Reducing In-Hospital and 60-Day Mortality in Critically Ill Patients after Surgery with Strict Nutritional Supplementation: A Prospective, Single-Labeled, Randomized Controlled Trial
Abstract
:1. Introduction
2. Participants and Methods
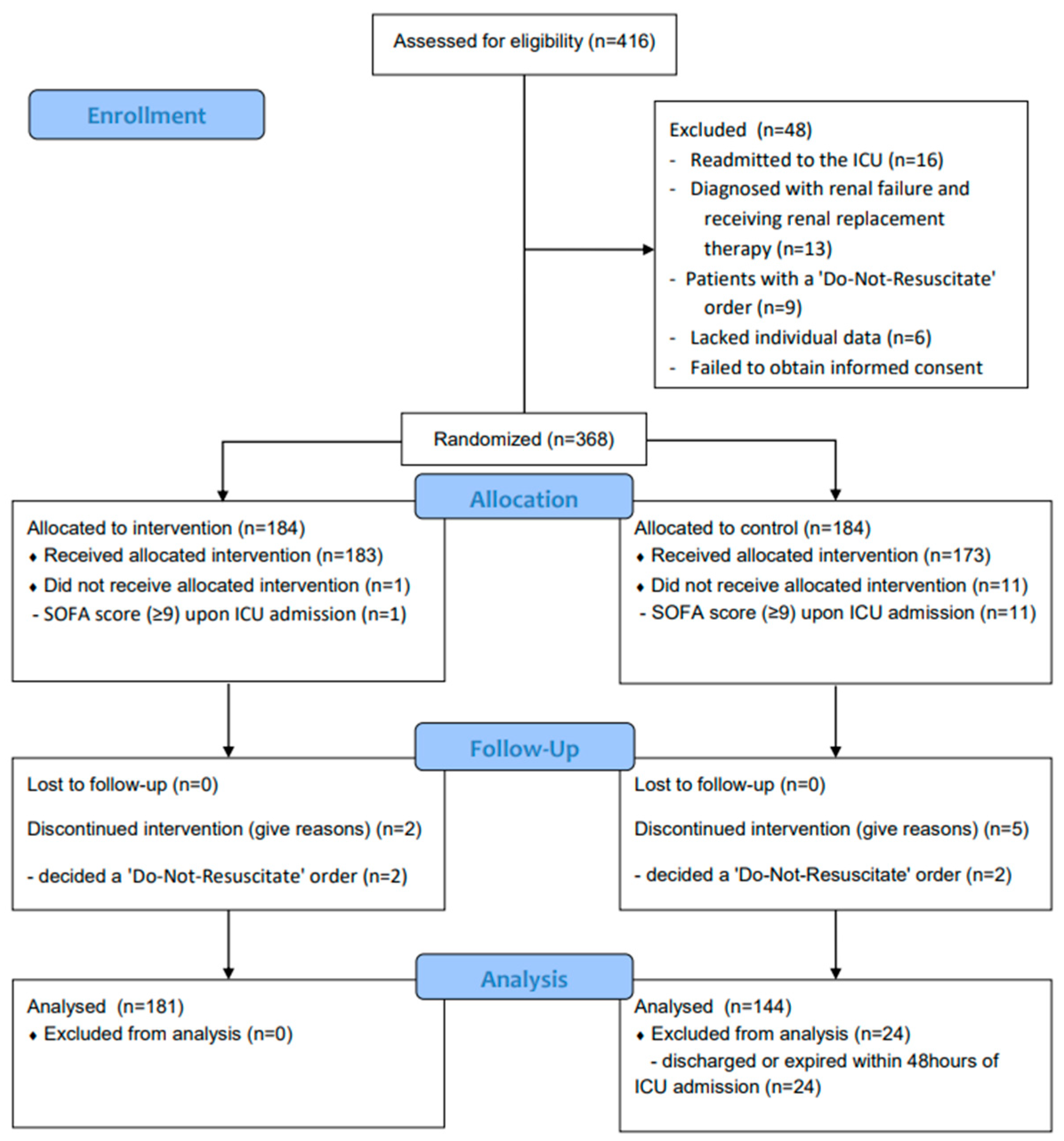
2.1. Patient Enrollment and Exclusion Criteria
2.2. Sample Size Calculation
2.3. Randomization and the Study Protocol
2.4. Data Collection and Outcome Measurement
2.5. Study Outcomes and Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maday, K.R. The importance of nutrition in critically ill patients. J. Am. Acad. PAs 2017, 30, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Alhazzani, W.; Calder, P.C.; Casaer, M.P.; Hiesmayr, M.; Mayer, K.; Montejo, J.C.; Pichard, C.; et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin. Nutr. 2019, 38, 48–79. [Google Scholar] [CrossRef]
- Villet, S.; Chiolero, R.L.; Bollmann, M.D.; Revelly, J.P.; Cayeux, R.N.M.; Delarue, J.; Berger, M.M. Negative impact of hypocaloric feeding and energy balance on clinical outcome in ICU patients. Clin. Nutr. 2005, 24, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Weissman, C. The metabolic response to stress: An overview and update. Anesthesiology 1990, 73, 308–327. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Xie, K.; Li, X.K.; Wang, G.M.; Luo, J.; Zhang, C.; Jiang, Z.S.; Wang, Y.L.; Luo, C.; Qiang, Y.; et al. The prognostic value of modified NUTRIC score for patients in cardiothoracic surgery recovery unit: A retrospective cohort study. J. Hum. Nutr. Diet. 2021, 34, 926–934. [Google Scholar] [CrossRef]
- Reisinger, K.W.; van Vugt, J.L.; Tegels, J.J.; Snijders, C.; Hulsewé, K.W.; Hoofwijk, A.G.; Stoot, J.H.; Von Meyenfeldt, M.F.; Beets, G.L.; Derikx, J.P.; et al. Functional compromise reflected by sarcopenia, frailty, and nutritional depletion predicts adverse postoperative outcome after colorectal cancer surgery. Ann. Surg. 2015, 261, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.W.; Chen, X.Y.; Fan, S.D.; Zhang, F.M.; Huang, D.D.; Li, B.; Shen, X.; Zhuang, C.L.; Yu, Z. Impact of sarcopenia on clinical outcomes after radical gastrectomy for patients without nutritional risk. Nutrition 2019, 61, 61–66. [Google Scholar] [CrossRef]
- Schwegler, I.; von Holzen, A.; Gutzwiller, J.P.; Schlumpf, R.; Mühlebach, S.; Stanga, Z. Nutritional risk is a clinical predictor of postoperative mortality and morbidity in surgery for colorectal cancer. Br. J. Surg. 2010, 97, 92–97. [Google Scholar] [CrossRef]
