Glycine and N-Acetylcysteine (GlyNAC) Combined with Body Weight Support Treadmill Training Improved Spinal Cord and Skeletal Muscle Structure and Function in Rats with Spinal Cord Injury
Abstract
1. Introduction
2. Materials and Methods
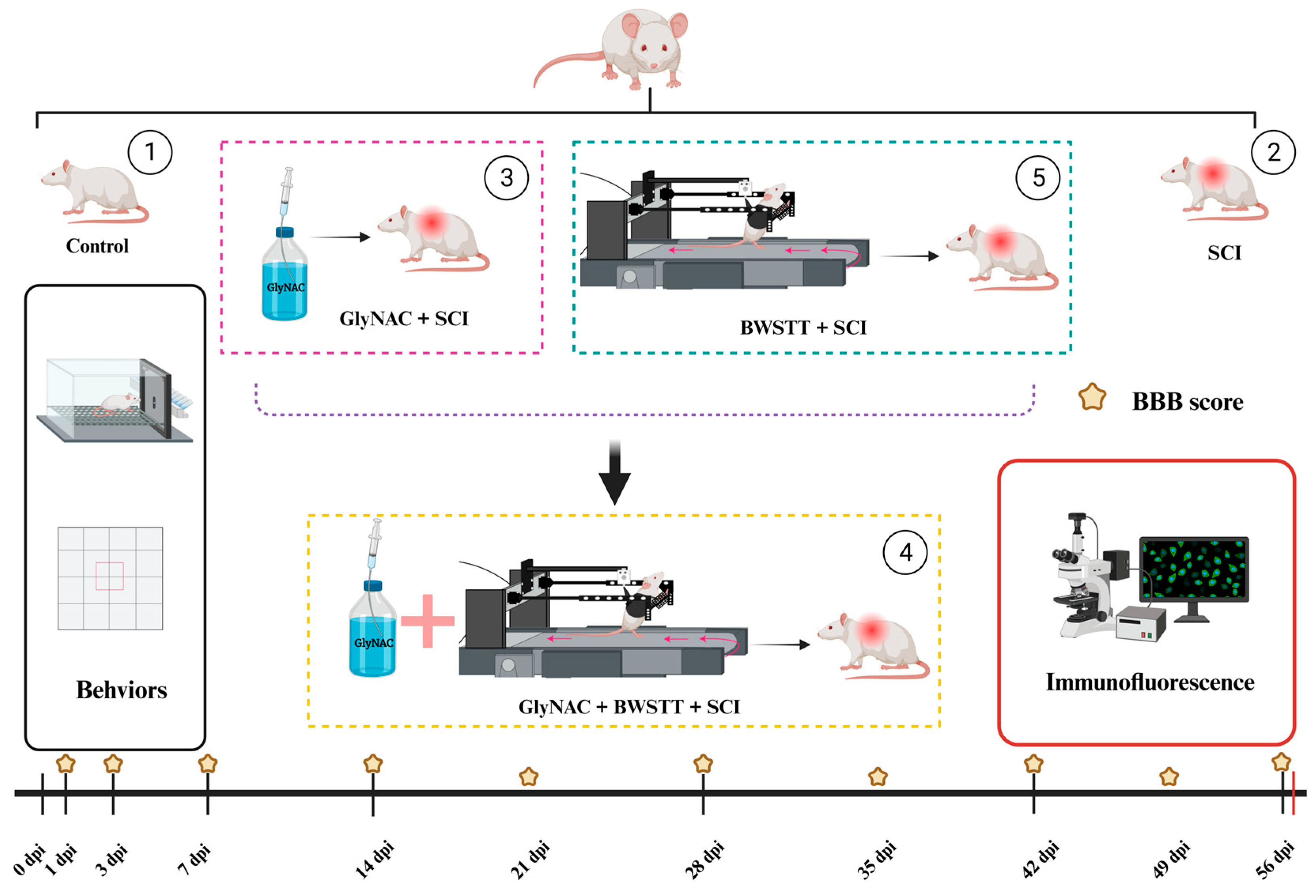
2.1. Animals and Grouping
2.2. Generation of the SCI Model and GlyNAC/BWSTT Intervention
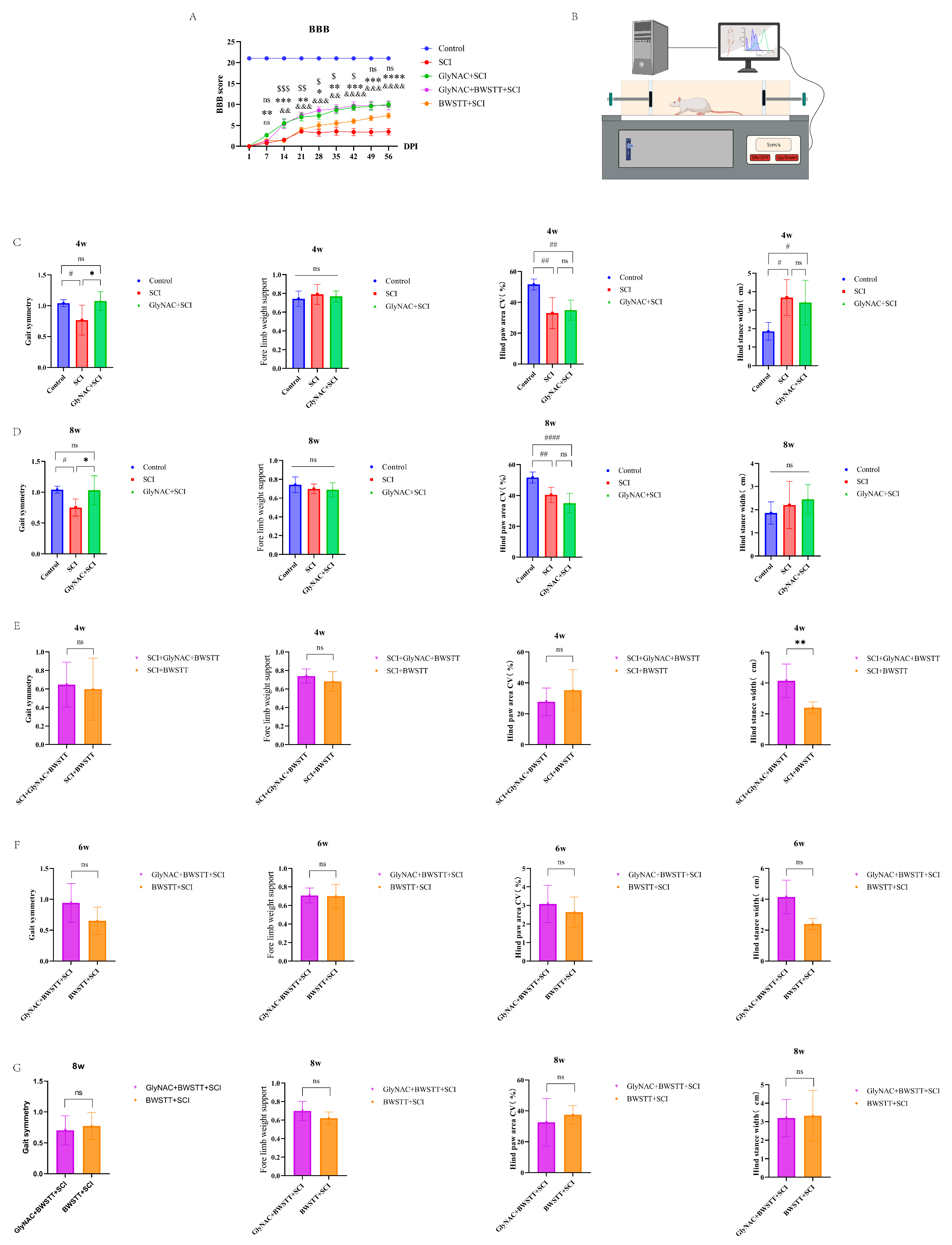
2.3. Basso–Beattie–Bresnahan (BBB) Locomotor Rating
2.4. Gait Analysis
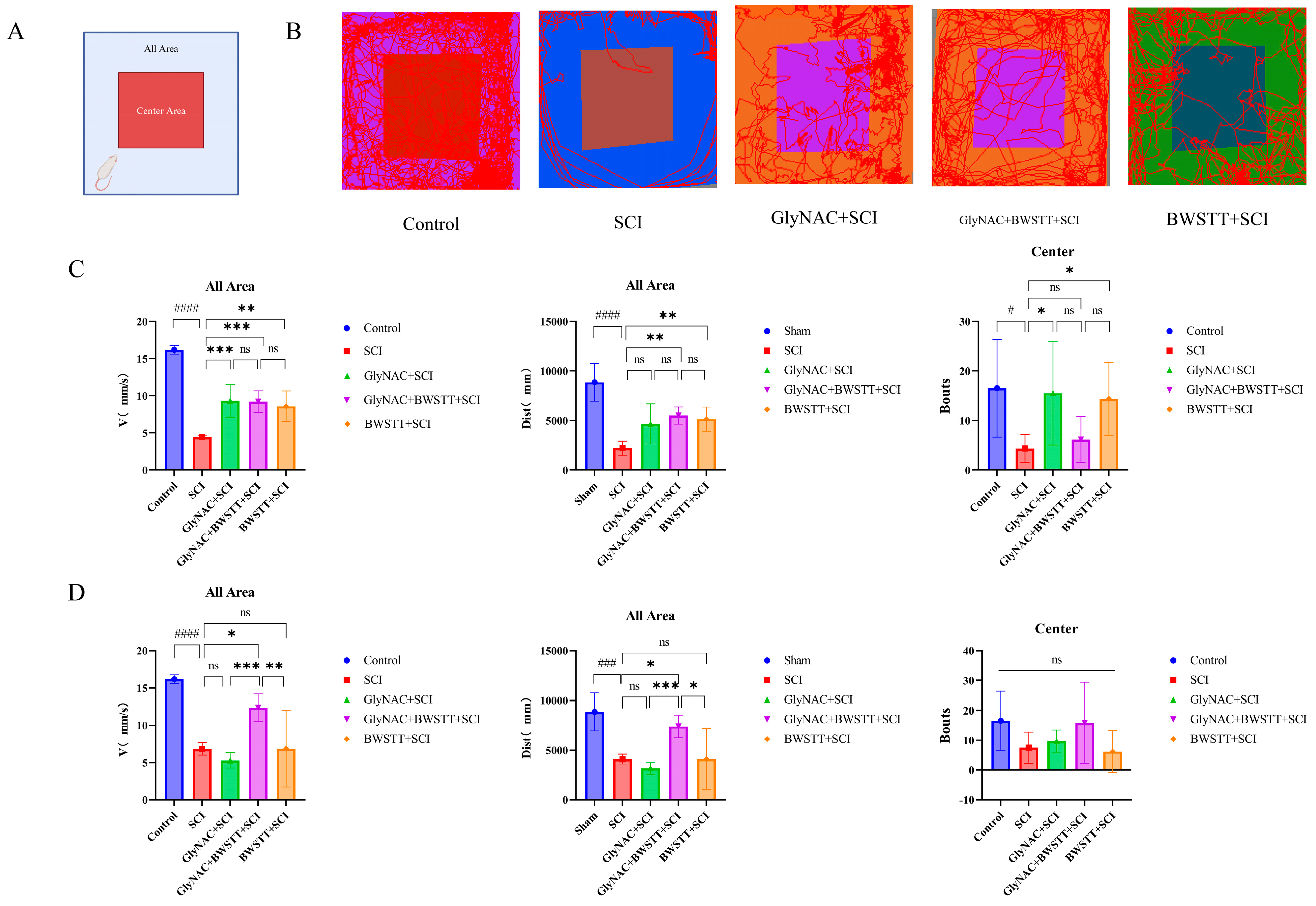
2.5. Open Field Test
2.6. Hindlimb Grip
2.7. Electrophysiologic Detection of Motor Neuron Excitability
2.8. Immunofluorescence Staining of the Spinal Cord and Skeletal Muscle
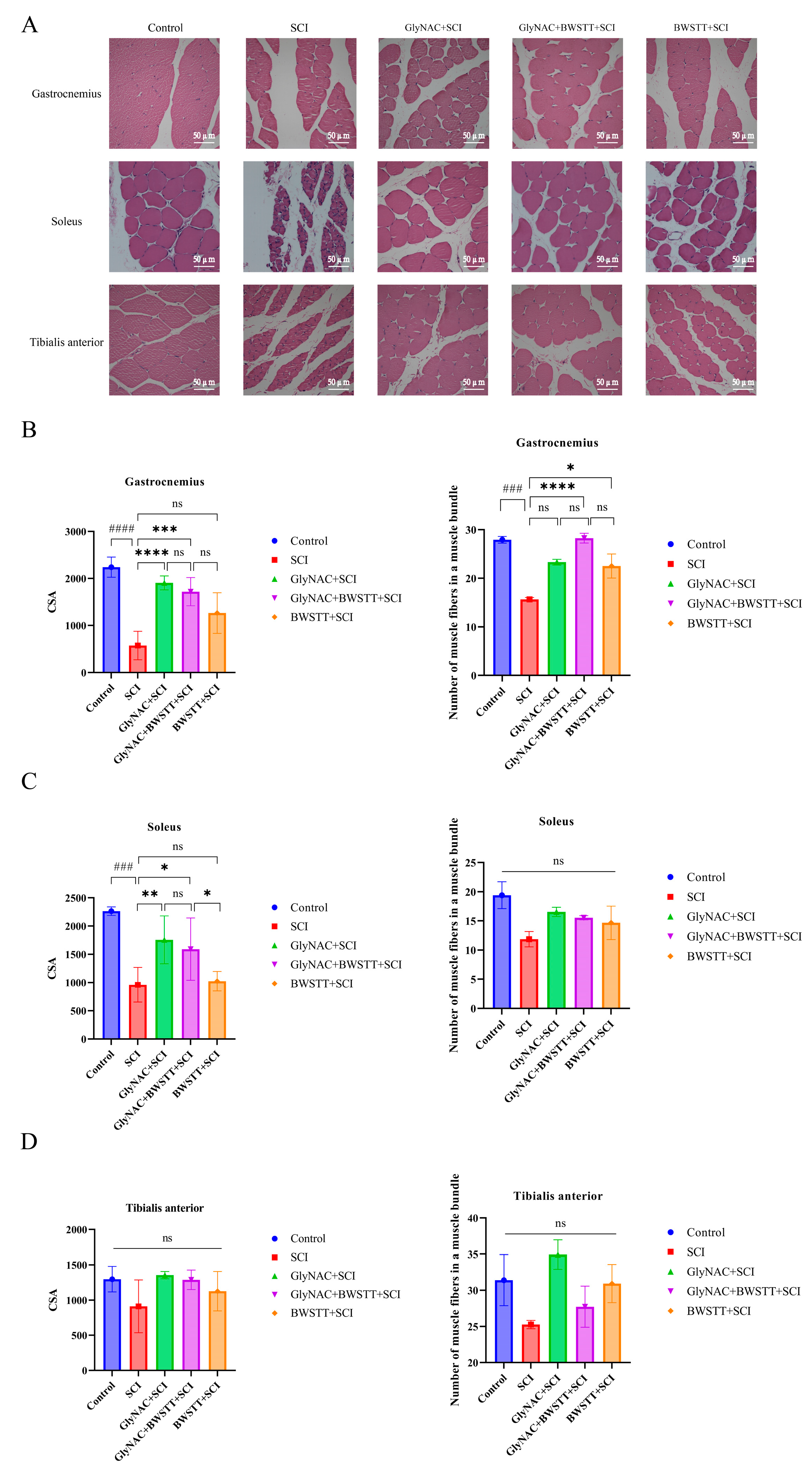
2.9. Hematoxylin and Eosin (H&E) Staining
2.10. Statistical Analysis
3. Results
3.1. GlyNAC Significantly Improved BBB Scores and Enhanced Gait Symmetry in Rats
3.2. Open Field Test Results
3.3. GlyNAC Combined with BWSTT Significantly Improved Hindlimb Grip in the Rats
3.4. GlyNAC Improved Motor Neuron Excitability in SCI Model Rats
3.5. Effects of GlyNAC Combined with BWSTT on Neurons and Motor Neurons at the Anterior Horn of the Lumbar Enlargement of the Spinal Cord
3.6. Therapeutic Effects of GlyNAC Combined with BWSTT on Skeletal Muscle Fiber CSA and Density
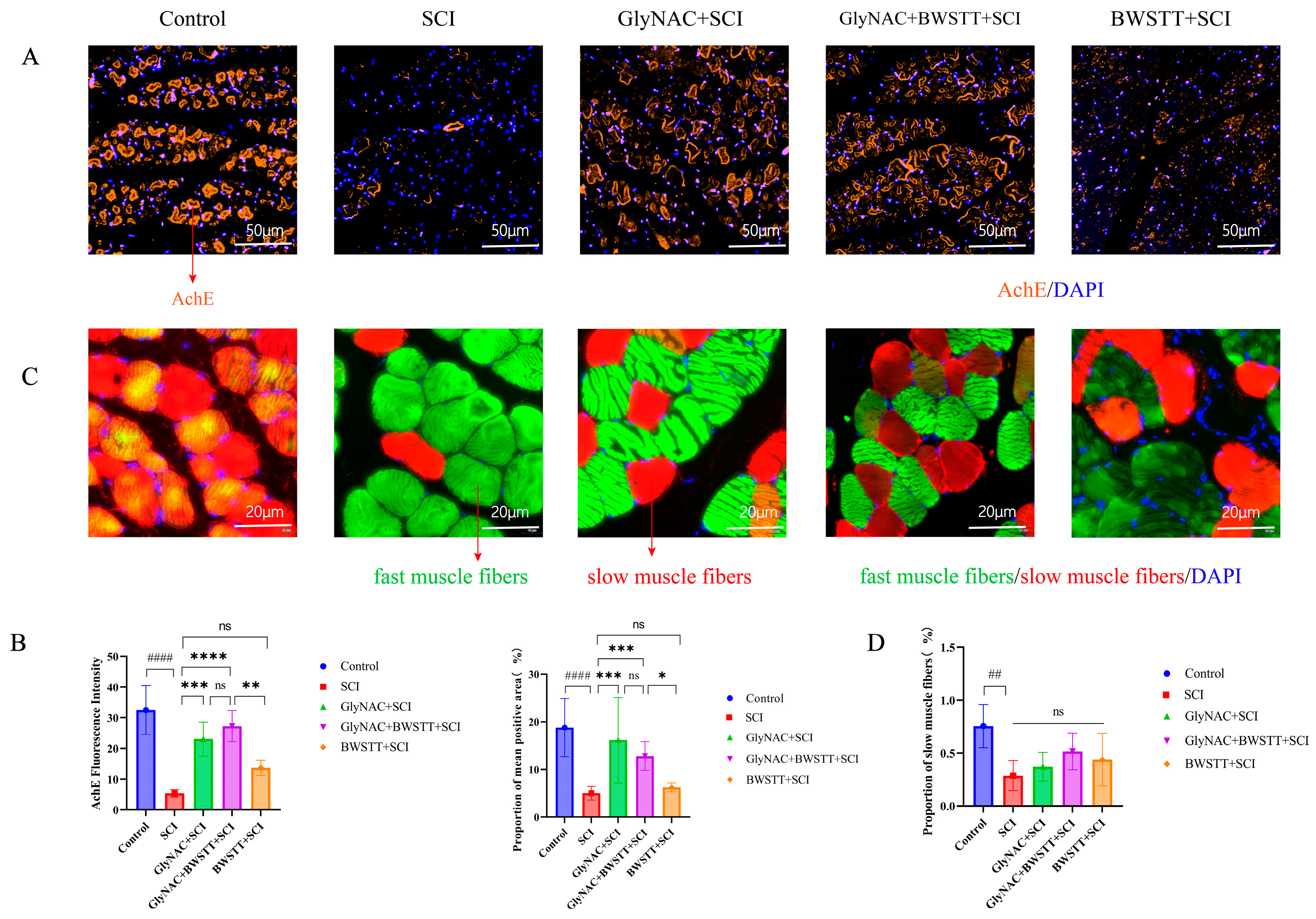
3.7. GlyNAC Combined with BWSTT Protects Motor Endplates in the Gastrocnemius after SCI
3.8. The Effect of GlyNAC in Combination with BWSTT on the Conversion of Fast and Slow Muscle Fibers in the Soleus
4. Discussion
Research Innovations and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McDonald, J.W.; Sadowsky, C. Spinal-cord injury. Lancet 2002, 359, 417–425. [Google Scholar] [CrossRef]
- Shi, Z.; Yuan, S.; Shi, L.; Li, J.; Ning, G.; Kong, X.; Feng, S. Programmed cell death in spinal cord injury pathogenesis and therapy. Cell Prolif. 2021, 54, e12992. [Google Scholar] [CrossRef]
- Shields, R.K. Muscular, skeletal, and neural adaptations following spinal cord injury. J. Orthop. Sports Phys. Ther. 2002, 32, 65–74. [Google Scholar] [CrossRef]
- Otzel, D.M.; Kok, H.J.; Graham, Z.A.; Barton, E.R.; Yarrow, J.F. Pharmacologic approaches to prevent skeletal muscle atrophy after spinal cord injury. Curr. Opin. Pharmacol. 2021, 60, 193–199. [Google Scholar] [CrossRef]
- Drasites, K.P.; Shams, R.; Zaman, V.; Matzelle, D.; Shields, D.C.; Garner, D.P.; Sole, C.J.; Haque, A.; Banik, N.L. Pathophysiology, Biomarkers, and Therapeutic Modalities Associated with Skeletal Muscle Loss Following Spinal Cord Injury. Brain Sci. 2020, 10, 933. [Google Scholar] [CrossRef]
