Effects of Walnut and Pumpkin on Selective Neurophenotypes of Autism Spectrum Disorders: A Case Study
Abstract
:1. Introduction
2. Case Study
2.1. Detailed History of the Case Study
- Child-X was the second child in his family. Child-X’s sleep had been brief and infrequent since birth, and when he began to speak, it was evident that his linguistic development was severely impaired. His speech was a mumble.
- Child-X enjoyed playing alone in a corner, arranging his cars from smallest to largest or arranging shoes. He did not respond to his name, so if called, he would not come, and if hugged or caressed, particularly his hair, he would not respond (Child-X’s mother stated).
- When Child-X was about a year and a half old, he began to pronounce “mama” and “papa,” but he did not mean either and he had no reaction to pain, even throughout the teething stage.
- Child-X was often frightened and could injure himself by crashing his head against the ground or a wall till he was harmed. He threw objects on the ground, and if they shattered, he went into a two- to three-hour-long panic attack. He slept on the ground, or even in the street, and he did not hurt anyone, but if anyone approached or touched him, he reacted violently. Child-X underwent a hearing test, which came out normal.
- Child-X went to a speech–language therapist when he was two years and two months old and, after careful examinations, she diagnosed him with moderate autism, recommending that he see a neurologist and go through speech, skill development, and behavior management sessions. The neurologist confirmed that he had moderate-grade autism and that he would never be normal.
2.2. Dietary Intervention
- Child-X was on a gluten- and casein-restricted diet and his mother changed to soy and almond products. To activate brain cells, the doctor suggested including pumpkin and walnuts in the diet.
- The therapy journey then began with Omega 3, GABA 500 mg/day, and a sedative, which was gradually tapered off because his parents did not want him to sleep for long periods of time. GABA was administered for only one year of the three years of the pumpkin/walnut-rich diet regimen. For over three years, the normal diet of child-X included blended mango juice with pumpkin, cooked pumpkin or soy products mixed with lentil and carrot, and daily servings of cake or pastries topped with chopped walnuts. Pumpkin was also used to make muffins, custards, and pancakes.
- Along with the food intervention, Child-X received speech, behavioral, and educational therapy twice a week for three years. A speech therapist measured the improvements and recorded them. Child-X’s mother recorded a remarkable improvement in speech going forward.
2.3. Language Development and CARS Score
- Then, at the age of four and a half, Child-X began to call his mother and say simple words without making usable phrases, and he began to speak intentionally and intelligibly at a rate of 30%. Now, he is speaking normally and, through behavioral and social interaction training, his CARS score has continued improving to reach 17. Table 1 displays the improvement in his CARS score during the course of the dietary intervention (from 2 years and 2 months to 6 years) and up until age 13 (Child X continued to prefer eating pumpkin and walnuts frequently).
