High ApoB/ApoA-I Ratio Predicts Post-Stroke Cognitive Impairment in Acute Ischemic Stroke Patients with Large Artery Atherosclerosis
Abstract
:1. Introduction
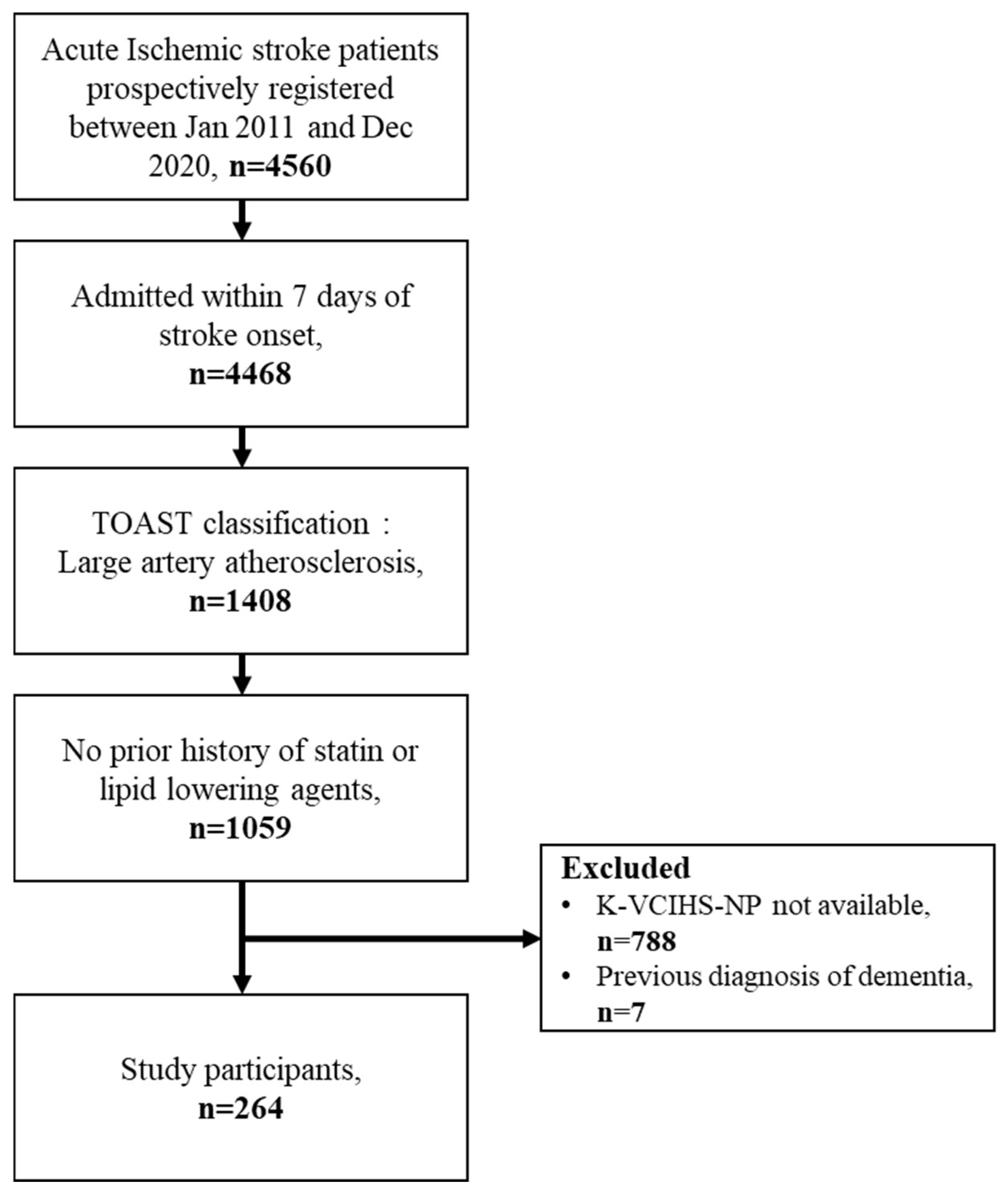
2. Materials and Methods
2.1. Study Design and Population
2.2. Main Exposure and Covariates
2.3. Neuropsychological Evaluation and Outcome Variables
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gorelick, P.B.; Scuteri, A.; Black, S.E.; Decarli, C.; Greenberg, S.M.; Iadecola, C.; Launer, L.J.; Laurent, S.; Lopez, O.L.; Nyenhuis, D.; et al. Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the american heart association/american stroke association. Stroke 2011, 42, 2672–2713. [Google Scholar] [CrossRef]
- Leys, D.; Henon, H.; Mackowiak-Cordoliani, M.A.; Pasquier, F. Poststroke dementia. Lancet Neurol. 2005, 4, 752–759. [Google Scholar] [CrossRef]
- Pendlebury, S.T.; Rothwell, P.M. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: A systematic review and meta-analysis. Lancet Neurol. 2009, 8, 1006–1018. [Google Scholar] [CrossRef]
- Sun, J.H.; Tan, L.; Yu, J.T. Post-stroke cognitive impairment: Epidemiology, mechanisms and management. Ann. Transl. Med. 2014, 2, 80. [Google Scholar] [CrossRef]
- Pendlebury, S.T.; Rothwell, P.M.; Oxford Vascular, S. Incidence and prevalence of dementia associated with transient ischaemic attack and stroke: Analysis of the population-based Oxford Vascular Study. Lancet Neurol. 2019, 18, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Sexton, E.; McLoughlin, A.; Williams, D.J.; Merriman, N.A.; Donnelly, N.; Rohde, D.; Hickey, A.; Wren, M.A.; Bennett, K. Systematic review and meta-analysis of the prevalence of cognitive impairment no dementia in the first year post-stroke. Eur. Stroke J. 2019, 4, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Lo, J.W.; Crawford, J.D.; Desmond, D.W.; Godefroy, O.; Jokinen, H.; Mahinrad, S.; Bae, H.J.; Lim, J.S.; Kohler, S.; Douven, E.; et al. Profile of and risk factors for poststroke cognitive impairment in diverse ethnoregional groups. Neurology 2019, 93, e2257–e2271. [Google Scholar] [CrossRef]
- Lee, M.; Lim, J.S.; Kim, Y.; Lee, J.H.; Kim, C.H.; Lee, S.H.; Jang, M.U.; Oh, M.S.; Lee, B.C.; Yu, K.H. Association between Geriatric Nutritional Risk Index and Post-Stroke Cognitive Outcomes. Nutrients 2021, 13, 1776. [Google Scholar] [CrossRef]
- Lee, M.; Lim, J.S.; Kim, C.H.; Lee, S.H.; Kim, Y.; Hun Lee, J.; Jang, M.U.; Sun Oh, M.; Lee, B.C.; Yu, K.H. High Neutrophil-Lymphocyte Ratio Predicts Post-stroke Cognitive Impairment in Acute Ischemic Stroke Patients. Front. Neurol. 2021, 12, 693318. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Lim, J.S.; Kim, Y.; Lee, J.H.; Kim, C.H.; Lee, S.H.; Jang, M.U.; Oh, M.S.; Lee, B.C.; Yu, K.H. Effects of Glycemic Gap on Post-Stroke Cognitive Impairment in Acute Ischemic Stroke Patients. Brain Sci. 2021, 11, 612. [Google Scholar] [CrossRef]
