Dietary Patterns, Nutritional Status and Inflammatory Biomarkers in Adolescents from the RPS Birth Cohort Consortium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Population and Sample
2.3. Data Collection
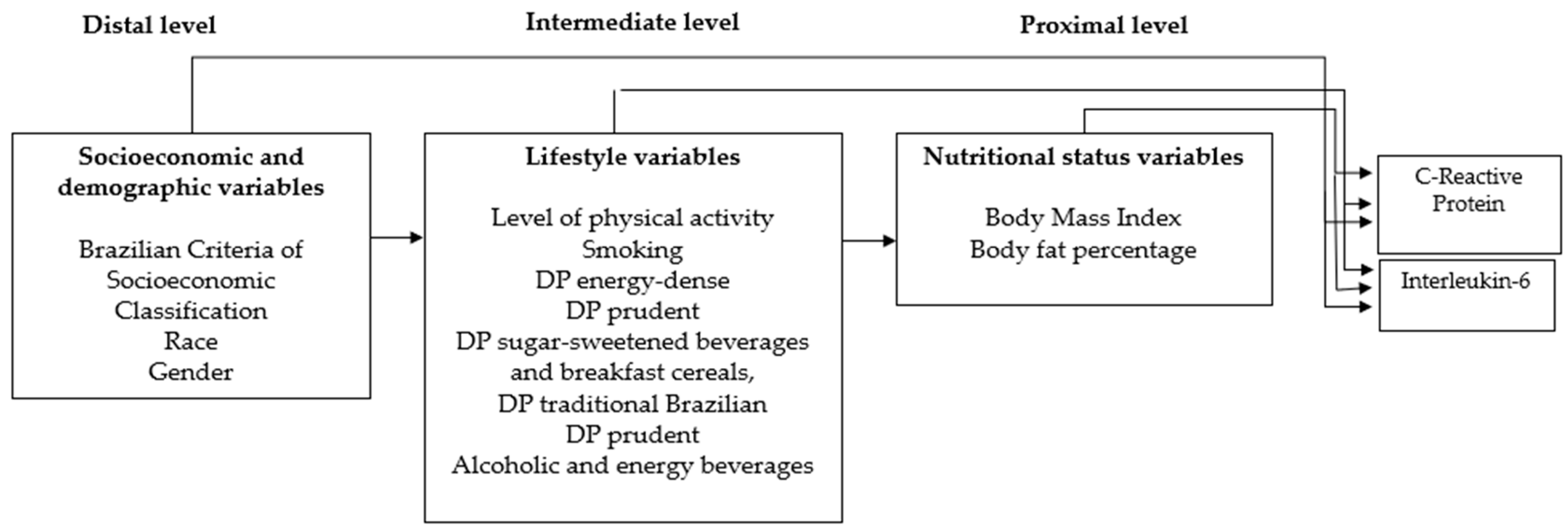
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saltiel, A.R.; Olefsky, J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef]
- Subirana, I.; Fitó, M.; Diaz, O.; Vila, J.; Francés, A.; Delpon, E.; Marrugat, J. Prediction of coronary disease incidence by biomarkers of inflammation, oxidation, and metabolism. Sci. Rep. 2018, 8, 3191. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.K.; Singh, H.V.; Raizada, A.; Singh, S.K. C-reactive protein, inflammation and coronary heart disease. Egypt. Heart J. 2015, 67, 89–97. [Google Scholar] [CrossRef]
- Medina-Leyte, D.J.; Zepeda-García, O.; Domínguez-Pérez, M.; González-Garrido, A.; Villarreal-Molina, T.; Jacobo-Albavera, L. Endothelial dysfunction, inflammation and coronary artery disease: Potential biomarkers and promising therapeutical approaches. Int. J. Mol. Sci. 2021, 22, 3850. [Google Scholar] [CrossRef]
- Allalou, A.; Peng, J.; Robinson, G.A.; Marruganti, C.; D’Aiuto, F.; Butler, G.; Ciurtin, C. Impact of puberty, sex determinants and chronic inflammation on cardiovascular risk in young people. Front. Cardiovasc. Med. 2023, 10, 1191119. [Google Scholar] [CrossRef]
- Meijel, R.L.; Blaak, E.E.; Goossens, G.H. Adipose tissue metabolism and inflammation in obesity. In Mechanisms and Manifestations of Obesity in Lung Disease; Academic Press: Cambridge, MA, USA, 2019; pp. 1–22. [Google Scholar]
- Sobrinho, C.A.; Moreira, C.M.M.; Mota, J.A.P.D.S.; Santos, R.M.R. Proteína C-reativa, atividade física e aptidão cardiorrespiratória em adolescentes portugueses: Um estudo transversal. Cad. Saúde Públ. 2015, 31, 1907–1915. [Google Scholar] [CrossRef]
- Blaudt, L.S.; Lopes, T.S.; Souza, A.M.; Yokoo, E.M.; Rocha, C.M.M.; Pereira, R.A. Association between dietary inflammatory index and anthropometric indicators of adiposity in Brazilian adolescents. Pediatr. Obes. 2023, 18, e13011. [Google Scholar] [CrossRef] [PubMed]