- Baligand, C.; Chen, Y.W.; Ye, F.; Pandey, S.N.; Lai, S.H.; Liu, M.; Vandenborne, K. Transcriptional Pathways Associated with Skeletal Muscle Changes after Spinal Cord Injury and Treadmill Locomotor Training. BioMed Res. Int. 2015, 2015, 387090. [Google Scholar] [CrossRef]
- Atkins, K.D.; Bickel, C.S. Effects of functional electrical stimulation on muscle health after spinal cord injury. Curr. Opin. Pharmacol. 2021, 60, 226–231. [Google Scholar] [CrossRef]
- Graham, Z.A.; Siedlik, J.A.; Harlow, L.; Sahbani, K.; Bauman, W.A.; Tawfeek, H.A.; Cardozo, C.P. Key Glycolytic Metabolites in Paralyzed Skeletal Muscle Are Altered Seven Days after Spinal Cord Injury in Mice. J. Neurotrauma 2019, 36, 2722–2731. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.D.; Craven, B.C.; Thabane, L.; Laing, A.C.; Frank-Wilson, A.W.; Kontulainen, S.A.; Papaioannou, A.; Adachi, J.D.; Giangregorio, L.M. Lower-extremity muscle atrophy and fat infiltration after chronic spinal cord injury. J. Musculoskelet. Neuronal Interact. 2015, 15, 32–41. [Google Scholar] [PubMed]
- Cardozo, C.P. Muscle biology after spinal cord injury: Recent advances and future challenges. Acta Physiol. 2018, 223, e13073. [Google Scholar] [CrossRef]
- Otzel, D.M.; Lee, J.; Ye, F.; Borst, S.E.; Yarrow, J.F. Activity-Based Physical Rehabilitation with Adjuvant Testosterone to Promote Neuromuscular Recovery after Spinal Cord Injury. Int. J. Mol. Sci. 2018, 19, 1701. [Google Scholar] [CrossRef]
- Yin, L.; Li, N.; Jia, W.; Wang, N.; Liang, M.; Yang, X.; Du, G. Skeletal muscle atrophy: From mechanisms to treatments. Pharmacol. Res. 2021, 172, 105807. [Google Scholar] [CrossRef]
- Ciciliot, S.; Rossi, A.C.; Dyar, K.A.; Blaauw, B.; Schiaffino, S. Muscle type and fiber type specificity in muscle wasting. Int. J. Biochem. Cell Biol. 2013, 45, 2191–2199. [Google Scholar] [CrossRef]
- Biering-Sørensen, B.; Kristensen, I.B.; Kjaer, M.; Biering-Sørensen, F. Muscle after spinal cord injury. Muscle Nerve 2009, 40, 499–519. [Google Scholar] [CrossRef]
- Ham, D.J.; Caldow, M.K.; Lynch, G.S.; Koopman, R. Leucine as a treatment for muscle wasting: A critical review. Clin. Nutr. 2014, 33, 937–945. [Google Scholar] [CrossRef]
- Savikj, M.; Kostovski, E.; Lundell, L.S.; Iversen, P.O.; Massart, J.; Widegren, U. Altered oxidative stress and antioxidant defence in skeletal muscle during the first year following spinal cord injury. Physiol. Rep. 2019, 7, e14218. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Liu, C.; Suliburk, J.; Hsu, J.W.; Muthupillai, R.; Jahoor, F.; Minard, C.G.; Taffet, G.E.; Sekhar, R.V. Supplementing Glycine and N-Acetylcysteine (GlyNAC) in Older Adults Improves Glutathione Deficiency, Oxidative Stress, Mitochondrial Dysfunction, Inflammation, Physical Function, and Aging Hallmarks: A Randomized Clinical Trial. J. Gerontology. Ser. A Biol. Sci. Med. Sci. 2023, 78, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Liu, C.; Hsu, J.W.; Chacko, S.; Minard, C.; Jahoor, F.; Sekhar, R.V. Glycine and N-acetylcysteine (GlyNAC) supplementation in older adults improves glutathione deficiency, oxidative stress, mitochondrial dysfunction, inflammation, insulin resistance, endothelial dysfunction, genotoxicity, muscle strength, and cognition: Results of a pilot clinical trial. Clin. Transl. Med. 2021, 11, e372. [Google Scholar] [CrossRef]
- Kumar, P.; Osahon, O.W.; Sekhar, R.V. GlyNAC (Glycine and N-Acetylcysteine) Supplementation in Mice Increases Length of Life by Correcting Glutathione Deficiency, Oxidative Stress, Mitochondrial Dysfunction, Abnormalities in Mitophagy and Nutrient Sensing, and Genomic Damage. Nutrients 2022, 14, 1114. [Google Scholar] [CrossRef] [PubMed]
- Giangregorio, L.M.; Hicks, A.L.; Webber, C.E.; Phillips, S.M.; Craven, B.C.; Bugaresti, J.M.; McCartney, N. Body weight supported treadmill training in acute spinal cord injury: Impact on muscle and bone. Spinal Cord 2005, 43, 649–657. [Google Scholar] [CrossRef]
- Zhao, B.L.; Li, W.T.; Zhou, X.H.; Wu, S.Q.; Cao, H.S.; Bao, Z.R.; An, L.B. Effective robotic assistive pattern of treadmill training for spinal cord injury in a rat model. Exp. Ther. Med. 2018, 15, 3283–3294. [Google Scholar] [CrossRef] [PubMed]
- Krogh-Madsen, R.; Thyfault, J.P.; Broholm, C.; Mortensen, O.H.; Olsen, R.H.; Mounier, R.; Plomgaard, P.; van Hall, G.; Booth, F.W.; Pedersen, B.K. A 2-wk reduction of ambulatory activity attenuates peripheral insulin sensitivity. J. Appl. Physiol. 2010, 108, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
- Sekhar, R.V.; Patel, S.G.; Guthikonda, A.P.; Reid, M.; Balasubramanyam, A.; Taffet, G.E.; Jahoor, F. Deficient synthesis of glutathione underlies oxidative stress in aging and can be corrected by dietary cysteine and glycine supplementation. Am. J. Clin. Nutr. 2011, 94, 847–853. [Google Scholar] [CrossRef]
- Weinberg, J.M.; Bienholz, A.; Venkatachalam, M.A. The role of glycine in regulated cell death. Cell. Mol. Life Sci. CMLS 2016, 73, 2285–2308. [Google Scholar] [CrossRef] [PubMed]
- Cantoria, M.J.; See, P.A.; Singh, H.; de Leon, R.D. Adaptations in glutamate and glycine content within the lumbar spinal cord are associated with the generation of novel gait patterns in rats following neonatal spinal cord transection. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 18598–18605. [Google Scholar] [CrossRef]