- Pascoe, M.; Ski, C.F.; Thompson, D.R.; Linden, T. Serum cholesterol, body mass index and smoking status do not predict long-term cognitive impairment in elderly stroke patients. J. Neurol. Sci. 2019, 406, 116476. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Shin, K.Y.; Chang, K.A. Potential Biomarkers for Post-Stroke Cognitive Impairment: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2022, 23, 602. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhu, H.; Chen, J.; Li, L.; Liu, C.; Gao, Y.; Sun, D. Serum TG/HDL-C level at the acute phase of ischemic stroke is associated with post-stroke cognitive impairment. Neurol. Sci. 2022, 43, 5977–5984. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Ding, M.; Cui, M.; Fang, M.; Gong, L.; Xu, Z.; Zhang, Y.; Wang, X.; Xu, X.; Liu, X.; et al. Development and validation of a clinical model (DREAM-LDL) for post-stroke cognitive impairment at 6 months. Aging 2021, 13, 21628–21641. [Google Scholar] [CrossRef]
- Rost, N.S.; Brodtmann, A.; Pase, M.P.; van Veluw, S.J.; Biffi, A.; Duering, M.; Hinman, J.D.; Dichgans, M. Post-Stroke Cognitive Impairment and Dementia. Circ. Res. 2022, 130, 1252–1271. [Google Scholar] [CrossRef]
- Gong, J.; Harris, K.; Peters, S.A.E.; Woodward, M. Serum lipid traits and the risk of dementia: A cohort study of 254,575 women and 214,891 men in the UK Biobank. eClinicalMedicine 2022, 54, 101695. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, D.; Song, Z.; Cui, X.; Liu, L.; Ding, Y.; Xue, J.; Zhang, X.; Ma, R.; Zhu, X.; et al. Elevated ApoB/ApoA-Iota ratio is associated with poor outcome in acute ischemic stroke. J. Clin. Neurosci. 2023, 107, 138–143. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, Y.; Wang, C.; Zuo, H. Association of the ApoB/ApoA-I ratio with stroke risk: Findings from the China Health and Nutrition Survey (CHNS). Nutr. Metab. Cardiovasc. Dis. 2022, 32, 203–209. [Google Scholar] [CrossRef]
- Park, J.H.; Hong, K.S.; Lee, E.J.; Lee, J.; Kim, D.E. High levels of apolipoprotein B/AI ratio are associated with intracranial atherosclerotic stenosis. Stroke 2011, 42, 3040–3046. [Google Scholar] [CrossRef]
- Sniderman, A.D.; Faraj, M. Apolipoprotein B, apolipoprotein A-I, insulin resistance and the metabolic syndrome. Curr. Opin. Lipidol. 2007, 18, 633–637. [Google Scholar] [CrossRef]
- Bielicki, J.K.; Oda, M.N. Apolipoprotein A-I(Milano) and apolipoprotein A-I(Paris) exhibit an antioxidant activity distinct from that of wild-type apolipoprotein A-I. Biochemistry 2002, 41, 2089–2096. [Google Scholar] [CrossRef] [PubMed]