- Rocha, N.P.; Milagres, L.C.; Longo, G.Z.; Ribeiro, A.Q.; Novaes, J.F. Association between dietary pattern and cardiometabolic risk in children and adolescents: A systematic review. J. Pediatr. 2017, 93, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Hilger-Kolb, J.; Bosle, C.; Motoc, I.; Hoffmann, K. Associations between dietary factors and obesity-related biomarkers in healthy children and adolescents-a systematic review. Nutr. J. 2017, 16, 85. [Google Scholar] [CrossRef]
- McCourt, H.J.; Draffin, C.R.; Woodside, J.V.; Cardwell, C.R.; Young, I.S.; Hunter, S.J. Dietary patterns and cardiovascular risk factors in adolescents and young adults: The Northern Ireland Young Hearts Project. Br. J. Nutr. 2014, 112, 1685–1698. [Google Scholar] [CrossRef]
- Bibiloni, M.M.; Maffeis, C.; Llompart, I.; Pons, A.; Tur, J.A. Dietary factors associated with subclinical inflammation among girls. Eur. J. Clin. Nutr. 2013, 67, 1264–1270. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.C.; Motta, J.V.D.S.; Muniz, L.C.; Bielemann, R.M.; Madruga, S.W.; Orlandi, S.P.; Assunção, M.C.F. Desenho de um questionário de frequência alimentar digital autoaplicado para avaliar o consumo alimentar de adolescentes e adultos jovens: Coortes de nascimentos de Pelotas, Rio Grande do Sul. Rev. Bras. Epidemiol. 2016, 19, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Bogea, E.G.; França, A.K.T.C.; Bragança, M.L.B.M.; Vaz, J.S.; Assunção, M.C.; Barbieri, M.A.; Silva, A.A.M. Relative validity of a food frequency questionnaire for adolescents from a capital in the Northeastern region of Brazil. Braz. J. Med. Biol. Res. 2020, 54, e00072618. [Google Scholar] [CrossRef]
- Núcleo de Estudos e Pesquisas em Alimentação (NEPA-UNICAMP). Tabela Brasileira de Composição de Alimentos—TACO-4a ed. Rev. e Ampl.; UNICAMP/NEPA: Campinas, Brazil, 2011. [Google Scholar]
- US Department of Agriculture. Nutrient Database for Standard Reference—SR14; US Department of Agriculture: Washington, DC, USA, 2011.
