis. Child. 2001, 84, 444–448. [Google Scholar] [CrossRef][Green Version]
- Rossi, A.; Rutten, M.G.S.; van Dijk, T.H.; Bakker, B.M.; Reijngoud, D.-J.; Oosterveer, M.H.; Derks, T.G.J. Dynamic Methods for Childhood Hypoglycemia Phenotyping: A Narrative Review. Front. Endocrinol. 2022, 13, 858832. [Google Scholar] [CrossRef]
- Reijngoud, D.-J. Flux analysis of inborn errors of metabolism. J. Inherit. Metab. Dis. 2018, 41, 309–328. [Google Scholar] [CrossRef]
- Exil, V.J.; Roberts, R.L.; Sims, H.; McLaughlin, J.E.; Malkin, R.A.; Gardner, C.D.; Ni, G.; Rottman, J.N.; Strauss, A.W. Very-Long-Chain Acyl-Coenzyme A Dehydrogenase Deficiency in Mice. Circ. Res. 2003, 93, 448–455. [Google Scholar] [CrossRef]
- van Dijk, T.H.; Boer, T.S.; Havinga, R.; Stellaard, F.; Kuipers, F.; Reijngoud, D.-J. Quantification of hepatic carbohydrate metabolism in conscious mice using serial blood and urine spots. Anal. Biochem. 2003, 322, 1–13. [Google Scholar] [CrossRef]
- Evers, B.; Gerding, A.; Boer, T.; Heiner-Fokkema, M.R.; Jalving, M.; Wahl, S.A.; Reijngoud, D.-J.; Bakker, B.M. Simultaneous Quantification of the Concentration and Carbon Isotopologue Distribution of Polar Metabolites in a Single Analysis by Gas Chromatography and Mass Spectrometry. Anal. Chem. 2021, 93, 8248–8256. [Google Scholar] [CrossRef]
- Millard, P.; Delépine, B.; Guionnet, M.; Heuillet, M.; Bellvert, F.; Létisse, F. IsoCor: Isotope correction for high-resolution MS labeling experiments. Bioinformatics 2019, 35, 4484–4487. [Google Scholar] [CrossRef]
- Vieira-Lara, M.A.; Reijne, A.C.; Koshian, S.; Ciapaite, J.; Abegaz, F.; Talarovicova, A.; van Dijk, T.H.; Versloot, C.J.; Bandsma, R.H.J.; Wolters, J.C.; et al. Age and Diet Modulate the Insulin-Sensitizing Effects of Exercise: A Tracer-Based Oral Glucose Tolerance Test. Diabetes 2023, 72, 872–883. [Google Scholar] [CrossRef] [PubMed]
- Hellerstein, M.K.; Neese, R.A. Mass isotopomer distribution analysis: A technique for measuring biosynthesis and turnover of polymers. Am. J. Physiol.-Endocrinol. Metab. 1992, 263, E988–E1001. [Google Scholar] [CrossRef]
- Dalla Man, C.; Yarasheski, K.E.; Caumo, A.; Robertson, H.; Toffolo, G.; Polonsky, K.S.; Cobelli, C. Insulin sensitivity by oral glucose minimal models: Validation against clamp. Am. J. Physiol.-Endocrinol. Metab. 2005, 289, E954–E959. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Venema, A.; Haarsma, P.; Feldbrugge, L.; Burghard, R.; Rodriguez-Buritica, D.; Parenti, G.; Oosterveer, M.H.; Derks, T.G.J. A Prospective Study on Continuous Glucose Monitoring in Glycogen Storage Disease Type Ia: Toward Glycemic Targets. J. Clin. Endocrinol. Metab. 2022, 107, e3612–e3623. [Google Scholar] [CrossRef]
- Kalemba, K.M.; Wang, Y.; Xu, H.; Chiles, E.; McMillin, S.M.; Kwon, H.; Su, X.; Wondisford, F.E. Glycerol induces G6pc in primary mouse hepatocytes and is the preferred substrate for gluconeogenesis both in vitro and in vivo. J. Biol. Chem. 2019, 294, 18017–18028. [Google Scholar] [CrossRef] [PubMed]