- Nissen, S.E.; Tsunoda, T.; Tuzcu, E.M.; Schoenhagen, P.; Cooper, C.J.; Yasin, M.; Eaton, G.M.; Lauer, M.A.; Sheldon, W.S.; Grines, C.L.; et al. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: A randomized controlled trial. JAMA 2003, 290, 2292–2300. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, E.M.; El Awady, M.A.E.S.; Sharaf, S.A.-A.; Selim, N.M.; Abdo, H.E.S.; Mohammed, S.S. Apolipoproteins A1 and B and their ratio in acute ischemic stroke patients with intracranial and extracranial arterial stenosis: An Egyptian study. Egypt. J. Neurol. Psychiatry Neurosurg. 2020, 56, 115. [Google Scholar] [CrossRef]
- Kostapanos, M.S.; Christogiannis, L.G.; Bika, E.; Bairaktari, E.T.; Goudevenos, J.A.; Elisaf, M.S.; Milionis, H.J. Apolipoprotein B-to-A1 ratio as a predictor of acute ischemic nonembolic stroke in elderly subjects. J. Stroke Cerebrovasc. Dis. 2010, 19, 497–502. [Google Scholar] [CrossRef]
- Walldius, G.; Jungner, I. The apoB/apoA-I ratio: A strong, new risk factor for cardiovascular disease and a target for lipid-lowering therapy—A review of the evidence. J. Intern. Med. 2006, 259, 493–519. [Google Scholar] [CrossRef]
- Adams, H.P., Jr.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E.E., 3rd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef]
- Fazekas, F.; Kleinert, R.; Offenbacher, H.; Schmidt, R.; Kleinert, G.; Payer, F.; Radner, H.; Lechner, H. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology 1993, 43, 1683–1689. [Google Scholar] [CrossRef] [PubMed]
- Scheltens, P.; Leys, D.; Barkhof, F.; Huglo, D.; Weinstein, H.C.; Vermersch, P.; Kuiper, M.; Steinling, M.; Wolters, E.C.; Valk, J. Atrophy of medial temporal lobes on MRI in “probable” Alzheimer’s disease and normal ageing: Diagnostic value and neuropsychological correlates. J. Neurol. Neurosurg. Psychiatry 1992, 55, 967–972. [Google Scholar] [CrossRef]
- Kang, Y.; Na, D.L.; Hahn, S. A validity study on the korean mini-mental state examination (K-MMSE) in dementia patients. J. Korean Neurol. Assoc. 1997, 15, 300–308. [Google Scholar]
- Kang, Y.; Park, J.S.; Yu, K.H.; Lee, B.C. A Reliability, Validity, and Normative Study of the Korean-Montreal Cognitive Assessment(K-MoCA) as an Instrument for Screening of Vascular Cognitive Impairment(VCI). Korean J. Clin. Psychol. 2009, 28, 549–562. [Google Scholar] [CrossRef]
- Park, M.H. Informant questionnaire on cognitive decline in the elderly (IQCODE) for classifying cognitive dysfunction as cognitively normal, mild cognitive impairment, and dementia. Int. Psychogeriatr. 2017, 29, 1461–1467. [Google Scholar] [CrossRef]
- Tian, Z.; Ji, X.; Liu, J. Neuroinflammation in Vascular Cognitive Impairment and Dementia: Current Evidence, Advances, and Prospects. Int. J. Mol. Sci. 2022, 23, 6224. [Google Scholar] [CrossRef]