- Child-X is now diagnosed as “out of autism” and he is being integrated into a regular school and achieving satisfactory learning outcomes in grade six. His mother was very keen to share her son’s management story with parents of individuals with ASD.
3. Etiological Mechanisms of ASD
3.1. Glutamate Excitotoxicity in ASD
3.2. Oxidative Stress and ASD
3.3. Neuroinflammation in ASD
3.4. Mitochondrial Dysfunction as a Central Etiological Mechanism in ASD
3.5. Autophagy and ASD
3.6. Altered Gut Microbiota in Autism
4. Walnut/Pumpkin Therapeutic and Neuroprotective Properties
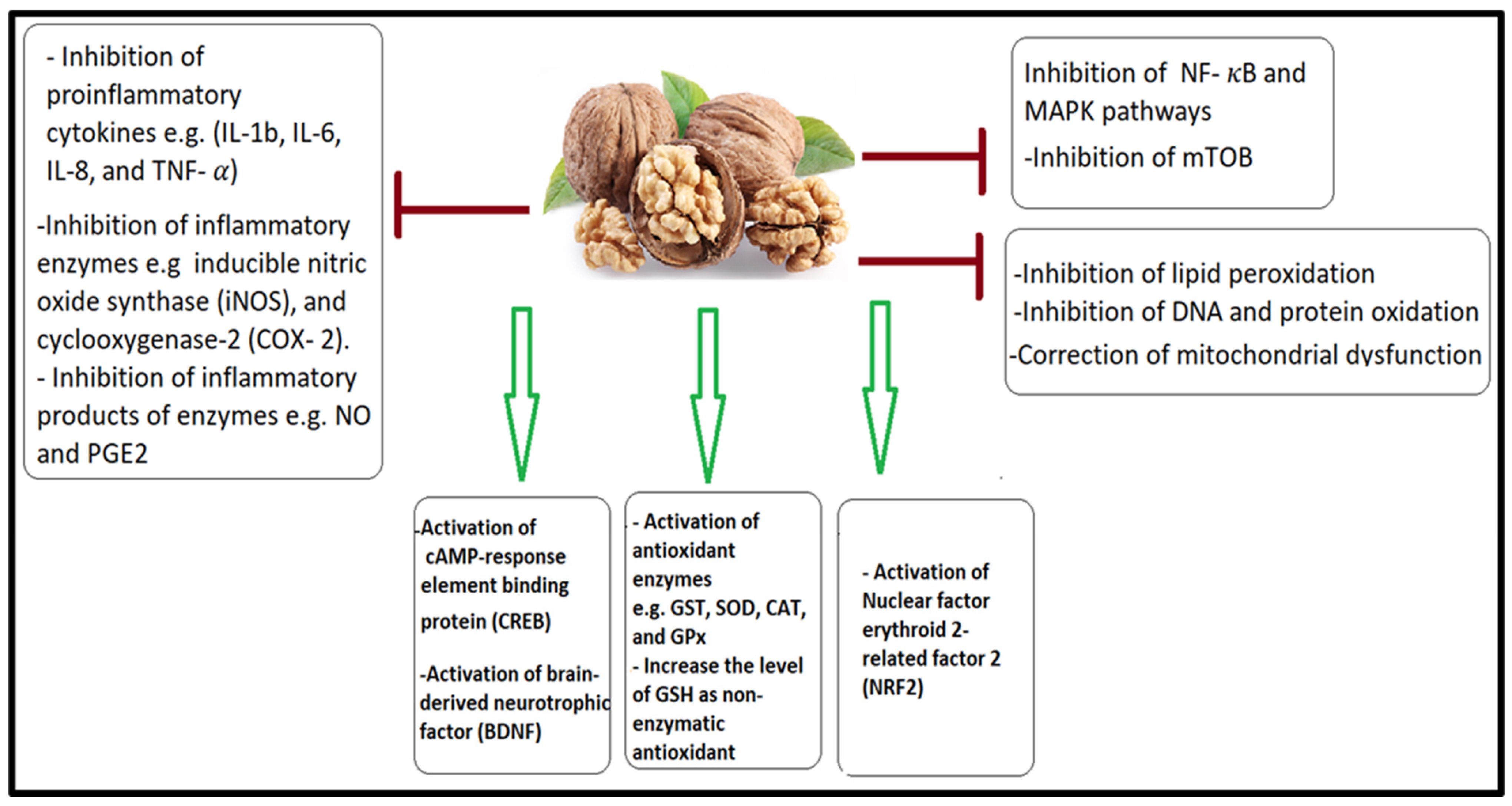
4.1. Antioxidant and Anti-Inflammatory Effects of a Walnut/Pumpkin-Rich Diet
4.2. Walnut/Pumpkin and the Treatment of Glutamate Excitotoxicity
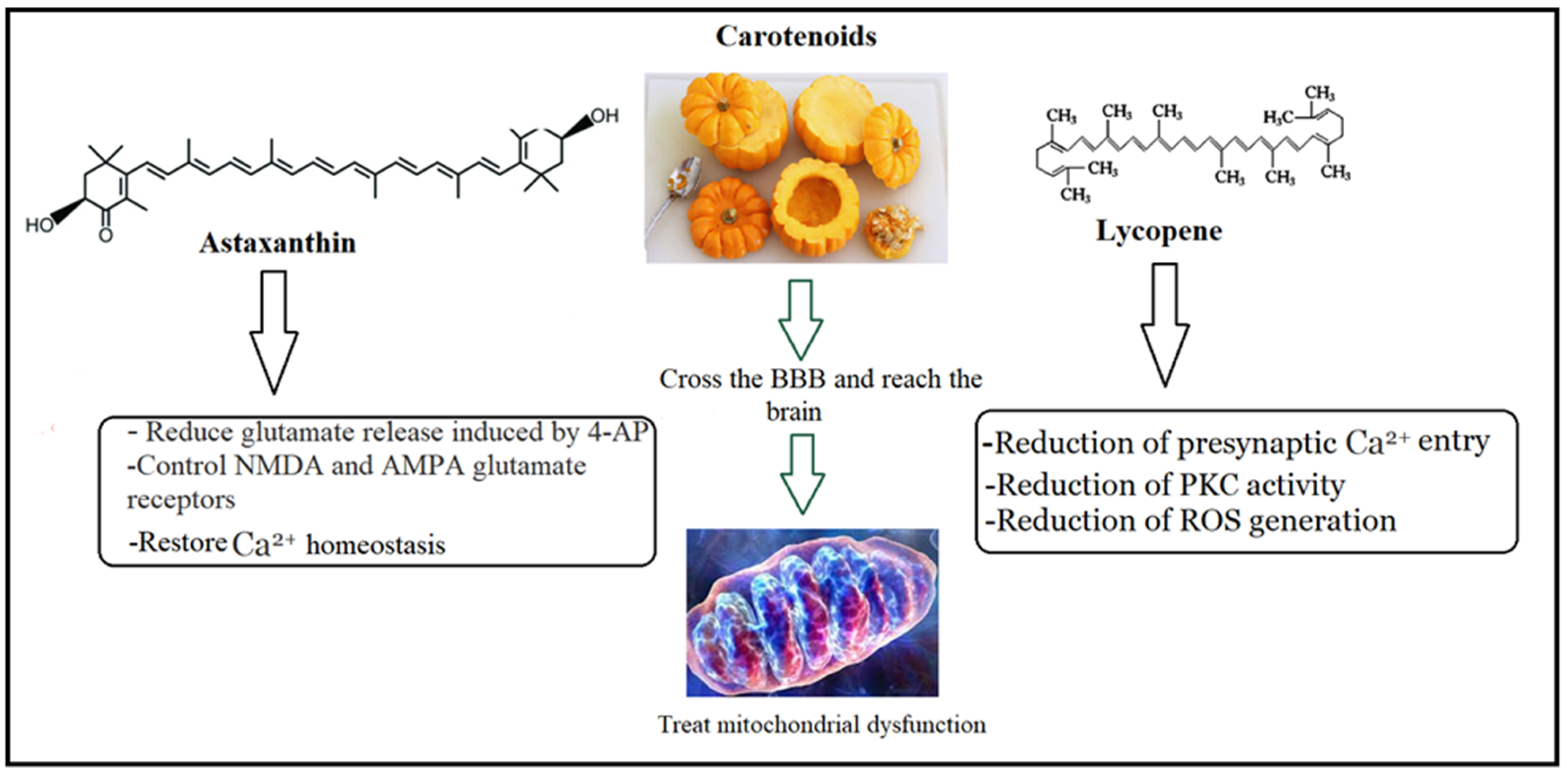
4.3. Walnut/Pumpkin and Correction of Mitochondrial Dysfunction in ASD
4.4. Walnut/Pumpkin and Healthy Gut Microbiota
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Yin, H.H.; Knowlton, B.J. The role of the basal ganglia in habit formation. Nat. Rev. Neurosci. 2006, 7, 464–476. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Fetit, R.; Hillary, R.F.; Price, D.J.; Lawrie, S.M. The neuropathology of autism: A systematic review of post-mortem studies of autism and related disorders. Neurosci. Biobehav. Rev. 2021, 129, 35–62. [Google Scholar] [CrossRef]
- Constantino, J.N.; Abbacchi, A.M.; Saulnier, C.; Klaiman, C.; Mandell, D.S.; Zhang, Y.; Hawks, Z.; Bates, J.; Klin, A.; Shattuck, P.; et al. Timing of the diagnosis of autism in African American children. Pediatrics 2020, 146, e20193629. [Google Scholar] [CrossRef]
- Marotta, R.; Risoleo, M.C.; Messina, G.; Parisi, L.; Carotenuto, M.; Vetri, L.; Roccella, M. The neurochemistry of autism. Brain Sci. 2020, 10, 163. [Google Scholar] [CrossRef]
- Pickles, A.; McCauley, J.B.; Pepa, L.A.; Huerta, M.; Lord, C. The adult outcome of children referred for autism: Typology and prediction from childhood. J. Child Psychol. Psychiatry 2020, 61, 760–767. [Google Scholar] [CrossRef]
- Belardo, A.; Gevi, F.; Zolla, L. The concomitant lower concentrations of vitamins B6, B9 and B12 may cause methylation deficiency in autistic children. J. Nutr. Biochem. 2019, 70, 38–46. [Google Scholar] [CrossRef]
- Robea, M.A.; Luca, A.C.; Ciobica, A. Relationship between Vitamin Deficiencies and Co-Occurring Symptoms in Autism Spectrum Disorder. Medicina 2020, 56, 245. [Google Scholar] [CrossRef]
- do Nascimento, P.K.d.S.B.; Oliveira Silva, D.F.; de Morais, T.L.S.A.; de Rezende, A.A. Zinc Status and Autism Spectrum Disorder in Children and Adolescents: A Systematic Review. Nutrients 2023, 15, 3663. [Google Scholar] [CrossRef]
- Alshamrani, A.A.; Alshehri, S.; Alqarni, S.S.; Ahmad, S.F.; Alghibiwi, H.; Al-Harbi, N.O.; Alqarni, S.A.; Al-Ayadhi, L.Y.; Attia, S.M.; Alfardan, A.S.; et al. DNA Hypomethylation Is Associated with Increased Inflammation in Peripheral Blood Neutrophils of Children with Autism Spectrum Disorder: Understanding the Role of Ubiquitous Pollutant Di(2-ethylhexyl) Phthalate. Metabolites 2023, 13, 458. [Google Scholar] [CrossRef]
- Stoccoro, A.; Conti, E.; Scaffei, E.; Calderoni, S.; Coppedè, F.; Migliore, L.; Battini, R. DNA Methylation Biomarkers for Young Children with Idiopathic Autism Spectrum Disorder: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 9138. [Google Scholar] [CrossRef]