- Myung, J.; Tang, Y.; Pitt, M.A. Evaluation and Comparison of Computational Models. Methods Enzym. 2009, 454, 287–304. [Google Scholar] [CrossRef]
- Inigo, M.; Deja, S.; Burgess, S.C. Ins and Outs of the TCA Cycle: The Central Role of Anaplerosis. Annu. Rev. Nutr. 2021, 41, 19–47. [Google Scholar] [CrossRef]
- Petersen, K.F.; Price, T.B.; Bergeron, R. Regulation of Net Hepatic Glycogenolysis and Gluconeogenesis during Exercise: Impact of Type 1 Diabetes. J. Clin. Endocrinol. Metab. 2004, 89, 4656–4664. [Google Scholar] [CrossRef]
- Houten, S.M.; Herrema, H.; te Brinke, H.; Denis, S.; Ruiter, J.P.N.; van Dijk, T.H.; Argmann, C.A.; Ottenhoff, R.; Müller, M.; Groen, A.K.; et al. Impaired amino acid metabolism contributes to fasting-induced hypoglycemia in fatty acid oxidation defects. Hum. Mol. Genet. 2013, 22, 5249–5261. [Google Scholar] [CrossRef]
- Tucci, S.; Herebian, D.; Sturm, M.; Seibt, A.; Spiekerkoetter, U. Tissue-Specific Strategies of the Very-Long Chain Acyl-CoA Dehydrogenase-Deficient (VLCAD−/−) Mouse to Compensate a Defective Fatty Acid β-Oxidation. PLoS ONE 2012, 7, e45429. [Google Scholar] [CrossRef] [PubMed]
- Diekman, E.F.; van Weeghel, M.; Suárez-Fariñas, M.; Argmann, C.; Ranea-Robles, P.; Wanders, R.J.A.; Visser, G.; van der Made, I.; Creemers, E.E.; Houten, S.M. Dietary restriction in the long-chain acyl-CoA dehydrogenase knockout mouse. Mol. Genet. Metab. Rep. 2021, 27, 100749. [Google Scholar] [CrossRef]
- Houten, S.M.; Violante, S.; Ventura, F.V.; Wanders, R.J.A. The Biochemistry and Physiology of Mitochondrial Fatty Acid β-Oxidation and Its Genetic Disorders. Annu. Rev. Physiol. 2016, 78, 23–44. [Google Scholar] [CrossRef] [PubMed]
- Brunengraber, H.; Roe, C.R. Anaplerotic molecules: Current and future. J. Inherit. Metab. Dis. 2006, 29, 327–331. [Google Scholar] [CrossRef]
- Tucci, S.; Behringer, S.; Spiekerkoetter, U. De novo fatty acid biosynthesis and elongation in very long-chain acyl-CoA dehydrogenase-deficient mice supplemented with odd or even medium-chain fatty acids. FEBS J. 2015, 282, 4242–4253. [Google Scholar] [CrossRef]
- Satapati, S.; Sunny, N.E.; Kucejova, B.; Fu, X.; He, T.T.; Méndez-Lucas, A.; Shelton, J.M.; Perales, J.C.; Browning, J.D.; Burgess, S.C. Elevated TCA cycle function in the pathology of diet-induced hepatic insulin resistance and fatty liver. J. Lipid Res. 2012, 53, 1080–1092. [Google Scholar] [CrossRef] [PubMed]
- Sunny, N.E.; Parks, E.J.; Browning, J.D.; Burgess, S.C. Excessive Hepatic Mitochondrial TCA Cycle and Gluconeogenesis in Humans with Nonalcoholic Fatty Liver Disease. Cell Metab. 2011, 14, 804–810. [Google Scholar] [CrossRef]
- Zöggeler, T.; Stock, K.; Jörg-Streller, M.; Spenger, J.; Konstantopoulou, V.; Hufgard-Leitner, M.; Scholl-Bürgi, S.; Karall, D. Long-term experience with triheptanoin in 12 Austrian patients with long-chain fatty acid oxidation disorders. Orphanet J. Rare Dis. 2021, 16, 28. [Google Scholar] [CrossRef]