- Scheijen, E.E.M.; Hendrix, S.; Wilson, D.M., 3rd. Oxidative DNA Damage in the Pathophysiology of Spinal Cord Injury: Seems Obvious, but Where Is the Evidence? Antioxidants 2022, 11, 1728. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wu, Z.; Dai, Z.; Yang, Y.; Wang, J.; Wu, G. Glycine metabolism in animals and humans: Implications for nutrition and health. Amino Acids 2013, 45, 463–477. [Google Scholar] [CrossRef]
- Koopman, R.; Caldow, M.K.; Ham, D.J.; Lynch, G.S. Glycine metabolism in skeletal muscle: Implications for metabolic homeostasis. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 237–242. [Google Scholar] [CrossRef]
- Ham, D.J.; Caldow, M.K.; Chhen, V.; Chee, A.; Wang, X.; Proud, C.G.; Lynch, G.S.; Koopman, R. Glycine restores the anabolic response to leucine in a mouse model of acute inflammation. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E970–E981. [Google Scholar] [CrossRef]
- Sun, K.; Wu, Z.; Ji, Y.; Wu, G. Glycine Regulates Protein Turnover by Activating Protein Kinase B/Mammalian Target of Rapamycin and by Inhibiting MuRF1 and Atrogin-1 Gene Expression in C2C12 Myoblasts. J. Nutr. 2016, 146, 2461–2467. [Google Scholar] [CrossRef]
- Karalija, A.; Novikova, L.N.; Kingham, P.J.; Wiberg, M.; Novikov, L.N. Neuroprotective effects of N-acetyl-cysteine and acetyl-L-carnitine after spinal cord injury in adult rats. PLoS ONE 2012, 7, e41086. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, X.; Wang, Z. Synergistic neuroprotective effects of hyperbaric oxygen and N-acetylcysteine against traumatic spinal cord injury in rat. J. Chem. Neuroanat. 2021, 118, 102037. [Google Scholar] [CrossRef]
- Guo, X.; He, J.; Zhang, R.; Wang, T.; Chen, J.; Wang, J.; Wang, Z.; Chang, G.; Niu, Y.; Niu, Z.; et al. N-Acetylcysteine alleviates spinal cord injury in rats after early decompression surgery by regulating inflammation and apoptosis. Neurol. Res. 2022, 44, 605–613. [Google Scholar] [CrossRef]
- Karalija, A.; Novikova, L.N.; Kingham, P.J.; Wiberg, M.; Novikov, L.N. The effects of N-acetyl-cysteine and acetyl-L-carnitine on neural survival, neuroinflammation and regeneration following spinal cord injury. Neuroscience 2014, 269, 143–151. [Google Scholar] [CrossRef] [PubMed]
- García-Campos, P.; Báez-Matus, X.; Jara-Gutiérrez, C.; Paz-Araos, M.; Astorga, C.; Cea, L.A.; Rodríguez, V.; Bevilacqua, J.A.; Caviedes, P.; Cárdenas, A.M. N-Acetylcysteine Reduces Skeletal Muscles Oxidative Stress and Improves Grip Strength in Dysferlin-Deficient Bla/J Mice. Int. J. Mol. Sci. 2020, 21, 4293. [Google Scholar] [CrossRef]