- Rovesti, G.; Valoriani, F.; Rimini, M.; Bardasi, C.; Ballarin, R.; Di Benedetto, F.; Menozzi, R.; Dominici, M.; Spallanzani, A. Clinical Implications of Malnutrition in the Management of Patients with Pancreatic Cancer: Introducing the Concept of the Nutritional Oncology Board. Nutrients 2021, 13, 3522. [Google Scholar] [CrossRef]
- Ziegler, T.R. Parenteral Nutrition in the Critically III Patient. N. Engl. J. Med. 2009, 361, 1088–1097. [Google Scholar] [CrossRef]
- Yeh, D.D.; Fuentes, E.; Quraishi, S.A.; Cropano, C.; Kaafarani, H.; Lee, J.; King, D.R.; DeMoya, M.; Fagenholz, P.; Butler, K.; et al. Adequate Nutrition May Get You Home: Effect of Caloric/Protein Deficits on the Discharge Destination of Critically Ill Surgical Patients. JPEN J. Parenter. Enter. Nutr. 2016, 40, 37–44. [Google Scholar] [CrossRef] [PubMed]
- McClave, S.A.; Taylor, B.E.; Martindale, R.G.; Warren, M.M.; Johnson, D.R.; Braunschweig, C.; McCarthy, M.S.; Davanos, E.; Rice, T.W.; Cresci, G.A.; et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically III Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J. Parenter. Enter. Nutr. 2016, 40, 159–211. [Google Scholar] [CrossRef] [PubMed]
- Weimann, A.; Braga, M.; Carli, F.; Higashiguchi, T.; Hübner, M.; Klek, S.; Laviano, A.; Ljungqvist, O.; Lobo, D.N.; Martindale, R.; et al. ESPEN guideline: Clinical nutrition in surgery. Clin. Nutr. 2017, 36, 623–650. [Google Scholar] [CrossRef] [PubMed]
- Chada, R.R.; Chidrawar, S.; Goud, B.A.; Maska, A.; Medanki, R.; Nagalla, B. Association Between Nutrition Delivery, Modified Nutrition Risk In Critically III Score, and 28-Day Mortality. Nutr. Clin. Pract. 2021, 36, 1020–1033. [Google Scholar] [CrossRef]
- Dhaliwal, R.; Cahill, N.; Lemieux, M.; Heyland, D.K. The Canadian critical care nutrition guidelines in 2013: An update on current recommendations and implementation strategies. Nutr. Clin. Pract. 2014, 29, 29–43. [Google Scholar] [CrossRef]
- Heyland, D.K.; Patel, J.; Compher, C.; Rice, T.W.; Bear, D.E.; Lee, Z.Y.; González, V.C.; O’Reilly, K.; Regala, R.; Wedemire, C.; et al. The effect of higher protein dosing in critically ill patients with high nutritional risk (EFFORT Protein): An international, multicentre, pragmatic, registry-based randomised trial. Lancet 2023, 401, 568–576. [Google Scholar] [CrossRef]
- De Waele, E.; Jakubowski, J.R.; Stocker, R.; Wischmeyer, P.E. Review of evolution and current status of protein requirements and provision in acute illness and critical care. Clin. Nutr. 2021, 40, 2958–2973. [Google Scholar] [CrossRef]