- Adams, J.B.; Audhya, T.; McDonough-Means, S.A.; Rubin, R.; Quig, D.; Geis, E.; Gehn, E.; Loresto, M.; Mitchell, J.; Atwood, S.; et al. Nutritional and metabolic status of children with autism vs. neurotypical children, and the association with autism severity. Nutr. Metab. 2011, 8, 34. [Google Scholar] [CrossRef]
- Arnold, G.L.; Hyman, S.L.; Mooney, R.A.; Kirby, R.S. Plasma amino acids profiles in children with autism: Potential risk of nutritional deficiencies. J. Autism Dev. Disord. 2003, 33, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Gevi, F.; Zolla, L.; Gabriele, S.; Persico, A.M. Urinary Metabolomics of Young Italian Autistic Children Supports Abnormal Tryptophan and Purine Metabolism. Mol. Autism 2016, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Horder, J.; Petrinovic, M.M.; Mendez, M.A.; Bruns, A.; Takumi, T.; Spooren, W.; Barker, G.J.; Künnecke, B.; Murphy, D.G. Glutamate and GABA in autism spectrum disorder-a translational magnetic resonance spectroscopy study in man and rodent models. Transl. Psychiatry 2018, 8, 106. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xu, Y.; Pang, D.; Zhao, Q.; Zhang, L.; Li, M.; Li, W.; Duan, G.; Zhu, C. Interrelation between homocysteine metabolism and the development of autism spectrum disorder in children. Front. Mol. Neurosci. 2022, 15, 947513. [Google Scholar] [CrossRef]
- Carpita, B.; Nardi, B.; Palego, L.; Cremone, I.; Massimetti, G.; Carmassi, C.; Dell’Osso, L. Kynurenine pathway and autism spectrum phenotypes: An investigation among adults with autism spectrum disorder and their first-degree relatives. CNS Spectr. 2023, 28, 374–385. [Google Scholar] [CrossRef]
- Nagwa, M.; Hebatalla, H.; Mohamed, G.; Samia, H.; Yuliya, S.; Saher, H.; Adel, H.; Ahmed, E.; Bjorklund, G. Evaluation of Branched-Chain Amino Acids in Children with Autism Spectrum Disorder and Epilepsy. Mol. Neurobiol. 2023, 60, 1997–2004. [Google Scholar] [CrossRef]
- Smith, A.M.; Natowicz, M.R.; Braas, D.; Ludwig, M.A.; Ney, D.M.; Donley, E.L.R.; Burrier, R.E.; Amaral, D.G. A Metabolomics Approach to Screening for Autism Risk in the Children’s Autism Metabolome Project. Autism Res. 2020, 13, 1270–1285. [Google Scholar] [CrossRef]
- Smith, D.E.; Clémençon, B.; Hediger, M.A. Proton-Coupled Oligopeptide Transporter Family SLC15: Physiological, Pharmacological and Pathological Implications. Mol. Asp. Med. 2013, 34, 323–336. [Google Scholar] [CrossRef]
- Xu, Q.; Yan, X.; Zhang, Y.; Wu, J. Current understanding of transport and bioavailability of bioactive peptides derived from dairy proteins: A review. Int. J. Food Sci. Technol. 2019, 54, 1930–1941. [Google Scholar] [CrossRef]
- Wijesinghe, A.; Kumari, S.; Booth, V. Conjugates for use in peptide therapeutics: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0255753. [Google Scholar] [CrossRef]
- Banks, W.A.; Audus, K.L.; Davis, T.P. Permeability of the blood-brain barrier to peptides: An approach to the development of therapeutically useful analogs. Peptides 1992, 13, 1289–1294. [Google Scholar] [CrossRef]
- Karaś, M.; Jakubczyk, A.; Szymanowska, U.; Materska, M.; Zielińska, E. Antioxidant activity of protein hydrolysates from raw and heat-treated yellow string beans (Phaseolus vulgaris L.). Acta Sci. Pol. Technol. Aliment. 2014, 13, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Ngoh, Y.Y.; Gan, C.Y. Enzyme-assisted extraction and identification of antioxidative and α-amylase inhibitory peptides from Pinto beans (Phaseolus vulgaris cv. Pinto). Food Chem. 2016, 190, 331–337. [Google Scholar] [CrossRef]