- Kumar, P.; Osahon, O.; Vides, D.B.; Hanania, N.; Minard, C.G.; Sekhar, R.V. Severe Glutathione Deficiency, Oxidative Stress and Oxidant Damage in Adults Hospitalized with COVID-19: Implications for GlyNAC (Glycine and N-Acetylcysteine) Supplementation. Antioxidants 2021, 11, 50. [Google Scholar] [CrossRef] [PubMed]
- Sekhar, R.V. GlyNAC (Glycine and N-Acetylcysteine) Supplementation Improves Impaired Mitochondrial Fuel Oxidation and Lowers Insulin Resistance in Patients with Type 2 Diabetes: Results of a Pilot Study. Antioxidants 2022, 11, 154. [Google Scholar] [CrossRef] [PubMed]
- Okawara, H.; Tashiro, S.; Sawada, T.; Sugai, K.; Matsubayashi, K.; Kawakami, M.; Nori, S.; Tsuji, O.; Nagoshi, N.; Matsumoto, M.; et al. Neurorehabilitation using a voluntary driven exoskeletal robot improves trunk function in patients with chronic spinal cord injury: A single-arm study. Neural Regen. Res. 2022, 17, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, J.; Du, L.; Yang, M.; Yang, D.; Li, J.; Gao, F.; Ma, K. Short-term effects of core stability training on the balance and ambulation function of individuals with chronic spinal cord injury: A pilot randomized controlled trial. Minerva Medica 2019, 110, 216–223. [Google Scholar] [CrossRef]
- Mehrholz, J.; Harvey, L.A.; Thomas, S.; Elsner, B. Is body-weight-supported treadmill training or robotic-assisted gait training superior to overground gait training and other forms of physiotherapy in people with spinal cord injury? A systematic review. Spinal Cord 2017, 55, 722–729. [Google Scholar] [CrossRef]
- Gong, C.; Zheng, X.; Guo, F.; Wang, Y.; Zhang, S.; Chen, J.; Sun, X.; Shah, S.Z.A.; Zheng, Y.; Li, X.; et al. Human spinal GABA neurons alleviate spasticity and improve locomotion in rats with spinal cord injury. Cell Rep. 2021, 34, 108889. [Google Scholar] [CrossRef]
- Grau, J.W.; Baine, R.E.; Bean, P.A.; Davis, J.A.; Fauss, G.N.; Henwood, M.K.; Hudson, K.E.; Johnston, D.T.; Tarbet, M.M.; Strain, M.M. Learning to promote recovery after spinal cord injury. Exp. Neurol. 2020, 330, 113334. [Google Scholar] [CrossRef]
- Zeman, R.J.; Zhao, J.; Zhang, Y.; Zhao, W.; Wen, X.; Wu, Y.; Pan, J.; Bauman, W.A.; Cardozo, C. Differential skeletal muscle gene expression after upper or lower motor neuron transection. Pflug. Arch. Eur. J. Physiol. 2009, 458, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Sangari, S.; Lundell, H.; Kirshblum, S.; Perez, M.A. Residual descending motor pathways influence spasticity after spinal cord injury. Ann. Neurol. 2019, 86, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Tamburin, S.; Filippetti, M.; Mantovani, E.; Smania, N.; Picelli, A. Spasticity following brain and spinal cord injury: Assessment and treatment. Curr. Opin. Neurol. 2022, 35, 728–740. [Google Scholar] [CrossRef] [PubMed]