- Heyland, D.K.; Weijs, P.J.; Coss-Bu, J.A.; Taylor, B.; Kristof, A.S.; O’Keefe, G.E.; Martindale, R.G. Protein Delivery in the Intensive Care Unit: Optimal or Suboptimal? Nutr. Clin. Pract. 2017, 32, 58s–71s. [Google Scholar] [CrossRef]
- Chapman, M.; Peake, S.L.; Bellomo, R.; Davies, A.; Deane, A.; Horowitz, M.; Hurford, S.; Lange, K.; Little, L.; Mackle, D.; et al. Energy-Dense versus Routine Enteral Nutrition in the Critically III. N. Engl. J. Med. 2018, 379, 1823–1834. [Google Scholar] [CrossRef]
- Im, K.M.; Kim, E.Y. Identification of ICU Patients with High Nutritional Risk after Abdominal Surgery Using Modified NUTRIC Score and the Association of Energy Adequacy with 90-Day Mortality. Nutrients 2022, 14, 946. [Google Scholar] [CrossRef]
- Chung, Y.J.; Kim, E.Y. Usefulness of Bioelectrical Impedance Analysis as a Guidance of Fluid Management in Critically Ill Patients after Major Abdomen Surgery; a Single Center, Prospective Cohort Study. Surg. Metab. Nutr. 2020, 11, 53–60. [Google Scholar] [CrossRef]
- Roza, A.M.; Shizgal, H.M. The Harris Benedict equation reevaluated: Resting energy requirements and the body cell mass. Am. J. Clin. Nutr. 1984, 40, 168–182. [Google Scholar] [CrossRef]
- Rahman, A.; Hasan, R.M.; Agarwala, R.; Martin, C.; Day, A.G.; Heyland, D.K. Identifying critically-ill patients who will benefit most from nutritional therapy: Further validation of the “modified NUTRIC” nutritional risk assessment tool. Clin. Nutr. 2016, 35, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Correia, M.I.; Hegazi, R.A.; Diaz-Pizarro Graf, J.I.; Gomez-Morales, G.; Fuentes Gutiérrez, C.; Goldin, M.F.; Navas, A.; Pinzón Espitia, O.L.; Tavares, G.M. Addressing Disease-Related Malnutrition in Healthcare: A Latin American Perspective. JPEN J. Parenter. Enter. Nutr. 2016, 40, 319–325. [Google Scholar] [CrossRef]
- Norman, K.; Pichard, C.; Lochs, H.; Pirlich, M. Prognostic impact of disease-related malnutrition. Clin. Nutr. 2008, 27, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Jensen, G.L.; Bistrian, B.; Roubenoff, R.; Heimburger, D.C. Malnutrition syndromes: A conundrum vs continuum. JPEN J. Parenter. Enter. Nutr. 2009, 33, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Veterans Affairs Total Parenteral Nutrition Cooperative Study Group. Perioperative total parenteral nutrition in surgical patients. N. Engl. J. Med. 1991, 325, 525–532. [Google Scholar] [CrossRef]