- Sniderman, A.D.; St-Pierre, A.C.; Cantin, B.; Dagenais, G.R.; Despres, J.P.; Lamarche, B. Concordance/discordance between plasma apolipoprotein B levels and the cholesterol indexes of atherosclerotic risk. Am. J. Cardiol. 2003, 91, 1173–1177. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Brewer, H.B., Jr.; Ansell, B.J.; Barter, P.; Chapman, M.J.; Heinecke, J.W.; Kontush, A.; Tall, A.R.; Webb, N.R. Dysfunctional HDL and atherosclerotic cardiovascular disease. Nat. Rev. Cardiol. 2016, 13, 48–60. [Google Scholar] [CrossRef]
- Walldius, G.; Jungner, I.; Holme, I.; Aastveit, A.H.; Kolar, W.; Steiner, E. High apolipoprotein B, low apolipoprotein A-I, and improvement in the prediction of fatal myocardial infarction (AMORIS study): A prospective study. Lancet 2001, 358, 2026–2033. [Google Scholar] [CrossRef]
- Faraj, M.; Messier, L.; Bastard, J.P.; Tardif, A.; Godbout, A.; Prud’homme, D.; Rabasa-Lhoret, R. Apolipoprotein B: A predictor of inflammatory status in postmenopausal overweight and obese women. Diabetologia 2006, 49, 1637–1646. [Google Scholar] [CrossRef]
- Umemoto, T.; Han, C.Y.; Mitra, P.; Averill, M.M.; Tang, C.; Goodspeed, L.; Omer, M.; Subramanian, S.; Wang, S.; Den Hartigh, L.J.; et al. Apolipoprotein AI and high-density lipoprotein have anti-inflammatory effects on adipocytes via cholesterol transporters: ATP-binding cassette A-1, ATP-binding cassette G-1, and scavenger receptor B-1. Circ. Res. 2013, 112, 1345–1354. [Google Scholar] [CrossRef]
- Carnevale Schianca, G.P.; Pedrazzoli, R.; Onolfo, S.; Colli, E.; Cornetti, E.; Bergamasco, L.; Fra, G.P.; Bartoli, E. ApoB/apoA-I ratio is better than LDL-C in detecting cardiovascular risk. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 406–411. [Google Scholar] [CrossRef]
- Sachdev, P.S.; Brodaty, H.; Valenzuela, M.J.; Lorentz, L.; Looi, J.C.; Wen, W.; Zagami, A.S. The neuropsychological profile of vascular cognitive impairment in stroke and TIA patients. Neurology 2004, 62, 912–919. [Google Scholar] [CrossRef]
- Yu, K.H.; Cho, S.J.; Oh, M.S.; Jung, S.; Lee, J.H.; Shin, J.H.; Koh, I.-S.; Cha, J.-K.; Park, J.-M.; Bae, H.-J.; et al. Cognitive impairment evaluated with vascular cognitive impairment harmonization standards in a multicenter prospective stroke cohort in Korea. Stroke 2013, 44, 786–788. [Google Scholar] [CrossRef]
- Kang, Y.W.; Chin, J.H.; Na, D.L.; Lee, J.H.; Park, J.S. A normative study of the Korean version of Controlled Oral Word Association Test (COWAT) in the elderly. Korean J. Clin. Psychol. 2000, 19, 385–392. [Google Scholar]
- Yum, T.H.; Park, Y.S.; Oh, K.J.; Kim, J.H.; Lee, Y.H. Manual for Korean-Wechsler Adult Intelligence Scale; Korea Guidance: Seoul, Republic of Korea, 1992. [Google Scholar]
- Yi, H.; Chin, J.H.; Lee, B.H.; Kang, Y.; Na, D.L. Development and validation of Korean version of trail making test for elderly persons. Dement. Neurocogn. Disord. 2007, 6, 54–66. [Google Scholar]