- Gorgey, A.S.; Dudley, G.A. Spasticity may defend skeletal muscle size and composition after incomplete spinal cord injury. Spinal Cord 2008, 46, 96–102. [Google Scholar] [CrossRef]
- Harris, R.L.; Bobet, J.; Sanelli, L.; Bennett, D.J. Tail muscles become slow but fatigable in chronic sacral spinal rats with spasticity. J. Neurophysiol. 2006, 95, 1124–1133. [Google Scholar] [CrossRef]
- Alizadeh, A.; Dyck, S.M.; Karimi-Abdolrezaee, S. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Front. Neurol. 2019, 10, 282. [Google Scholar] [CrossRef]
- Li, C.; Wu, Z.; Zhou, L.; Shao, J.; Hu, X.; Xu, W.; Ren, Y.; Zhu, X.; Ge, W.; Zhang, K.; et al. Temporal and spatial cellular and molecular pathological alterations with single-cell resolution in the adult spinal cord after injury. Signal Transduct. Target. Ther. 2022, 7, 65. [Google Scholar] [CrossRef]
- Grumbles, R.M.; Liu, Y.; Thomas, C.M.; Wood, P.M.; Thomas, C.K. Acute stimulation of transplanted neurons improves motoneuron survival, axon growth, and muscle reinnervation. J. Neurotrauma 2013, 30, 1062–1069. [Google Scholar] [CrossRef]
- Pizzi, M.; Brunelli, G.; Barlati, S.; Spano, P. Glutamatergic innervation of rat skeletal muscle by supraspinal neurons: A new paradigm in spinal cord injury repair. Curr. Opin. Neurobiol. 2006, 16, 323–328. [Google Scholar] [CrossRef]
- Tosolini, A.P.; Mohan, R.; Morris, R. Targeting the full length of the motor end plate regions in the mouse forelimb increases the uptake of fluoro-gold into corresponding spinal cord motor neurons. Front. Neurol. 2013, 4, 58. [Google Scholar] [CrossRef]
- Wang, J.; Sun, J.; Tang, Y.; Guo, G.; Zhou, X.; Chen, Y.; Shen, M. Basic fibroblast growth factor attenuates the degeneration of injured spinal cord motor endplates. Neural Regen. Res. 2013, 8, 2213–2224. [Google Scholar] [CrossRef]
- Mohan, R.; Tosolini, A.P.; Morris, R. Segmental distribution of the motor neuron columns that supply the rat hindlimb: A muscle/motor neuron tract-tracing analysis targeting the motor end plates. Neuroscience 2015, 307, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Negredo, P.; Rivero, J.L.; González, B.; Ramón-Cueto, A.; Manso, R. Slow- and fast-twitch rat hind limb skeletal muscle phenotypes 8 months after spinal cord transection and olfactory ensheathing glia transplantation. J. Physiol. 2008, 586, 2593–2610. [Google Scholar] [CrossRef][Green Version]