- Muthaiyah, B.; Essa, M.M.; Lee, M.; Chauhan, V.; Kaur, K.; Chauhan, A. Dietary supplementation of walnuts improves memory deficits and learning skills in transgenic mouse model of Alzheimer’s disease. J. Alzheimer’s Dis. 2014, 42, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- Cintesun, S.; Ozman, Z.; Kocyigit, A.; Mansuroglu, B.; Kocacaliskan, I. Effects of walnut (Juglans regia L.) kernel extract and juglone on dopamine levels and oxidative stress in rats. Food Biosci. 2023, 51, 102327. [Google Scholar] [CrossRef]
- Ventola, P.E.; Kleinman, J.; Pandey, J.; Barton, M.; Allen, S.; Green, J.; Robins, D.; Fein, D. Agreement among Four Diagnostic Instruments for Autism Spectrum Disorders in Toddlers. J. Autism Dev. Disord. 2006, 36, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Kausar, T.; Sehar, S.; Sarwar, A.; Ashraf, A.H.; Jamil, M.A.; Noreen, S.; Rafique, A.; Iftikhar, K.; Aslam, J.; et al. Utilization of pumpkin, pumpkin powders, extracts, isolates, purified bioactives and pumpkin based functional food products: A key strategy to improve health in current post COVID-19 period: An updated review. Appl. Food Res. 2022, 2, 100241. [Google Scholar] [CrossRef]
- Frangiamone, M.; Cimbalo, A.; Font, G.; Alonso-Garrido, M.; Manyes, L. In vitro exposure to pumpkin extract induces a protective transcriptomic profile in blood brain barrier electron transport chain. Rev. Toxicol. 2021, 38, 1–7. [Google Scholar]
- Choudhury, P.R.; Lahiri, S.; Rajamma, U. Glutamate mediated signaling in the pathophysiology of autism spectrum disorders. Pharmacol. Biochem. Behav. 2012, 100, 841–849. [Google Scholar] [CrossRef]
- Rojas, D.C. The role of glutamate and its receptors in autism and the use of glutamate receptor antagonists in treatment. J. Neural Transm. 2014, 121, 891–905. [Google Scholar] [CrossRef] [PubMed]
- Carlson, G.C. Glutamate receptor dysfunction and drug targets across models of autism spectrum disorders. Pharmacol. Biochem. Behav. 2012, 100, 850–854. [Google Scholar] [CrossRef]
- Guimaraes, I.M.; Carvalho, T.G.; Ferguson, S.S.; Pereira, G.S.; Ribeiro, F.M. The metabotropic glutamate receptor 5 role on motor behavior involves specific neural substrates. Mol. Brain 2015, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Gécz, J. Glutamate receptors and learning and memory. Nat. Genet. 2010, 42, 925–926. [Google Scholar] [CrossRef] [PubMed]
- Zoicas, I.; Kornhuber, J. The role of metabotropic glutamate receptors in social behavior in rodents. Int. J. Mol. Sci. 2019, 20, 1412. [Google Scholar] [CrossRef]
- Tomé, D. The roles of dietary glutamate in the intestine. Ann. Nutr. Metab. 2018, 73 (Suppl. S5), 15–20. [Google Scholar] [CrossRef] [PubMed]
- Kirchgessner, A.L. Glutamate in the enteric nervous system. Curr. Opin. Pharmacol. 2001, 1, 591–596. [Google Scholar] [CrossRef]
- Montanari, M.; Martella, G.; Bonsi, P.; Meringolo, M. Autism Spectrum Disorder: Focus on Glutamatergic Neurotransmission. Int. J. Mol. Sci. 2022, 23, 3861. [Google Scholar] [CrossRef]
- Verma, M.; Wills, Z.; Chu, C.T. Excitatory Dendritic Mitochondrial Calcium Toxicity: Implications for Parkinson’s and Other Neurodegenerative Diseases. Front. Neurosci. 2018, 12, 523. [Google Scholar] [CrossRef]