- Kang, Y.; Kim, H.H.; Na, D.L. A short form of the Korean-Boston Naming Test (K-BNT) for using in dementia patients. Korean J. Clin. Psych. 1999, 18, 125–138. [Google Scholar]
- Kang, Y.; Na, D.L. Seoul Neuropsychological Screening Battery; Professional manual; Human Brain Research and Consulting: Seoul, Republic of Korea, 2003. [Google Scholar]
- Lee, D.W.; Lee, J.Y.; Ryu, S.G.; Cho, S.J.; Hong, C.H.; Lee, J.H.; Choi, Y.M.; Kim, B.S.; Park, E.J.; Park, S.H. Validity of the Korean version of Informant Questionnaire on Cognitive Decline in the Elderly(IQCODE). J. Korean Geriatr. Soc. 2005, 9, 196–204. [Google Scholar]
- Kang, Y. A normative study of the Korean-Mini Mental State Examination(K-MMSE) in the elderly. Korean J. Psych. 2006, 25, 1–12. [Google Scholar]
- Kang, S.J.; Choi, S.H.; Lee, B.H.; Kwon, J.C.; Na, D.L.; Han, S.H.; Korean Dementia Research Group. The reliability and validity of the Korean Instrumental Activities of Daily Living (K-IADL). J. Korean Neurol. Assoc. 2002, 20, 8–14. [Google Scholar]
| ApoB/ApoA-I Ratio Quintile Groups | Q1 (n = 52) | Q2 (n = 53) | Q3 (n = 52) | Q4 (n = 53) | Q5 (n = 53) | p-Value |
|---|---|---|---|---|---|---|
| PSCI | 13 (25.0%) | 18 (34.0%) | 20 (38.5%) | 17 (32.1%) | 23 (43.4%) | 0.351 |
| ApoB | 73.3 ± 13.0 | 82.0 ± 15.0 | 100.8 ± 17.0 | 106.5 ± 15.7 | 132.2 ± 20.5 | <0.001 |
| ApoA1 | 145.0 ± 24.0 | 123.4 ± 23.4 | 127.8 ± 22.6 | 114.0 ± 17.3 | 106.8 ± 17.3 | <0.001 |
| ApoB/ApoA1 ratio | 0.5 ± 0.1 | 0.7 ± 0.0 | 0.8 ± 0.0 | 0.9 ± 0.0 | 1.3 ± 0.2 | <0.001 |
| Demographics | ||||||
| Age | 68.5 ± 12.4 | 66.9 ± 11.4 | 63.6 ± 11.2 | 64.6 ± 10.9 | 65.8 ± 12.1 | 0.240 |
| Sex (male) | 28 (53.8%) | 35 (66.0%) | 34 (65.4%) | 39 (73.6%) | 33 (62.3%) | 0.325 |
| Education Level | 0.718 | |||||
| 0 year | 4 (7.7%) | 1 (1.9%) | 6 (11.5%) | 1 (1.9%) | 3 (5.7%) | |
| 1–6 years | 15 (28.8%) | 18 (34.0%) | 14 (26.9%) | 14 (26.4%) | 17 (32.1%) | |
| 6–12 years | 22 (42.3%) | 26 (49.1%) | 22 (42.3%) | 29 (54.7%) | 24 (45.3%) | |
| >12 years | 11 (21.2%) | 8 (15.1%) | 10 (19.2%) | 9 (17.0%) | 9 (17.0%) | |
| BMI | 23.7 ± 3.6 | 24.2 ± 3.3 | 24.4 ± 3.5 | 24.3 ± 3.2 | 24.1 ± 2.7 | 0.807 |
| Previous mRS | 0.2 ± 0.6 | 0.2 ± 0.8 | 0.2 ± 0.6 | 0.2 ± 0.6 | 0.1 ± 0.4 | 0.739 |
| Stroke Characteristics | ||||||
| Initial NIHSS Stroke Scale | 2.0 [1.0;5.0] | 2.0 [0.0;5.0] | 2.0 [1.0;5.0] | 2.0 [1.0;5.0] | 3.0 [1.0;6.0] | 0.499 |
| Stroke Volume(mm3) | 11.2 [0.6;95.5] | 31.2 [1.9;120.3] | 6.6 [0.6;39.2] | 4.7 [0.6;70.0] | 5.4 [0.5;61.2] | 0.202 |
| Thrombolysis | 0.494 | |||||
| none | 43 (82.7%) | 48 (90.6%) | 48 (92.3%) | 45 (84.9%) | 47 (88.7%) | |
| IVT | 6 (11.5%) | 5 (9.4%) | 4 (7.7%) | 7 (13.2%) | 5 (9.4%) | |
| IVT + IAT | 3 (5.8%) | 0 (0.0%) | 0 (0.0%) | 1 (1.9%) | 1 (1.9%) | |