- Jayaraman, A.; Liu, M.; Ye, F.; Walter, G.A.; Vandenborne, K. Regenerative responses in slow- and fast-twitch muscles following moderate contusion spinal cord injury and locomotor training. Eur. J. Appl. Physiol. 2013, 113, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Byers, J.S.; Huguenard, A.L.; Kuruppu, D.; Liu, N.K.; Xu, X.M.; Sengelaub, D.R. Neuroprotective effects of testosterone on motoneuron and muscle morphology following spinal cord injury. J. Comp. Neurol. 2012, 520, 2683–2696. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Tripathy, D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 2009, 32 (Suppl. S2), S157–S163. [Google Scholar] [CrossRef]
- Kraemer, W.J.; Ratamess, N.A.; Hymer, W.C.; Nindl, B.C.; Fragala, M.S. Growth Hormone(s), Testosterone, Insulin-Like Growth Factors, and Cortisol: Roles and Integration for Cellular Development and Growth With Exercise. Front. Endocrinol. 2020, 11, 33. [Google Scholar] [CrossRef]
- Scholpa, N.E.; Simmons, E.C.; Tilley, D.G.; Schnellmann, R.G. β2-adrenergic receptor-mediated mitochondrial biogenesis improves skeletal muscle recovery following spinal cord injury. Exp. Neurol. 2019, 322, 113064. [Google Scholar] [CrossRef] [PubMed]
- Gorgey, A.S.; Witt, O.; O’Brien, L.; Cardozo, C.; Chen, Q.; Lesnefsky, E.J.; Graham, Z.A. Mitochondrial health and muscle plasticity after spinal cord injury. Eur. J. Appl. Physiol. 2019, 119, 315–331. [Google Scholar] [CrossRef] [PubMed]
- Gorgey, A.S.; Dudley, G.A. Skeletal muscle atrophy and increased intramuscular fat after incomplete spinal cord injury. Spinal Cord 2007, 45, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Talifu, Z.; Zhang, C.J.; Gao, F.; Ke, H.; Pan, Y.Z.; Gong, H.; Du, H.Y.; Yu, Y.; Jing, Y.L.; et al. Mechanism of skeletal muscle atrophy after spinal cord injury: A narrative review. Front. Nutr. 2023, 10, 1099143. [Google Scholar] [CrossRef]
- Gorgey, A.S.; Khalil, R.E.; Gill, R.; Gater, D.R.; Lavis, T.D.; Cardozo, C.P.; Adler, R.A. Low-Dose Testosterone and Evoked Resistance Exercise after Spinal Cord Injury on Cardio-Metabolic Risk Factors: An Open-Label Randomized Clinical Trial. J. Neurotrauma 2019, 36, 2631–2645. [Google Scholar] [CrossRef]
- Gorgey, A.S.; Graham, Z.A.; Chen, Q.; Rivers, J.; Adler, R.A.; Lesnefsky, E.J.; Cardozo, C.P. Sixteen weeks of testosterone with or without evoked resistance training on protein expression, fiber hypertrophy and mitochondrial health after spinal cord injury. J. Appl. Physiol. 2020, 128, 1487–1496. [Google Scholar] [CrossRef]