- Sallis, J.F.; Strikmiller, P.K.; Harsha, D.W.; Feldman, H.A.; Ehlinger, S.; Stone, E.J. Validation of interviewer-and self-administered physical activity checklists for fifth grade students. Med. Sci. Sports Exerc. 1996, 28, 840–851. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Haskell, W.L.; Leon, A.S.; Jacobs, D.R.; Montoye, H.J.; Sallis, J.F. Compendium of physical activities: Classification of energy costs of human physical activities. Med. Sci. Sports Exerc. 1993, 25, 71–80. [Google Scholar] [CrossRef]
- Benedetti, T.R.B.; Antunes, P.C.; Rodriguez-Añez, C.R.; Mazo, G.Z.; Petroski, E.L. Reprodutibilidade e validade do Questionário Internacional de Atividade Física (IPAQ) em homens idosos. Rev. Bras. Med. Esporte 2007, 13, 11–16. [Google Scholar] [CrossRef]
- Yao, Z.; Zhang, Y.; Wu, H. Regulation of C-reactive protein conformation in inflammation. Inflamm. Res. 2019, 68, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Aliyu, M.; Zohora, F.T.; Anka, A.U.; Ali, K.; Maleknia, S.; Saffarioun, M. Interleukin-6 cytokine: An overview of the immune regulation, immune dysregulation, and therapeutic approach. Int. Immunopharmacol. 2022, 111, 109130. [Google Scholar] [CrossRef]
- Kreiner, F.F.; Kraaijenhof, J.M.; Herrath, M.; Hovingh, G.K.K.; Von Scholten, B.J. Interleukin 6 in diabetes, chronic kidney disease, and cardiovascular disease: Mechanisms and therapeutic perspectives. Expert Rev. Clin. Immunol. 2022, 18, 377–389. [Google Scholar] [CrossRef]
- Newby, P.K.; Tucker, K.L. Empirically derived eating patterns using factor or cluster analysis: A review. Nutr. Rev. 2004, 62, 177–203. [Google Scholar] [CrossRef]
- Salvatti, A.G.; Escrivão, M.A.M.S.; Taddei, J.A.A.C.; Bracco, M.M. Eating patterns of eutrophic and overweight adolescents in the city of São Paulo, Brazil. Rev. Nutr. 2011, 24, 703–713. [Google Scholar] [CrossRef]
- Victora, C.G.; Huttly, S.R.; Fuchs, S.C.; Olinto, M.T.A. The role of conceptual frameworks in epidemiological analysis: A hierarchical approach. Int. J. Epidemiol. 1997, 24, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.; López-Cheda, A.; Santos, A.C.; Barros, H.; Fraga, S. How do early socioeconomic circumstances impact inflammatory trajectories? Findings from Generation XXI. Psychoneuroendocrinology 2020, 119, 104755. [Google Scholar] [CrossRef]
- Elisia, I.; Lam, V.; Cho, B.; Hay, M.; Li, M.Y.; Yeung, M. The effect of smoking on chronic inflammation, immune function and blood cell composition. Sci. Rep. 2020, 10, 19480. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrova, K.; Koelman, L.; Rodrigues, C.E. Dietary patterns and biomarkers of oxidative stress and inflammation: A systematic review of observational and intervention studies. Redox Biol. 2021, 42, 101869. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Kautz, A. An evidence review of the association of immune and inflammatory markers with obesity-related eating behaviors. Front. Immunol. 2022, 13, 902114. [Google Scholar] [CrossRef]