| Vascular Risk Factors | ||||||
| Previous stroke or TIA | 4 (7.7%) | 6 (11.3%) | 6 (11.5%) | 6 (11.3%) | 4 (7.5%) | 0.908 |
| Hypertension | 28 (53.8%) | 32 (60.4%) | 36 (69.2%) | 32 (60.4%) | 30 (56.6%) | 0.570 |
| Diabetes Mellitus | 15 (28.8%) | 15 (28.3%) | 16 (30.8%) | 13 (24.5%) | 16 (30.2%) | 0.961 |
| Hyperlipidemia | 5 (9.6%) | 9 (17.0%) | 17 (32.7%) | 18 (34.0%) | 22 (41.5%) | 0.001 |
| Smoking | 15 (28.8%) | 22 (41.5%) | 21 (40.4%) | 20 (37.7%) | 27 (50.9%) | 0.239 |
| Laboratory Results | ||||||
| Total Cholesterol | 164.4 ± 30.2 | 167.5 ± 34.4 | 199.3 ± 50.6 | 196.0 ± 30.5 | 227.8 ± 38.1 | <0.001 |
| Low-density lipoprotein | 92.5 ± 22.0 | 101.6 ± 23.2 | 121.0 ± 29.6 | 131.8 ± 23.0 | 158.8 ± 29.3 | <0.001 |
| Creatinine | 1.0 ± 1.7 | 0.7 ± 0.1 | 0.8 ± 0.3 | 0.8 ± 0.2 | 0.9 ± 0.3 | 0.503 |
| Hemoglobin | 13.6 ± 1.8 | 14.0 ± 1.3 | 14.2 ± 1.7 | 14.0 ± 1.7 | 14.2 ± 1.5 | 0.369 |
| Fasting blood sugar | 131.0 ± 49.5 | 121.1 ± 39.6 | 130.6 ± 52.8 | 122.8 ± 41.2 | 123.0 ± 40.4 | 0.679 |
| Systolic Blood Pressure | 148.7 ± 23.6 | 145.4 ± 23.9 | 154.8 ± 27.5 | 150.0 ± 23.7 | 143.6 ± 25.3 | 0.173 |
| Lesion Characteristics | ||||||
| Left Sided Lesion | 30 (57.7%) | 24 (45.3%) | 24 (46.2%) | 29 (54.7%) | 30 (56.6%) | 0.566 |
| Multiples Lesion | 7 (13.5%) | 4 (7.5%) | 4 (7.7%) | 4 (7.5%) | 4 (7.5%) | 0.771 |
| Cortical Lesion | 26 (50.0%) | 25 (47.2%) | 21 (40.4%) | 23 (43.4%) | 23 (43.4%) | 0.881 |
| Subcortical lesion | 16 (30.8%) | 21 (39.6%) | 22 (42.3%) | 19 (35.8%) | 17 (32.1%) | 0.705 |
| Infratentorial Lesion | 20 (38.5%) | 16 (30.2%) | 18 (34.6%) | 19 (35.8%) | 22 (41.5%) | 0.800 |
| Strategic Lesion | 16 (30.8%) | 16 (30.2%) | 17 (32.7%) | 21 (39.6%) | 12 (22.6%) | 0.456 |
| Modified Fazekas Scale | 0.929 | |||||
| mFS 0 | 10 (19.2%) | 8 (15.1%) | 9 (17.3%) | 12 (22.6%) | 12 (22.6%) | |
| mFS 1 | 23 (44.2%) | 25 (47.2%) | 26 (50.0%) | 21 (39.6%) | 26 (49.1%) | |
| mFS 2 | 9 (17.3%) | 14 (26.4%) | 10 (19.2%) | 13 (24.5%) | 8 (15.1%) | |
| mFS 3 | 10 (19.2%) | 6 (11.3%) | 7 (13.5%) | 7 (13.2%) | 7 (13.2%) | |
| Any CMB | 13 (25.0%) | 10 (18.9%) | 8 (15.4%) | 9 (16.9%) | 8 (15.1%) | 0.681 |
| Total MTLA score | 2.50 ± 1.83 | 2.38 ± 1.61 | 2.31 ± 1.73 | 1.98 ± 1.72 | 2.68 ± 1.82 | 0.326 |
| NCI (n = 172) | PSCI (n = 92) | p-Value | |
|---|---|---|---|
| ApoB | 96.9 ± 25.2 | 102.3 ± 28.6 | 0.124 |
| ApoA1 | 123.9 ± 24.9 | 122.3 ± 24.2 | 0.616 |
| ApoB/ApoA1 ratio | 0.81 ± 0.26 | 0.87 ± 0.31 | 0.117 |
| Demographics | |||
| Age | 65.8 ± 11.9 | 66.1 ± 11.2 | 0.838 |
| Sex (male) | 108 (62.8%) | 62 (67.4%) | 0.543 |
| Education Level | 0.142 | ||
| 0 year | 12 (7.0%) | 3 (3.3%) | |
| 1–6 years | 49 (28.5%) | 30 (32.6%) | |
| 6–12 years | 75 (43.6%) | 48 (52.2%) | |
| >12 years | 36 (20.9%) | 11 (12.0%) | |