- Warby, S.C.; Doty, C.N.; Graham, R.K.; Carroll, J.B.; Yang, Y.-Z.; Singaraja, R.R.; Overall, C.M.; Hayden, M.R. Activated Caspase-6 and Caspase-6-Cleaved Fragments of Huntingtin Specifically Colocalize in the Nucleus. Hum. Mol. Genet. 2008, 17, 2390–2404. [Google Scholar] [CrossRef]
- El-Ansary, A. GABA and Glutamate Imbalance in Autism and Their Reversal as Novel Hypothesis for Effective Treatment Strategy. Autizm i narusheniya razvitiya. Autism Dev. Disord. 2020, 18, 46–63. [Google Scholar] [CrossRef]
- Hawkins, R.A. The blood-brain barrier and glutamate. Am. J. Clin. Nutr. 2009, 90, 867S–874S. [Google Scholar] [CrossRef]
- Hawkins, R.A.; Viña, J.R. How Glutamate Is Managed by the Blood-Brain Barrier. Biology 2016, 5, 37. [Google Scholar] [CrossRef] [PubMed]
- Moussawi, K.; Riegel, A.; Nair, S.; Kalivas, P.W. Extracellular glutamate: Functional compartments operate in different concentration ranges. Front. Syst. Neurosci. 2011, 5, 94. [Google Scholar] [CrossRef] [PubMed]
- Ghanizadeh, A. Increased glutamate and homocysteine and decreased glutamine levels in autism: A review and strategies for future studies of amino acids in autism. Dis. Markers 2013, 35, 281–286. [Google Scholar] [CrossRef] [PubMed]
- El-Ansary, A.; Al-Ayadhi, L. GABAergic/glutamatergic imbalance relative to excessive neuroinflammation in autism spectrum disorders. J. Neuroinflamm. 2014, 11, 189. [Google Scholar] [CrossRef]
- Cai, J.; Ding, L.; Zhang, J.-S.; Xue, J.; Wang, L.-Z. Elevated plasma levels of glutamate in children with autism spectrum disorders. NeuroReport 2016, 27, 272–276. [Google Scholar] [CrossRef]
- Zaki, M.M.; Abdel-Al, H.; Al-Sawi, M. Assessment of plasma amino acid profile in autism using cation-exchange chromatography with postcolumn derivatization by ninhydrin. Turk. J. Med. Sci. 2017, 47, 260–267. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Gruenbaum, B.F.; Zlotnik, A.; Frenkel, A.; Fleidervish, I.; Boyko, M. Glutamate Efflux across the Blood-Brain Barrier: New Perspectives on the Relationship between Depression and the Glutamatergic System. Metabolites 2022, 12, 459. [Google Scholar] [CrossRef]
- Choi, D.W. Excitotoxicity: Still Hammering the Ischemic Brain in 2020. Front. Neurosci. 2020, 14, 579953. [Google Scholar] [CrossRef]
- Hollander, E.; Uzunova, G. Are there new advances in the pharmacotherapy of autism spectrum disorders? World Psychiatry 2017, 16, 101–102. [Google Scholar] [CrossRef] [PubMed]
- Mehta, M.V.; Gandal, M.J.; Siegel, S.J. mGluR5-antagonist mediated reversal of elevated stereotyped, repetitive behaviors in the VPA model of autism. PLoS ONE 2011, 6, e26077. [Google Scholar] [CrossRef] [PubMed]
- Nisar, S.; Bhat, A.A.; Masoodi, T.; Hashem, S.; Akhtar, S.; Ali, T.A.; Amjad, S.; Chawla, S.; Bagga, P.; Frenneaux, M.P.; et al. Genetics of glutamate and its receptors in autism spectrum disorder. Mol. Psychiatry 2022, 27, 2380–2392. [Google Scholar] [CrossRef]
- Kim, Y.S.; Choi, J.; Yoon, B.E. Neuron-glia interactions in neurodevelopmental disorders. Cells 2020, 9, 2176. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Meguid, N.A.; El-Bana, M.A.; Tinkov, A.A.; Saad, K.; Dadar, M.; Hemimi, M.; Skalny, A.V.; Hosnedlová, B.; Kizek, R.; et al. Oxidative Stress in Autism Spectrum Disorder. Mol. Neurobiol. 2020, 57, 2314–2332. [Google Scholar] [CrossRef]