- Bargetzi, L.; Brack, C.; Herrmann, J.; Bargetzi, A.; Hersberger, L.; Bargetzi, M.; Kaegi-Braun, N.; Tribolet, P.; Gomes, F.; Hoess, C.; et al. Nutritional support during the hospital stay reduces mortality in patients with different types of cancers: Secondary analysis of a prospective randomized trial. Ann. Oncol. 2021, 32, 1025–1033. [Google Scholar] [CrossRef]
- Richards, J.; Arensberg, M.B.; Thomas, S.; Kerr, K.W.; Hegazi, R.; Bastasch, M. Impact of Early Incorporation of Nutrition Interventions as a Component of Cancer Therapy in Adults: A Review. Nutrients 2020, 12, 3403. [Google Scholar] [CrossRef]
- Lin, P.Y.; Yen, Y.T.; Lam, C.T.; Li, K.C.; Lu, M.J.; Hsu, H.S. Use of modified-NUTRIC score to assess nutritional risk in surgical intensive care unit. J. Chin. Med. Assoc. 2021, 84, 860–864. [Google Scholar] [CrossRef] [PubMed]
- Lee, Z.Y.; Hasan, M.S.; Day, A.G.; Ng, C.C.; Ong, S.P.; Yap, C.S.L.; Engkasan, J.P.; Barakatun-Nisak, M.Y.; Heyland, D.K. Initial development and validation of a novel nutrition risk, sarcopenia, and frailty assessment tool in mechanically ventilated critically ill patients: The NUTRIC-SF score. JPEN J. Parenter. Enter. Nutr. 2022, 46, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Bian, Y.; Tang, Z.; Wang, F. Use of Nutrition Risk in Critically Ill (NUTRIC) Scoring System for Nutrition Risk Assessment and Prognosis Prediction in Critically Ill Neurological Patients: A Prospective Observational Study. JPEN J. Parenter. Enter. Nutr. 2021, 45, 1032–1041. [Google Scholar] [CrossRef] [PubMed]
| Variables | All Patients | Intervention Group | Control Group | p-Value |
|---|---|---|---|---|
| n = 325 | n = 181 | n = 144 | ||
| Demographics | ||||
| Age (years) | 65 ± 14.6 | 67.9 ± 13.6 | 61.3 ± 15.1 | <0.001 |
| Gender (male, %) | 217 (66.8) | 112 (61.9) | 105 (72.9) | 0.044 |
| Body mass index (kg/m−2) | 23.5 ± 4.1 | 23.3 ± 4.1 | 23.7 ± 4.2 | 0.309 |
| Use of vasopressors (%) | 75 (23.1) | 42 (56) | 33 (22.9) | 1.000 |
| SOFA score | 5.1 ± 3.5 | 4.8 ± 3.5 | 5.6 ± 3.5 | 0.041 |
| APACHE II score | 14.1 ± 7.2 | 12.5 ± 6.5 | 16 ± 7.5 | <0.001 |
| mNUTRIC score | 3.8 ± 1.8 | 3.9 ± 1.8 | 3.8 ± 1.7 | 0.640 |
| Patients with high mNUTRIC score ≥ 5 | 108 (27) | 65 (25.4) | 43 (29.9) | 0.349 |
| SGA (%) | 0.409 | |||
| well-nourished | 234 (72) | 131 (72.4) | 103 (71.5) | 0.901 |
| moderately malnourished | 60 (18.5) | 30 (16.6) | 30 (20.8) | 0.388 |
| severely malnourished | 31 (9.5) | 20 (11) | 11 (7.6) | 0.345 |
| Clinical outcomes | ||||
| Postoperative complication (%) | 113 (34.8) | 45 (24.9) | 68 (47.2) | <0.001 |