| BMI | 24.3 ± 3.1 | 23.9 ± 3.5 | 0.281 |
| Previous mRS | 0.13 ± 0.54 | 0.22 ± 0.76 | 0.314 |
| Stroke Characteristics | |||
| Initial NIHSS Stroke Scale | 2.0 [0.5;4.0] | 4.0 [1.0;7.0] | <0.001 |
| Stroke Volume(mm3) | 6.2 [0.81;46.1] | 20.7 [0.50;157.3] | 0.046 |
| Thrombolysis | 0.693 | ||
| none | 151 (87.8%) | 80 (87.0%) | |
| IVT | 17 (9.9%) | 11 (12.0%) | |
| IVT + IAT | 4 (2.3%) | 1 (1.1%) | |
| Vascular Risk Factors | |||
| Previous stroke or TIA | 14 (8.1%) | 12 (13.0%) | 0.290 |
| Hypertension | 102 (59.3%) | 57 (62.0%) | 0.773 |
| Diabetes Mellitus | 43 (25.0%) | 32 (34.8%) | 0.124 |
| Hyperlipidemia | 44 (25.6%) | 27 (29.3%) | 0.609 |
| Smoking | 59 (34.3%) | 47 (51.1%) | 0.012 |
| Laboratory Results | |||
| Total Cholesterol | 187.6 ± 39.0 | 196.7 ± 51.9 | 0.140 |
| Low-density lipoprotein | 119.2 ± 33.2 | 124.7 ± 37.5 | 0.227 |
| Creatinine | 0.8 ± 0.2 | 1.0 ± 1.3 | 0.302 |
| Hemoglobin | 14.0 ± 1.7 | 14.0 ± 1.6 | 0.844 |
| Fasting blood sugar | 121.8 ± 40.7 | 132.6 ± 50.9 | 0.082 |
| Systolic Blood Pressure | 150.6 ± 25.4 | 144.6 ± 23.6 | 0.064 |
| Lesion Characteristics | |||
| Left Sided Lesion | 86 (50.0%) | 52 (56.5%) | 0.378 |
| Multiple Lesion | 16 (9.3%) | 7 (7.6%) | 0.813 |
| Cortical Lesion | 62 (36.0%) | 57 (62.0%) | <0.001 |
| Subcortical lesion | 60 (34.9%) | 35 (38.0%) | 0.708 |
| Infratentorial Lesion | 77 (44.8%) | 18 (19.6%) | <0.001 |
| Strategic Lesion | 44 (25.6%) | 39 (42.4%) | 0.008 |
| Modified Fazekas Scale | 0.015 | ||
| mFS 0 | 38 (22.1%) | 13 (14.1%) | |
| mFS 1 | 80 (46.5%) | 42 (45.7%) | |
| mFS 2 | 38 (22.1%) | 16 (17.4%) | |
| mFS 3 | 16 (9.3%) | 21 (22.8%) | |
| Any CMB | 30 (17.4%) | 18 (19.6%) | 0.796 |
| Total MTLA score | 2.24 ± 1.70 | 2.61 ± 1.8 | 0.105 |
| Crude OR (95% CI) | p-Value | Adjusted OR (95% CI) | p-Value | |
|---|---|---|---|---|
| ApoB/ApoA1 ratio | ||||
| Q1 | Reference | Reference | ||
| Q2 | 1.54 (0.66–3.60 | 0.316 | 1.73 (0.61–4.91) | 0.303 |
| Q3 | 1.87 (1.81–4.34) | 0.143 | 2.23 (0.82–6.06) | 0.117 |
| Q4 | 1.42 (0.60–3.32) | 0.423 | 1.56 (0.54–4.47) | 0.412 |
| Q5 | 2.30 (1.00–5.28) | 0.049 | 3.00 (1.10–8.20) | 0.032 |
| Age | 1.00 (0.98–1.02) | 0.837 | 0.97 (0.94–1.01) | 0.172 |
| Sex (male) | 1.22 (0.72–2.09) | 0.457 | 0.93 (0.43–2.01) | 0.859 |
| BMI | 0.95 (0.88–1.04) | 0.281 | 1.07 (0.97–1.18) | 0.196 |
| Education years | 0.92 (0.67–1.25) | 0.587 | 0.88 (0.54–1.43) | 0.614 |
| Systolic Blood Pressure | 0.99 (0.98–1.00) | 0.066 | 0.99 (0.98–1.00) | 0.099 |
| History of DM | 1.60 (0.92–2.77) | 0.094 | 1.49 (0.66–3.36) | 0.338 |
| Smoking | 2.00 (1.19–3.35) | 0.008 | 1.85 (0.87–3.93) | 0.111 |
| Fasting blood sugar | 1.01 (1.00–1.01) | 0.066 | 1.01 (1.00–1.02) | 0.022 |
| Initial NIHSS | 1.13 (1.06–1.21) | <0.001 | 1.18 (1.00–1.17) | 0.057 |