- Gutiérrez-Pliego, L.E.; Del Socorro, C.R.E.; Montenegro-Morales, L.P.; García, J.G. Dietary patterns associated with body mass index (BMI) and lifestyle in Mexican adolescents. BMC Public Health 2016, 16, 850. [Google Scholar] [CrossRef]
- Pinho, M.G.M.D.; Adami, F.; Benedet, J.; Vasconcelos, F.D.A.G.D. Association between screen time and dietary patterns and overweight/obesity among adolescents. Rev. Nutr. 2017, 30, 377–389. [Google Scholar] [CrossRef]
- Appannah, G.; Pot, G.K.; Huang, R.C.; Oddy, W.H.; Beilin, L.J.; Mori, T.A. Identification of a dietary pattern associated with greater cardiometabolic risk in adolescence. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 643–650. [Google Scholar] [CrossRef]
- Oddy, W.H.; Allen, K.L.; Trapp, G.S.; Ambrosini, G.L.; Black, L.J.; Huang, R.C. Dietary patterns, body mass index and inflammation: Pathways to depression and mental health problems in adolescents. Brain Behav. Immun. 2018, 69, 428–439. [Google Scholar] [CrossRef]
- Avelino, G.F.; Previdelli, A.N.; Castro, M.A.; Marchioni, D.M.L.; Fisberg, R.M. Sub-relato da ingestão energética e fatores associados em estudo de base populacional. Cad. Saúde Públ. 2014, 30, 663–668. [Google Scholar] [CrossRef]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhang, Y.; Ma, J.; Liu, Y.; Li, W.; Wang, T. Interleukin-6 absence triggers intestinal microbiota dysbiosis and mucosal immunity in mice. Cytokine 2022, 153, 155841. [Google Scholar] [CrossRef]
- Umoh, F.I.; Kato, I.; Ren, J.; Wachowiak, P.L.; Ruffin, M.T.; Turgeon, D.K.; Djuric, Z. Markers of systemic exposures to products of intestinal bacteria in a dietary intervention study. Eur. J. Nutr. 2016, 55, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Medina-Remon, A.; Casas, R.; Tressserra-Rimbau, A.; Ros, E.; Martinez-Gonzalez, M.A.; Fito, M. Polyphenol intake from a Mediterranean diet decreases inflammatory biomarkers related to atherosclerosis: A substudy of the PREDIMED trial. Br. J. Clin. Pharmacol. 2017, 83, 114–128. [Google Scholar] [CrossRef]
- Jaceldo-Siegl, K.; Haddad, E.; Knutsen, S.; Fan, J.; Lloren, J.; Bellinger, D. Lower C-reactive protein and IL-6 associated with vegetarian diets are mediated by BMI. Nutr. Metab. Cardiovasc. Dis. 2018, 8, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Previdelli, A.N.; Andrade, S.C.; Fisberg, R.M.; Marchioni, D. Using Two Different Approaches to Assess Dietary Patterns: Hypothesis-Driven and Data-Driven Analysis. Nutrients 2016, 8, 593. [Google Scholar] [CrossRef]
- Wang, H.J.; Zakhari, S.; Jung, M.K. Alcohol, inflammation, and gut-liver-brain interactions in tissue damage and disease development. World J. Gastroenterol. 2010, 16, 1304–1313. [Google Scholar] [CrossRef]