- Gu, L.; Zhang, G.-F.; Kombu, R.S.; Allen, F.; Kutz, G.; Brewer, W.-U.; Roe, C.R.; Brunengraber, H. Parenteral and enteral metabolism of anaplerotic triheptanoin in normal rats. II. Effects on lipolysis, glucose production, and liver acyl-CoA profile. Am. J. Physiol.-Endocrinol. Metab. 2010, 298, E362–E371. [Google Scholar] [CrossRef]
- Gonzalez, J.T.; Fuchs, C.J.; Betts, J.A.; van Loon, L.J.C. Liver glycogen metabolism during and after prolonged endurance-type exercise. Am. J. Physiol.-Endocrinol. Metab. 2016, 311, E543–E553. [Google Scholar] [CrossRef]
- Jin, E.S.; Sherry, A.D.; Malloy, C.R. Metabolism of Glycerol, Glucose, and Lactate in the Citric Acid Cycle Prior to Incorporation into Hepatic Acylglycerols. J. Biol. Chem. 2013, 288, 14488–14496. [Google Scholar] [CrossRef]
- Lee, J.; Choi, J.; Selen Alpergin, E.S.; Zhao, L.; Hartung, T.; Scafidi, S.; Riddle, R.C.; Wolfgang, M.J. Loss of Hepatic Mitochondrial Long-Chain Fatty Acid Oxidation Confers Resistance to Diet-Induced Obesity and Glucose Intolerance. Cell Rep. 2017, 20, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Hasenour, C.M.; Wall, M.L.; Ridley, D.E.; Hughey, C.C.; James, F.D.; Wasserman, D.H.; Young, J.D. Mass spectrometry-based microassay of 2H and 13C plasma glucose labeling to quantify liver metabolic fluxes in vivo. Am. J. Physiol.-Endocrinol. Metab. 2015, 309, E191–E203. [Google Scholar] [CrossRef] [PubMed]
- Hasenour, C.M.; Rahim, M.; Young, J.D. In Vivo Estimates of Liver Metabolic Flux Assessed by 13C-Propionate and 13C-Lactate Are Impacted by Tracer Recycling and Equilibrium Assumptions. Cell Rep. 2020, 32, 107986. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nurjanah, S.; Gerding, A.; Vieira-Lara, M.A.; Evers, B.; Langelaar-Makkinje, M.; Spiekerkoetter, U.; Bakker, B.M.; Tucci, S. Heptanoate Improves Compensatory Mechanism of Glucose Homeostasis in Mitochondrial Long-Chain Fatty Acid Oxidation Defect. Nutrients 2023, 15, 4689. https://doi.org/10.3390/nu15214689
Nurjanah S, Gerding A, Vieira-Lara MA, Evers B, Langelaar-Makkinje M, Spiekerkoetter U, Bakker BM, Tucci S. Heptanoate Improves Compensatory Mechanism of Glucose Homeostasis in Mitochondrial Long-Chain Fatty Acid Oxidation Defect. Nutrients. 2023; 15(21):4689. https://doi.org/10.3390/nu15214689
Chicago/Turabian StyleNurjanah, Siti, Albert Gerding, Marcel A. Vieira-Lara, Bernard Evers, Miriam Langelaar-Makkinje, Ute Spiekerkoetter, Barbara M. Bakker, and Sara Tucci. 2023. "Heptanoate Improves Compensatory Mechanism of Glucose Homeostasis in Mitochondrial Long-Chain Fatty Acid Oxidation Defect" Nutrients 15, no. 21: 4689. https://doi.org/10.3390/nu15214689
APA StyleNurjanah, S., Gerding, A., Vieira-Lara, M. A., Evers, B., Langelaar-Makkinje, M., Spiekerkoetter, U., Bakker, B. M., & Tucci, S. (2023). Heptanoate Improves Compensatory Mechanism of Glucose Homeostasis in Mitochondrial Long-Chain Fatty Acid Oxidation Defect. Nutrients, 15(21), 4689. https://doi.org/10.3390/nu15214689