- Chauhan, A.; Chauhan, V. Oxidative stress in autism. Pathophysiology 2006, 13, 171–181. [Google Scholar] [CrossRef]
- Morimoto, M.; Hashimoto, T.; Tsuda, Y.; Nakatsu, T.; Kitaoka, T.; Kyotani, S. Assessment of oxidative stress in autism spectrum disorder using reactive oxygen metabolites and biological antioxidant potential. PLoS ONE 2020, 15, e0233550. [Google Scholar] [CrossRef]
- Wen, Y.; Yao, Y. Autism Spectrum Disorders: The Mitochondria Connection; Grabrucker, A.M., Ed.; Exon Publications: Brisbane, AU, USA, 2021; Chapter 7. Available online: https://www.ncbi.nlm.nih.gov/books/NBK573616/ (accessed on 25 June 2023).
- Frye, R.E.; Lionnard, L.; Singh, I.; Karim, M.A.; Chajra, H.; Frechet, M.; Kissa, K.; Racine, V.; Ammanamanchi, A.; McCarty, P.J.; et al. Mitochondrial morphology is associated with respiratory chain uncoupling in autism spectrum disorder. Transl. Psychiatry 2021, 11, 527. [Google Scholar] [CrossRef]
- Rossignol, D.A.; Frye, R.E. Mitochondrial dysfunction in autism spectrum disorders: A systematic review and meta-analysis. Mol. Psychiatry 2012, 17, 290–314. [Google Scholar] [CrossRef]
- Cheng, N.; Rho, J.M.; Masino, S.A. Metabolic Dysfunction Underlying Autism Spectrum Disorder and Potential Treatment Approaches. Front. Mol. Neurosci. 2017, 10, 34. [Google Scholar] [CrossRef]
- Chauhan, A.; Gu, F.; Essa, M.M.; Wegiel, J.; Kaur, K.; Brown, W.T.; Chauhan, V. Brain region-specific deficit in mitochondrial electron transport chain complexes in children with autism. J. Neurochem. 2011, 117, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Usui, N.; Kobayashi, H.; Shimada, S. Neuroinflammation and Oxidative Stress in the Pathogenesis of Autism Spectrum Disorder. Int. J. Mol. Sci. 2023, 24, 5487. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Tsilioni, I.; Patel, A.B.; Doyle, R. Atopic diseases and inflammation of the brain in the pathogenesis of autism spectrum disorders. Transl. Psychiatry 2016, 6, e844. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Asadi, S.; Patel, A.B. Focal brain inflammation and autism. J. Neuroinflammation 2013, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Kordulewska, N.K.; Kostyra, E.; Piskorz-Ogórek, K.; Moszyńska, M.; Cieślińska, A.; Fiedorowicz, E.; Jarmołowska, B. Serum cytokine levels in children with spectrum autism disorder: Differences in pro- and anti-inflammatory balance. J. Neuroimmunol. 2019, 337, 577066. [Google Scholar] [CrossRef] [PubMed]
- Ashwood, P.; Krakowiak, P.; Hertz-Picciotto, I.; Hansen, R.; Pessah, I.; Van de Water, J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav. Immun. 2011, 25, 40–45. [Google Scholar] [CrossRef]
- Krakowiak, P.; Goines, P.E.; Tancredi, D.J.; Ashwood, P.; Hansen, R.L.; Hertz-Picciotto, I.; Van de Water, J. Neonatal Cytokine Profiles Associated with Autism Spectrum Disorder. Biol. Psychiatry 2017, 81, 442–451. [Google Scholar] [CrossRef]
- Sidenkova, A. Mitochondrial disorders and ASD. Mechanisms of mitochondrial dysfunction in ASD. Eur. Psychiatry 2023, 66, S100–S101. [Google Scholar] [CrossRef]
- Kushnireva, L.; Korkotian, E. Mitochondria-Endoplasmic Reticulum Interaction in Central Neurons. In Updates on Endoplasmic Reticulum; IntechOpen: London, UK, 2022. [Google Scholar]
- Cowan, K.; Anichtchik, O.; Luo, S. Mitochondrial integrity in neurodegeneration. CNS Neurosci. Ther. 2019, 25, 825–836. [Google Scholar] [CrossRef]