| Length of ICU stay (days) | 5.5 ± 6.2 | 5 ± 5.7 | 6.1 ± 6.8 | 0.115 |
| Length of hospital stay (days) | 28.6 ± 23.9 | 23.5 ± 20.5 | 35.1 ± 26.2 | <0.001 |
| In-hospital mortality (%) | 34 (10.5) | 9 (5) | 25 (17.4) | <0.001 |
| 60-day mortality (%) | 30 (9.2) | 8 (4.4) | 22 (15.3) | 0.001 |
| Overall mortality (%) | 36 (11.1) | 9 (5) | 27 (18.8) | <0.001 |
| Laboratory test | ||||
| Total protein (g/dL) | 5.5 ± 1.2 | 5.7 ± 1.3 | 5.3 ± 1.1 | 0.005 |
| Albumin (g/dL) | 3.2 ± 0.9 | 3.2 ± 1.1 | 3.2 ± 0.6 | 0.965 |
| Prealbumin (mg/dL) | 15.7 ± 7.6 | 15.9 ± 7.3 | 15 ± 8.4 | 0.435 |
| Transferrin (mg/dL) | 154.9 ± 59.7 | 163.3 ± 60.8 | 140.1 ± 55 | 0.040 |
| Total cholesterol (mg/dL) | 105.5 ± 43.2 | 107.7 ± 43 | 101.8 ± 43.4 | 0.303 |
| Triglycerides (mg/dL) | 75.3 ± 51.5 | 73.6 ± 47.8 | 78.1 ± 57.4 | 0.519 |
| HDL (mg/dL) | 28.8 ± 13.3 | 30.4 ± 13.8 | 26.2 ± 12.1 | 0.019 |
| LDL (mg/dL) | 55.8 ± 29.5 | 56.6 ± 31.6 | 54.3 ± 25.7 | 0.571 |
| Variables | All Patients | Intervention Group | Control Group | p-Value |
|---|---|---|---|---|
| n = 108 | n = 65 | n = 43 | ||
| Demographics | ||||
| Age (years) | 70.6 ± 12.8 | 72.7 ± 12.2 | 67.4 ± 13.2 | 0.034 |
| Gender (male, %) | 69 (63.9) | 41 (63.1) | 28 (65.1) | 1.000 |
| Body mass index (kg/m−2) | 23 ± 4.5 | 22.7 ± 4.6 | 23.4 ± 4.5 | 0.466 |
| Use of vasopressors (%) | 53 (49.1) | 35 (53.8) | 18 (41.9) | 0.244 |
| SOFA score | 8.1 ± 3.3 | 8.2 ± 3.1 | 8 ± 3.5 | 0.756 |
| APACHE II score | 16.7 ± 6.7 | 17.1 ± 5.9 | 16.1 ± 7.8 | 0.439 |
| mNUTRIC score | 5.9 ± 1 | 6 ± 1 | 6 ± 1 | 0.902 |
| Patients with high mNUTRIC score ≥ 5 | 0.209 | |||
| SGA (%) | 65 (60.2) | 36 (55.4) | 29 (67.4) | 0.234 |
| well-nourished | 25 (23.1) | 15 (23.1) | 10 (23.3) | 1.000 |
| moderately malnourished | 18 (16.7) | 14 (21.5) | 4 (9.3) | 0.118 |
| severely malnourished | 70.6 ± 12.8 | 72.7 ± 12.2 | 67.4 ± 13.2 | 0.034 |
| Clinical outcomes | ||||
| Postoperative complication (%) | 65 (60.2) | 35 (53.8) | 30 (69.8) | 0.112 |
| Length of ICU stay (days) | 8.3 ± 8.7 | 8.4 ± 7.8 | 8.3 ± 10 | 0.954 |
| Length of hospital stay (days) | 35.4 ± 23.6 | 33.6 ± 22.8 | 38.2 ± 24.7 | 0.327 |
| In-hospital mortality (%) | 21 (19.4) | 8 (12.3) | 13 (30.2) | 0.027 |
| 60-day mortality (%) | 19 (17.6) | 7 (10.8) | 12 (27.9) | 0.037 |
| Overall mortality (%) | 21 (19.4) | 8 (12.3) | 13 (30.2) | 0.027 |
| Laboratory test | ||||
| Total protein (g/dL) | 5 ± 1.1 | 4.9 ± 1.2 | 5 ± 1 | 0.532 |
| Albumin (g/dL) | 3 ± 1.2 | 2.9 ± 1.5 | 3.1 ± 0.5 | 0.430 |