- Arevalo, S.P.; Bazzi, A.R.; Falcon, L.M.; Tucker, K.L. Alcohol use and cardiovascular risk in a prospective cohort study of Latino Adults: The mediating effect of inflammation. FASEB J. 2016, 30, 115. [Google Scholar]
- Alcohol and Health: All, None, or Somewhere In-Between? Lancet Rheumatol. 2023, 5, e167. Available online: https://www.thelancet.com/action/showPdf?pii=S2665-9913%2823%2900073-5 (accessed on 18 August 2023). [CrossRef]
- Giannini, D.T.; Kuschnir, M.C.C.; Oliveira, C.L.; Bloch, K.V.; Schaan, B.D.; Cureau, F.V. C-reactive protein in Brazilian adolescents: Distribution and association with metabolic syndrome in ERICA survey. Eur. J. Clin. Nutr. 2017, 71, 1206. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.R.; Stefanello, J.M.F.; Pizzi, J.; Timossi, L.S.; Leite, N. Aterosclerose subclínica e marcadores inflamatórios em crianças e adolescentes obesos e não obesos. Rev. Bras. Epidemiol. 2012, 15, 804–816. [Google Scholar] [CrossRef] [PubMed]

| Food Group | Dietary Pattern | ||||
|---|---|---|---|---|---|
| Energy-Dense | Sugar Sweetened Beverages and Cereal Breakfast | Prudent | Traditional Brazilian | Alcoholic and Energy Beverages | |
| Junk food | 0.789 | ||||
| Sweets | 0.642 | ||||
| Cakes and cookies | 0.614 | ||||
| Processed meats | 0.608 | ||||
| Sauces | 0.551 | ||||
| Soda/Industrialized juice | 0.430 | ||||
| Canned food | 0.381 | ||||
| Natural Juice | 0.803 | ||||
| Added sugar | 0.785 | ||||
| Breakfast cereals | 0.488 | ||||
| Milk and derivatives | 0.401 | ||||
| Fish | 0.647 | ||||
| Vegetables | 0.598 | ||||
| Red meat and offal | 0.579 | ||||
| Chicken, birds | 0.473 | ||||
| Nuts | 0.441 | ||||
| Fruit | 0.406 | ||||
| Eggs | 0.380 | ||||
| Tapioca/couscous | 0.346 | ||||
| Tubers | 0.322 | ||||
| Rice | 0.578 | ||||
| Breads | 0.561 | ||||
| Fats | 0.541 | ||||
| Coffe | 0.533 | ||||
| Beans | 0.385 | ||||
| Flours | 0.363 | ||||
| Energy Beverages | 0.716 | ||||
| Alcoholic Beverages | 0.600 | ||||
| Guarana powder | 0.339 | ||||
| Number of items | 7 | 4 | 9 | 6 | 3 |
| Proportional Variance | 9.42% | 7.52% | 7.24% | 6.76% | 5.12% |
| Accumulated Variance | 9.42% | 16.94% | 24.18% | 30.94% | 36.06% |
| KMO * coefficient | 0.723 | ||||
| Energy-Dense | Sugar-Sweetened Beverages and Breakfast Cereals | Prudent | |||||||
|---|---|---|---|---|---|---|---|---|---|
| T1 | T3 | p Value | T1 | T3 | p Value | T1 | T3 | p Valor | |
| Physical activity (%) | 0.048 * | 0.137 | 0.002 * | ||||||
| Sedentary | 38.9 | 26.9 | 38.9 | 29.1 | 40.0 | 23.4 | |||
| Active | 30.3 | 37.0 | 30.3 | 35.1 | 30.3 | 38.4 | |||
| Nutritional Status (BMI) 1 | 0.113 | 0.459 | 0.075 | ||||||
| Without overweight | 31.5 | 31.0 | 32.2 | 34.1 | 35.6 | 32.7 | |||
| Overweight | 41.7 | 32.9 | 38.5 | 29.2 | 24.0 | 35.4 | |||
| Body fat (%) | 18.3 (10.6–28.5) | 16.6 (8.4–25.5) | 0.119 | 16.6 (8.6–27.3) | 16.8 (8.4–25.1) | 0.602 | 18.1 (8.5–25.8) | 15.7 (7.9 –24.0) | 0.035 * |