- Korkotian, E.; Meshcheriakova, A.; Segal, M. Presenilin 1 regulates [Ca2+]i and mitochondria/ER interaction in cultured rat hippocampal neurons. Oxidative Med. Cell. Longev. 2019, 2019, 7284967. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, S.; Gharagozloo, M.; Simard, C.; Gris, D. Astrocytes maintain glutamate homeostasis in the CNS by controlling the balance between glutamate uptake and release. Cells 2019, 8, 184. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A.R.; Binder, D.K. Astrocyte glutamate uptake and signaling as novel targets for antiepileptogenic therapy. Front. Neurol. 2020, 11, 1006. [Google Scholar] [CrossRef] [PubMed]
- Bantle, C.M.; Hirst, W.D.; Weihofen, A.; Shlevkov, E. Mitochondrial dysfunction in astrocytes: A role in Parkinson’s disease? Front. Cell Dev. Biol. 2021, 8, 608026. [Google Scholar] [CrossRef]
- Guo, R.; Gu, J.; Zong, S.; Wu, M.; Yang, M. Structure and mechanism of mitochondrial electron transport chain. Biomed. J. 2018, 41, 9–20. [Google Scholar] [CrossRef]
- Frye, R.E. Mitochondrial Dysfunction in Autism Spectrum Disorder: Unique Abnormalities and Targeted Treatments. Semin. Pediatr. Neurol. 2020, 35, 100829. [Google Scholar] [CrossRef]
- Mahalaxmi, I.; Subramaniam, M.D.; Gopalakrishnan, A.V.; Vellingiri, B. Dysfunction in Mitochondrial Electron Transport Chain Complex I, Pyruvate Dehydrogenase Activity, and Mutations in ND1 and ND4 Gene in Autism Spectrum Disorder Subjects from Tamil Nadu Population, India. Mol. Neurobiol. 2021, 58, 5303–5311. [Google Scholar] [CrossRef]
- Singh, K.; Singh, I.N.; Diggins, E.; Connors, S.L.; Karim, M.A.; Lee, D.; Zimmerman, A.W.; Frye, R.E. Developmental regression and mitochondrial function in children with autism. Ann. Clin. Transl. Neurol. 2020, 7, 683–694. [Google Scholar] [CrossRef]
- Levine, B.; Kroemer, G. Biological Functions of Autophagy Genes: A Disease Perspective. Cell 2019, 176, 11–42. [Google Scholar] [CrossRef]
- Nikoletopoulou, V.; Tavernarakis, N. Regulation and Roles of Autophagy at Synapses. Trends Cell Biol. 2018, 28, 646–661. [Google Scholar] [CrossRef]
- Tomoda, T.; Yang, K.; Sawa, A. Neuronal Autophagy in Synaptic Functions and Psychiatric Disorders. Biol. Psychiatry 2020, 87, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-Y.; Yan, J.; Zukin, R.S. Autophagy and Synaptic Plasticity: Epigenetic Regulation. Curr. Opin. Neurobiol. 2019, 59, 207–212. [Google Scholar] [CrossRef]
- Deng, Z.; Zhou, X.; Lu, J.-H.; Yue, Z. Autophagy Deficiency in Neurodevelopmental Disorders. Cell Biosci. 2021, 11, 214. [Google Scholar] [CrossRef] [PubMed]
- Gianchecchi, E.; Delfino, D.V.; Fierabracci, A. Recent Insights on the Putative Role of Autophagy in Autoimmune Diseases. Autoimmun. Rev. 2014, 13, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.; Ammitzboell, M.; Nys, K.; Seidelin, J.B.; Nielsen, O.H. ATG16L1: A Multifunctional Susceptibility Factor in Crohn Disease. Autophagy 2015, 11, 585–594. [Google Scholar] [CrossRef]
- Foerster, E.G.; Mukherjee, T.; Cabral-Fernandes, L.; Rocha, J.D.B.; Girardin, S.E.; Philpott, D.J. How Autophagy Controls the Intestinal Epithelial Barrier. Autophagy 2022, 18, 86–103. [Google Scholar] [CrossRef]
- Chernikova, M.A.; Flores, G.D.; Kilroy, E.; Labus, J.S.; Mayer, E.A.; Aziz-Zadeh, L. The Brain-Gut-Microbiome System: Pathways and Implications for Autism Spectrum Disorder. Nutrients 2021, 13, 4497. [Google Scholar] [CrossRef]