| Prealbumin (mg/dL) | 11.3 ± 6 | 11.1 ± 5.4 | 12.2 ± 8.4 | 0.591 |
| Transferrin (mg/dL) | 122.7 ± 43.2 | 123.7 ± 47.8 | 120.4 ± 30.3 | 0.737 |
| Total cholesterol (mg/dL) | 83.9 ± 33.7 | 82.5 ± 34.3 | 88.6 ± 32.9 | 0.636 |
| Triglycerides (mg/dL) | 70.6 ± 50.3 | 68.3 ± 49.9 | 75.4 ± 52.1 | 0.584 |
| HDL (mg/dL) | 23.4 ± 12.9 | 24.1 ± 14.1 | 21.9 ± 10.1 | 0.469 |
| LDL (mg/dL) | 40.8 ± 21.8 | 38.6 ± 22.5 | 45.4 ± 19.9 | 0.225 |
| (A) Total Participants | ||||
|---|---|---|---|---|
| Variables | All Patients | Intervention Group | Control Group | p-Value |
| n = 325 | n = 181 | n = 144 | ||
| Total calorie need | 1543.5 ± 233.6 | 1619.9 ± 257.5 | 1447.4 ± 153.2 | <0.001 |
| Energy delivered per day (kcal/day) | 993.3 ± 525 | 1307.9 ± 456.8 | 597.9 ± 281.3 | <0.001 |
| Energy adequacy (%) | 63.7 ± 29.4 | 80.7 ± 23 | 42.2 ± 21.5 | <0.001 |
| Average energy delivered (kcal/kg/day) | 16.9 ± 8.6 | 22 ± 7.4 | 10.5 ±5.2 | <0.001 |
| Protein delivered per day (g/day) | 51.6 ± 32.6 | 70.7 ± 30.5 | 27.6 ± 13.7 | <0.001 |
| Average protein delivered (g/kg/day) | 0.87 ± 0.5 | 1.17 ± 0.43 | 0.49 ± 0.25 | <0.001 |
| (B) High mNUTRIC Group (mNUTRIC Score ≥ 5) | ||||
| Variables | All Patients | Intervention Group | Control Group | p-Value |
| n = 108 | n = 65 | n = 43 | ||
| Total calorie need | 1444.9 ± 188.4 | 1470.7 ± 204 | 1405.9 ± 156.2 | 0.080 |
| Energy delivered per day (kcal/day) | 877 ± 418 | 1033 ± 399.4 | 641.3 ± 327.9 | <0.001 |
| Energy adequacy (%) | 61.7 ± 28.7 | 70.8 ± 25.5 | 48 ± 28.1 | <0.001 |
| Average energy delivered (kcal/kg/day) | 15.8 ± 7.8 | 18.5 ± 7.4 | 11.8 ± 6.6 | <0.001 |
| Protein delivered per day (g/day) | 43.7 ± 24.6 | 53.5 ± 24.3 | 29 ± 16.5 | <0.001 |
| Average protein delivered (g/kg/day) | 0.78 ± 0.44 | 1 ± 0.4 | 0.5 ± 0.3 | <0.001 |
| (A) Total Participants | ||||
|---|---|---|---|---|
| Variable | Univariate | Multivariate | ||
| OR (95% CI) | p | OR (95% CI) | p | |
| Age | 0.989 (0.974–1.005) | 0.183 | ||
| SOFA score | 1.513 (1.376–1.664) | <0.001 | 1.338 (1.197–1.496) | <0.001 |
| APACHE II score | 1.086 (1.050–1.122) | <0.001 | 1.008 (0.967–1.051) | 0.708 |
| mNUTRIC score | 1.861 (1.580–2.193) | <0.001 | 1.272 (1.044–1.549) | 0.017 |
| Emergent surgery | 3.175 (2.044–4.933) | <0.001 | 1.726 (0.979–3.042) | 0.059 |
| Average energy delivered ≥ 20 (kcal/kg/day) | 0.189 (0.112–0.317) | <0.001 | 1.226 (0.430–3.499) | 0.703 |
| Average protein delivered ≥ 1.2 (g/kg/day) | 0.170 (0.104–0.278) | <0.001 | 0.303 (0.113–0.814) | 0.018 |