| Dietary Intake | |||||||||
| Calories (kcal/day) | 2082.7 (1539.5–2768.0) | 3962.8 (3235.4–5244.3) | <0.001 * | 2576.9 (1787.0–3661.2) | 3661.2 (2671.1–4784.3) | <0.001 * | 2575.2 (1671.6–3564.3) | 3508.5 (2754.5–4716.1) | <0.001 * |
| Carbohydrates (g/day) | 460.6 (423.6–486.0) | 431.5 (401.0–459.6) | <0.001 * | 427.7 (400.8–459.3) | 455.7 (427.4–481.3) | <0.001 * | 448.7 (418.0–73.6) | 431.5 (403.5–62.2) | 0.008 * |
| Protein (g/day) | 100.7 (85.4–119.2) | 96.1 (83.7–110.0) | 0.040 * | 103.7 (90.4–123.3) | 93.4 (82.0–109.8) | 0.001 * | 89.6 (79.3–99.5) | 114.5 (97.1–131.5) | <0.001 * |
| Lipid (g/day) | 66.3 (57.2–75.9) | 81.2 (70.8–92.0) | <0.001 * | 77.9 (68.2–91.6) | 69.8 (61.0–78.3) | <0.001 * | 77.6 (66.7–90.1) | 72.0 (61.6–81.6) | 0.003 * |
| Saturated fat (g/day) | 24.5 (20.5–30.0) | 30.3 (26.1–34.8) | <0.001 * | 28.2 (24.0–32.6) | 26.9 (22.2–30.7) | 0.076 | 29.1 (25.7–24.5) | 25.9 (22.9–30.3) | <0.001 * |
| Omega 6 (g/day) | 7.2 (5.7–9.3) | 8.8 (7.2–11.0) | <0.001 * | 8.8 (7.0–11.0) | 7.3 (5.6–9.0) | <0.001 * | 8.2 (6.4–10.2) | 7.6 (6.3–9.7) | 0.191 |
| Omega 3 (g/day) | 0.8 (0.7–1.0) | 0.8 (0.7–1.1) | 0.752 | 0.9 (0.7–1.1) | 0.8 (0.6–1.0) | 0.055 | 0.8 (0.6–1.0) | 0.9 (0.7–1.1) | 0.019 * |
| Fiber (g/day) | 80.1 (64.9–101.6) | 66.2 (56.5–79.9) | <0.001 * | 65.9 (56.6–79.9) | 76.4 (65.3–94.2) | <0.001 * | 65.4 (55.1–79.9) | 76.8 (64.8–95.1) | <0.001 * |
| Calcium (mg/day) | 737.1 (528.8–932.2) | 688.8 (565.0–863.1) | 0.170 | 626.2 (495.2–772.1) | 837.4 (649.7–1013.4) | <0.001 * | 765.5 (573.2–980.2) | 709.3 (569.9–876.6) | 0.080 |
| Iron (mg/day) | 12.6 (10.8–15.1) | 13.0 (11.1–15.0) | 0.887 | 13.2 (11.1–15.5) | 12.6 (10.9–14.7) | 0.460 | 12.0 (10.2–14.5) | 13.9 (11.6–16.7) | <0.001 * |
| Thiamine (mg/day) | 1.4 (1.2–1.7) | 1.5 (1.3–1.7) | 0.675 | 1.4 (1.2–1.7) | 1.5 (1.3–1.8) | 0.075 | 1.4 (1.2–1.7) | 1.5 (1.4–1.7) | 0.076 |
| Niacin (mg/day) | 15.0 (11.4–18.9) | 17.1 (13.3–21.4) | 0.001 * | 17.2 (13.4–21.2) | 15.2 (12.0–18.7) | 0.004 * | 14.3 (11.7–17.7) | 18.2 (15.1–18.2) | <0.001 * |
| Vitamin C (mg/day) | 132.7 (86.0–224.1) | 114.4 (63.0–167.6) | 0.001 * | 85.4 (51.5–135.0) | 164.5 (112.7–238.5) | <0.001 * | 97.0 (61.2–149.5) | 144.0 (92.2–213.0) | <0.001 |
| Sodium (mg/day) | 1716.3 (1458.6–2026.5) | 2084.7 (1823.4–2506.0) | <0.001 * | 2043.2 (1681.0–1510.0) | 1807.3 (1562.8–2140.8) | <0.001 * | 2100.7 (1681.0–2603.5) | 1811.3 (1547.3–2089.3) | <0.001 |
| Cholesterol (mg/day) | 377.6 (275.8–473.8) | 374.5 (300.5–511.4) | 0.563 | 418.5 (309.8–560.8) | 343.6 (261.8–466.1) | <0.001 * | 310.4 (251.5–378.8) | 446.0 (343.5–574.5) | <0.001 |
| Traditional Brazilian | Alcoholic and Energy Beverages | |||||
|---|---|---|---|---|---|---|
| T1 | T3 | p Value | T1 | T3 | p Valor | |
| Physical activity (%) | 0.006 * | 0.001 * | ||||
| Sedentary | 41.1 | 24.6 | 36.0 | 22.9 | ||
| Active | 29.7 | 37.4 | 31.8 | 38.7 | ||