- Bauman, W.A.; Cirnigliaro, C.M.; La Fountaine, M.F.; Jensen, A.M.; Wecht, J.M.; Kirshblum, S.C.; Spungen, A.M. A small-scale clinical trial to determine the safety and efficacy of testosterone replacement therapy in hypogonadal men with spinal cord injury. Horm. Metab. Res. 2011, 43, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhao, J.; Zhao, W.; Pan, J.; Bauman, W.A.; Cardozo, C.P. Nandrolone normalizes determinants of muscle mass and fiber type after spinal cord injury. J. Neurotrauma 2012, 29, 1663–1675. [Google Scholar] [CrossRef]
- Myatich, A.; Haque, A.; Sole, C.; Banik, N.L. Clemastine in remyelination and protection of neurons and skeletal muscle after spinal cord injury. Neural Regen. Res. 2023, 18, 940–946. [Google Scholar] [CrossRef]


| Antibody | Host | Supplier | Catalog No. | Dilution | Application |
|---|---|---|---|---|---|
| ChAT | Rabbit | Abcam, Cambridge, UK | ab181023 | 1:1000 | IF |
| NeuN | Rabbit | Servicebio | GB11138 | 1:200 | IF |
| Fast | Rabbit | Servicebio | GB112130 | 1:3000 | IF |
| Slow | Rabbit | Servicebio | GB112131 | 1:500 | 1:500 |
| AChE | Rabbit | Servicebio | GB11038-1 | 1:500 | 1:500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Du, H.-Y.; Talifu, Z.; Zhang, C.-J.; Li, Z.-H.; Liu, W.-B.; Liang, Y.-X.; Xu, X.-L.; Zhang, J.-M.; Yang, D.-G.; et al. Glycine and N-Acetylcysteine (GlyNAC) Combined with Body Weight Support Treadmill Training Improved Spinal Cord and Skeletal Muscle Structure and Function in Rats with Spinal Cord Injury. Nutrients 2023, 15, 4578. https://doi.org/10.3390/nu15214578
Xu X, Du H-Y, Talifu Z, Zhang C-J, Li Z-H, Liu W-B, Liang Y-X, Xu X-L, Zhang J-M, Yang D-G, et al. Glycine and N-Acetylcysteine (GlyNAC) Combined with Body Weight Support Treadmill Training Improved Spinal Cord and Skeletal Muscle Structure and Function in Rats with Spinal Cord Injury. Nutrients. 2023; 15(21):4578. https://doi.org/10.3390/nu15214578
Chicago/Turabian StyleXu, Xin, Hua-Yong Du, Zuliyaer Talifu, Chun-Jia Zhang, Ze-Hui Li, Wu-Bo Liu, Yi-Xiong Liang, Xu-Luan Xu, Jin-Ming Zhang, De-Gang Yang, and et al. 2023. "Glycine and N-Acetylcysteine (GlyNAC) Combined with Body Weight Support Treadmill Training Improved Spinal Cord and Skeletal Muscle Structure and Function in Rats with Spinal Cord Injury" Nutrients 15, no. 21: 4578. https://doi.org/10.3390/nu15214578
APA StyleXu, X., Du, H.-Y., Talifu, Z., Zhang, C.-J., Li, Z.-H., Liu, W.-B., Liang, Y.-X., Xu, X.-L., Zhang, J.-M., Yang, D.-G., Gao, F., Du, L.-J., Yu, Y., Jing, Y.-L., & Li, J.-J. (2023). Glycine and N-Acetylcysteine (GlyNAC) Combined with Body Weight Support Treadmill Training Improved Spinal Cord and Skeletal Muscle Structure and Function in Rats with Spinal Cord Injury. Nutrients, 15(21), 4578. https://doi.org/10.3390/nu15214578