| Intervention | 0.370 (0.231–0.592) | <0.001 | 0.594 (0.294–1.200) | 0.147 |
| (B) High mNUTRIC Group (mNUTRIC Score ≥ 5) | ||||
| Variable | Univariate | Multivariate | ||
| OR (95% CI) | p | OR (95% CI) | p | |
| Age | 0.958 (0.926–0.992) | 0.015 | 0.976 (0.936–1.016) | 0.238 |
| SOFA score | 1.338 (1.155–1.550) | <0.001 | 1.197 (0.991–1.447) | 0.062 |
| APACHE II score | 0.998 (0.942–1.058) | 0.959 | ||
| mNUTRIC score | 2.223 (1.376–3.591) | 0.001 | 1.660 (0.900–3.059) | 0.104 |
| Emergent surgery | 3.175 (2.044–4.933) | <0.001 | 1.799 (0.727–4.449) | 0.204 |
| Average energy delivered ≥ 20 (kcal/kg/day) | 0.314 (0.132–0.752) | 0.009 | 0.990 (0.197–4.984) | 0.990 |
| Average protein delivered ≥ 1.2 (g/kg/day) | 0.371 (0.164–0.838) | 0.017 | 0.830 (0.179–3.844) | 0.812 |
| Intervention | 0.506 (0.224–1.140) | 0.100 | 0.468 (0.160–1.365) | 0.164 |
| (A) Total Participants | ||||
|---|---|---|---|---|
| Variable | Univariate | Multivariate | ||
| OR (95% CI) | p | OR (95% CI) | p | |
| Age | 1.010 (0.985–1.036) | 0.442 | ||
| SOFA score | 1.295 (1.167–1.437) | <0.001 | 1.119 (0.974–1.284) | 0.112 |
| APACHE II score | 1.046 (1.004–1.090) | 0.030 | 0.979 (0.928–1.033) | 0.439 |
| mNUTRIC score | 1.733 (1.393–2.155) | <0.001 | 1.419 (1.086–1.855) | 0.010 |
| Emergent surgery | 5.541 (2.614–11.743) | <0.001 | 3.842 (1.691–8.725) | 0.001 |
| Average energy delivered ≥ 20 (kcal/kg/day) | 0.223 (0.091–0.546) | 0.001 | 1.256 (0.286–5.520) | 0.763 |
| Average protein delivered ≥ 1.2 (g/kg/day) | 0.235 (0.105–0.525) | <0.001 | 0.619 (0.166–2.306) | 0.475 |
| Intervention | 0.249 (0.112–0.553) | 0.001 | 0.300 (0.122–0.739) | 0.009 |
| (B) High mNUTRIC Group (mNUTRIC Score ≥ 5) | ||||
| Variable | Univariate | Multivariate | ||
| OR (95% CI) | p | OR (95% CI) | p | |
| Age | 1.009 (0.971–1.049) | 0.634 | ||
| SOFA score | 1.183 (1.004–1.393) | 0.044 | 1.017 (0.832–1.243) | 0.870 |
| APACHE II score | 0.991 (0.921–1.065) | 0.796 | ||
| mNUTRIC score | 2.483 (1.444–4.272) | 0.001 | 2.792 (1.340–5.817) | 0.006 |
| Emergent surgery | 3.872 (1.204–12.458) | 0.023 | 4.563 (1.207–17.250) | 0.025 |
| Average energy delivered ≥ 20 (kcal/kg/day) | 0.370 (0.101–1.364) | 0.135 | ||
| Average protein delivered ≥ 1.2 (g/kg/day) | 0.367 (0.114–1.183) | 0.093 | ||
| Intervention | 0.324 (0.121–0.868) | 0.025 | 0.197 (0.059–0.657) | 0.008 |
| (A) Total Participants | ||||
|---|---|---|---|---|
| Variable | Univariate | Multivariate | ||
| OR (95% CI) | p | OR (95% CI) | p | |
| Age | 1.008 (0.982–1.035) | 0.556 | ||