| Nutritional Status (BMI) 1 | 0.010 * | 0.147 | ||||
| Without overweight | 30.8 | 35.8 | 35.3 | 32.0 | ||
| Overweight | 44.8 | 21.9 | 25.0 | 38.5 | ||
| Body fat (%) | 20.6 (12.4–28.5) | 12.9 (6.9–20.7) | <0.001 * | 17.8 (8.6–25.0) | 15.4 (8.4–24.8) | 0.332 |
| Dietary Intake | ||||||
| Calories (kcal/day) | 2128.9 (1601.2–3318.0) | 3526.4 (2899.2–4768.9) | <0.001 * | 2971.1 (2161.4–4091.1) | 3319.5 (2414.8–4027.9) | <0.001 * |
| Carbohydrates (g/day) | 435.1 (404.4–462.9) | 448.0 (419.4–475.3) | 0.037 | 428.0 (398.8–453.0) | 458.8 (20.7–481.3) | <0.001 * |
| Protein (g/day) | 98.0 (85.4–116.4) | 95.3 (84.7–112.3) | 0.869 | 107.6 (89.9–125.2) | 92.6 (80.7–105.5) | <0.001 * |
| Lipid (g/day) | 75.4 (66.3–90.7) | 71.9 (61.9–81.3) | 0.021 * | 75.8 (67.9–89.3) | 70.8 (61.0–82.7) | 0.004 * |
| Saturated fat (g/day) | 29.6 (25.1–33.8) | 26.7 (22.0–30.4) | 0.001 * | 29.2 (25.8–33.4) | 26.8 (22.0–31.1) | 0.001 * |
| Omega 6 (g/day) | 8.4 (6.5–10.6) | 7.9 (6.1–10.0) | 0.358 | 7.8 (6.6–10.7) | 8.0 (6.0–10.0) | 0.687 |
| Omega 3 (g/day) | 0.8 (0.6–1.0) | 0.9 (0.7–1.0) | 0.360 | 0.9 (0.7–1.0) | 0.8 (0.6–1.0) | 0.100 |
| Fiber (g/day) | 73.3 (59.6–86.9) | 71.5 (60.6–88.2) | 0.831 | 74.5 (62.0–96.9) | 68.6 (58.5–81.8) | 0.053 |
| Calcium (mg/day) | 708.2 (545.2–889.3) | 733.4 (586.1–888.3) | 0.352 | 812.3(629.6–1036.3) | 647.3 (519.0–785.6) | <0.001 * |
| Iron (mg/day) | 13.3 (11.4–15.5) | 12.5 (10.4–14.8) | 0.053 | 13.9 (11.6–16.1) | 12.2 (10.7–13.8) | <0.001 * |
| Thiamine (mg/day) | 1.5 (1.3–1.8) | 1.5 (1.3–1.7) | 0.312 | 1.6 (1.4–1.8) | 1.4 (1.2–1.6) | <0.001 * |
| Niacin (mg/day) | 17.1 (14.2–22.0) | 15.0 (12.1–18.3) | <0.001 * | 15.9 (12.8–20.1) | 16.4 (12.6–21.4) | 0.056 |
| Vitamin C (mg/day) | 157.9 (103.1–245.6) | 98.8 (59.4–163.9) | <0.001 * | 107.5 (71.3–159.4) | 139.3 (84.1–220.5) | 0.011 * |
| Sodium (mg/day) | 1875.9 (1569.1–2184.7) | 1984.0 (1689.1–2320.2) | 0.178 | 2025.3 (1705.3–2345.7) | 1847.6 (1584.0–2297.6) | 0.061 |
| Cholesterol (mg/day) | 375.8 (300.5–539.9) | 338.6 (263.6–457.8) | 0.021 * | 410.8 (321.0–535.9) | 350.0 (261.8–455.9) | <0.001 * |
| Interleukin 6 | |||||||
|---|---|---|---|---|---|---|---|
| Non-Adjusted | Adjusted | ||||||
| Beta | IC 1 95% | p Value | Beta | IC 1 95% | p Value | ||
| Distal level *: variables | Color | 0.01 | −0.12–0.15 | 0.883 | 0.30 | −0.11–0.18 | 0.690 |
| socioeconomic and | CEB 2 | −0.09 | −0.26–0.08 | 0.283 | −0.11 | −0.29–0.18 | 0.220 |
| demographics | Gender | 0.13 | −0.09–0.34 | 0.250 | 0.09 | −0.15–0.33 | 0.468 |
| Energy Dense | −0.05 | −0.15–0.06 | 0.376 | −0.04 | −0.15–0.06 | 0.445 | |
| Sugar sweetened beverages and cereal breakfast | −0.05 | −0.15–0.06 | 0.383 | −0.04 | −0.15–0.06 | 0.4183 | |
| Prudent | −0.12 | −0.22–−0.01 | 0.029 ¥ | −0.11 | −0.22–−0.01 | 0.040 ¥ | |
| Intermediate level **: lifestyle variables | Traditional Brazilian | −0.12 | −0.23–−0.02 | 0.020 ¥ | −0.12 | −0.22–−0.01 | 0.027 ¥ |