| Stroke Volume (mm3) | 1.00 (1.00–1.01) | <0.001 | 1.00 (1.00–1.01) | 0.003 |
| Cortical lesions | 2.89 (1.71–4.88) | <0.001 | 1.94 (0.84–4.52) | 0.122 |
| Infratentorial lesions | 0.30 (0.17–0.54) | <0.001 | 0.62 (0.24–1.60) | 0.321 |
| Strategic lesions | 2.14 (1.25–3.66) | 0.006 | 2.49 (1.16–5.38) | 0.020 |
| Modified Fazekas Scale | ||||
| mFS 0 | Reference | Reference | ||
| mFS 1 | 1.53 (0.74–3.19) | 0.252 | 2.53 (0.98–6.51) | 0.055 |
| mFS 2 | 1.23 (0.52–2.91) | 0.636 | 2.58 (0.80–8.31) | 0.112 |
| mFS 3 | 3.84 (1.55–9.49) | 0.004 | 11.92 (3.05–46.57) | <0.001 |
| Total MTLA score | 1.13 (0.97–1.30) | 0.106 | 1.02 (0.83–1.27) | 0.831 |
| ApoB/ApoAI Ratio | Q1 (n = 52) | Q2 (n = 53) | Q3 (n = 52) | Q4 (n = 53) | Q5 (n = 53) | p-Value * |
|---|---|---|---|---|---|---|
| K-MMSE Raw score | 25.0 ± 4.9 | 24.8 ± 5.1 | 24.7 ± 4.2 | 25.2 ± 4.8 | 23.6 ± 4.7 | 0.689 |
| K-MMSE standardized Z-score | −1.1 ± 1.9 | −1.2 ± 2.1 | −1.3 ± 1.7 | −1.3 ± 2.5 | −1.8 ± 1.7 | 0.414 |
| K-MoCA standardized Z-score | −0.6 ± 1.8 | −0.9 ± 1.9 | −0.7 ± 1.7 | −0.8 ± 2.7 | −1.0 ± 1.6 | 0.689 |
| Frontal domain Z-score | −0.6 ± 0.9 | −0.8 ± 1.0 | −0.6 ± 1.0 | −0.9 ± 0.9 | −1.0 ± 1.0 | 0.045 |
| Language domain Z-score | −0.2 ± 1.2 | −0.1 ± 1.2 | −0.1 ± 1.0 | −0.3 ± 1.2 | −0.3 ± 1.2 | 0.743 |
| Memory domain Z-score | −0.7 ± 1.1 | −0.9 ± 1.1 | −0.9 ± 1.0 | −0.9 ± 1.0 | −1.1 ± 1.0 | 0.311 |
| Visuospatial domain Z-score | −0.8 ± 1.5 | −1.3 ± 2.2 | −1.4 ± 2.2 | −1.2 ± 2.0 | −1.3 ± 1.8 | 0.446 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.; Lim, J.-S.; Kim, Y.; Park, S.H.; Lee, S.-H.; Kim, C.; Lee, B.-C.; Yu, K.-H.; Lee, J.-J.; Oh, M.S. High ApoB/ApoA-I Ratio Predicts Post-Stroke Cognitive Impairment in Acute Ischemic Stroke Patients with Large Artery Atherosclerosis. Nutrients 2023, 15, 4670. https://doi.org/10.3390/nu15214670
Lee M, Lim J-S, Kim Y, Park SH, Lee S-H, Kim C, Lee B-C, Yu K-H, Lee J-J, Oh MS. High ApoB/ApoA-I Ratio Predicts Post-Stroke Cognitive Impairment in Acute Ischemic Stroke Patients with Large Artery Atherosclerosis. Nutrients. 2023; 15(21):4670. https://doi.org/10.3390/nu15214670
Chicago/Turabian StyleLee, Minwoo, Jae-Sung Lim, Yerim Kim, Soo Hyun Park, Sang-Hwa Lee, Chulho Kim, Byung-Chul Lee, Kyung-Ho Yu, Jae-Jun Lee, and Mi Sun Oh. 2023. "High ApoB/ApoA-I Ratio Predicts Post-Stroke Cognitive Impairment in Acute Ischemic Stroke Patients with Large Artery Atherosclerosis" Nutrients 15, no. 21: 4670. https://doi.org/10.3390/nu15214670
APA StyleLee, M., Lim, J.-S., Kim, Y., Park, S. H., Lee, S.-H., Kim, C., Lee, B.-C., Yu, K.-H., Lee, J.-J., & Oh, M. S. (2023). High ApoB/ApoA-I Ratio Predicts Post-Stroke Cognitive Impairment in Acute Ischemic Stroke Patients with Large Artery Atherosclerosis. Nutrients, 15(21), 4670. https://doi.org/10.3390/nu15214670