| SOFA score | 1.258 (1.131–1.399) | <0.001 | 1.063 (0.921–1.227) | 0.406 |
| APACHE II score | 1.045 (1.001–1.091) | 0.046 | 0.981 (0.927–1.039) | 0.514 |
| mNUTRIC score | 1.771 (1.405–2.234) | <0.001 | 1.538 (1.154–2.052) | 0.003 |
| Emergent surgery | 5.547 (2.304–13.353) | <0.001 | 3.575 (1.510–8.465) | 0.004 |
| Average energy delivered ≥ 20 (kcal/kg/day) | 0.215 (0.081–0.567) | 0.002 | 2.278 (0.406–12.775) | 0.349 |
| Average protein delivered ≥ 1.2 (g/kg/day) | 0.156 (0.059–0.413) | <0.001 | 0.228 (0.047–1.119) | 0.069 |
| Intervention | 0.256 (0.111–0.595) | 0.002 | 0.351 (0.138–0.897) | 0.029 |
| (B) High mNUTRIC Group (mNUTRIC Score ≥ 5) | ||||
| Variable | Univariate | Multivariate | ||
| OR (95% CI) | p | OR (95% CI) | p | |
| Age | 1.000 (0.962–1.040) | 0.981 | ||
| SOFA score | 1.167 (0.987–1.381) | 0.071 | ||
| APACHE II score | 0.993 (0.921–1.070) | 0.857 | ||
| mNUTRIC score | 2.658 (1.498–4.716) | 0.001 | 3.106 (1.532–6.298) | 0.002 |
| Emergent surgery | 3.271 (1.005–10.644) | 0.049 | 4.242 (1.054–17.076) | 0.042 |
| Average energy delivered ≥ 20 (kcal/kg/day) | 0.256 (0.066–1.186) | 0.082 | ||
| Average protein delivered ≥ 1.2 (g/kg/day) | 0.173 (0.038–0.796) | 0.024 | 0.233 (0.040–1.369) | 0.107 |
| Intervention | 0.312 (0.111–0.873) | 0.026 | 0.254 (0.070–0.918) | 0.037 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Im, K.M.; Kim, E.Y. Reducing In-Hospital and 60-Day Mortality in Critically Ill Patients after Surgery with Strict Nutritional Supplementation: A Prospective, Single-Labeled, Randomized Controlled Trial. Nutrients 2023, 15, 4684. https://doi.org/10.3390/nu15214684
Im KM, Kim EY. Reducing In-Hospital and 60-Day Mortality in Critically Ill Patients after Surgery with Strict Nutritional Supplementation: A Prospective, Single-Labeled, Randomized Controlled Trial. Nutrients. 2023; 15(21):4684. https://doi.org/10.3390/nu15214684
Chicago/Turabian StyleIm, Kyoung Moo, and Eun Young Kim. 2023. "Reducing In-Hospital and 60-Day Mortality in Critically Ill Patients after Surgery with Strict Nutritional Supplementation: A Prospective, Single-Labeled, Randomized Controlled Trial" Nutrients 15, no. 21: 4684. https://doi.org/10.3390/nu15214684
APA StyleIm, K. M., & Kim, E. Y. (2023). Reducing In-Hospital and 60-Day Mortality in Critically Ill Patients after Surgery with Strict Nutritional Supplementation: A Prospective, Single-Labeled, Randomized Controlled Trial. Nutrients, 15(21), 4684. https://doi.org/10.3390/nu15214684