| Alcoholic and energy beverages | 0.09 | −0.13–0.20 | 0.085 | 0.11 | 0.01–0.22 | 0.041 ¥ | |
| smoking | −0.21 | −0.58–0.15 | 0.250 | −0.25 | −0.62–0.12 | 0.193 | |
| Physical activity | −0.04 | −0.13–0.04 | 0.345 | −0.02 | −0.11–0.07 | 0.696 | |
| Proximal level ***: adiposity status variables | BMI 3 | 0.12 | 0.00–0.23 | 0.042 | 0.03 | −0.11–0.17 | 0.670 |
| Body Fat | 0.01 | 0.00–0.02 | 0.006 ¥ | 0.01 | 0.00–0.21 | 0.077 | |
| C-Reactive Protein | |||||||
|---|---|---|---|---|---|---|---|
| Non-Adjusted | Adjusted | ||||||
| Beta | IC ¹ 95% | p Value | Beta | IC 95% | p Value | ||
| Distal level *: variables | Color | −0.02 | −0.18–0.13 | 0.775 | −0.02 | −0.19–0.15 | 0.809 |
| Socioeconomic and demographics | CEB 2 | −0.01 | −0.20–0.18 | 0.926 | −0.01 | −0.21–0.19 | 0.938 |
| Gender | 0.06 | −0.19–0.31 | 0.635 | 0.02 | −0.25–0.30 | 0.850 | |
| Energy-Dense | 0.05 | −0.07–0.17 | 0.420 | 0.04 | −0.09–0.16 | 0.569 | |
| Sugar sweetened beverages and cereal breakfast | 0.04 | −0.08–0.16 | 0.493 | 0.03 | −0.09–0.15 | 0.625 | |
| Prudent | 0.03 | −0.09– 0.15 | 0.590 | 0.02 | −0.14–−0.11 | 0.784 | |
| Intermediate level **: lifestyle variables | Traditional Brazilian | −0.03 | −0.15–0.09 | 0.607 | −0.03 | −0.15–−0.09 | 0.640 |
| Alcoholic and energy beverages | 0.11 | −0.01–0.24 | 0.062 | 0.10 | −0.03–0.23 | 0.130 | |
| smoking | 0.05 | −0.37–0.46 | 0.825 | −0.06 | −0.49–0.37 | 0.791 | |
| Physical activity | 0.08 | −0.02–0.18 | 0.107 | −0.06 | −0.04–0.17 | 0.255 | |
| Proximal level ***: adiposity status variables | BMI 3 | 0.48 | 0.35–0.60 | <0.001 ¥ | 0.36 | 0.21–0.51 | <0.001 ¥ |
| Body Fat | 0.03 | 0.02–0.04 | <0.001 ¥ | 0.02 | 0.00–0.03 | 0.014 ¥ | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogea, E.G.; Martins, M.L.B.; França, A.K.T.d.C.; Silva, A.A.M.d. Dietary Patterns, Nutritional Status and Inflammatory Biomarkers in Adolescents from the RPS Birth Cohort Consortium. Nutrients 2023, 15, 4640. https://doi.org/10.3390/nu15214640
Bogea EG, Martins MLB, França AKTdC, Silva AAMd. Dietary Patterns, Nutritional Status and Inflammatory Biomarkers in Adolescents from the RPS Birth Cohort Consortium. Nutrients. 2023; 15(21):4640. https://doi.org/10.3390/nu15214640
Chicago/Turabian StyleBogea, Eduarda Gomes, Maylla Luanna Barbosa Martins, Ana Karina Teixeira da Cunha França, and Antônio Augusto Moura da Silva. 2023. "Dietary Patterns, Nutritional Status and Inflammatory Biomarkers in Adolescents from the RPS Birth Cohort Consortium" Nutrients 15, no. 21: 4640. https://doi.org/10.3390/nu15214640
APA StyleBogea, E. G., Martins, M. L. B., França, A. K. T. d. C., & Silva, A. A. M. d. (2023). Dietary Patterns, Nutritional Status and Inflammatory Biomarkers in Adolescents from the RPS Birth Cohort Consortium. Nutrients, 15(21), 4640. https://doi.org/10.3390/nu15214640