- Mitrea, L.; Nemeş, S.-A.; Szabo, K.; Teleky, B.-E.; Vodnar, D.-C. Guts Imbalance Imbalances the Brain: A Review of Gut Microbiota Association with Neurological and Psychiatric Disorders. Front. Med. 2022, 9, 813204. [Google Scholar] [CrossRef]
- Saeed, N.K.; Al-Beltagi, M.; Bediwy, A.S.; El-Sawaf, Y.; Toema, O. Gut Microbiota in Various Childhood Disorders: Implication and Indications. World J. Gastroenterol. 2022, 28, 1875–1901. [Google Scholar] [CrossRef]
- Santocchi, E.; Guiducci, L.; Fulceri, F.; Billeci, L.; Buzzigoli, E.; Apicella, F.; Calderoni, S.; Grossi, E.; Morales, M.A.; Muratori, F. Gut to brain interaction in Autism Spectrum Disorders: A randomized controlled trial on the role of probiotics on clinical, biochemical and neurophysiological parameters. BMC Psychiatry 2016, 16, 183. [Google Scholar] [CrossRef]
- Morton, J.T.; Jin, D.M.; Mills, R.H.; Shao, Y.; Rahman, G.; McDonald, D.; Zhu, Q.; Balaban, M.; Jiang, Y.; Cantrell, K.; et al. Multi-level analysis of the gut-brain axis shows autism spectrum disorder-associated molecular and microbial profiles. Nat. Neurosci. 2023, 26, 1208–1217. [Google Scholar] [CrossRef]
- Karimi, P.; Kamali, E.; Mousavi, S.M.; Karahmadi, M. Environmental factors influencing the risk of autism. J. Res. Med. Sci. 2017, 22, 27. [Google Scholar] [PubMed]
- Kohane, I.S.; McMurry, A.; Weber, G.; MacFadden, D.; Rappaport, L.; Kunkel, L.; Bickel, J.; Wattanasin, N.; Spence, S.; Murphy, S.; et al. The co-morbidity burden of children and young adults with autism spectrum disorders. PLoS ONE 2012, 7, e33224. [Google Scholar] [CrossRef] [PubMed]
- Wasilewska, J.; Klukowski, M. Gastrointestinal symptoms and autism spectrum disorder: Links and risks–a possible new overlap syndrome. Pediatr. Health Med. Ther. 2015, 6, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, E.Y. Gastrointestinal issues in autism spectrum disorder. Harv. Rev. Psychiatry 2014, 22, 104–111. [Google Scholar] [CrossRef]
- Strati, F.; Cavalieri, D.; Albanese, D.; De Felice, C.; Donati, C.; Hayek, J.; Jousson, O.; Leoncini, S.; Renzi, D.; Calabrò, A.; et al. New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome 2017, 5, 24. [Google Scholar] [CrossRef]
- Sgritta, M.; Dooling, S.W.; Buffington, S.A.; Momin, E.N.; Francis, M.B.; Britton, R.A.; Costa-Mattioli, M. Mechanisms underlying microbial-mediated changes in social behavior in mouse models of autism spectrum disorder. Neuron 2019, 101, 246–259. [Google Scholar] [CrossRef]
- Adams, J.B.; Johansen, L.J.; Powell, L.D.; Quig, D.; Rubin, R.A. Gastrointestinal flora and gastrointestinal status in children with autism–comparisons to typical children and correlation with autism severity. BMC Gastroenterol. 2011, 11, 22. [Google Scholar] [CrossRef]
- Song, Y.; Liu, C.; Finegold, S.M. Real-time PCR quantitation of clostridia in feces of autistic children. Appl. Environ. Microbiol. 2004, 70, 6459–6465. [Google Scholar] [CrossRef]
- Kang, D.W.; Adams, J.B.; Coleman, D.M.; Pollard, E.L.; Maldonado, J.; McDonough-Means, S.; Caporaso, J.G.; Krajmalnik-Brown, R. Long-term benefit of Microbiota Transfer Therapy on autism symptoms and gut microbiota. Sci. Rep. 2019, 9, 5821. [Google Scholar] [CrossRef]
- Kang, D.-W.; Adams, J.B.; Gregory, A.C.; Borody, T.; Chittick, L.; Fasano, A.; Khoruts, A.; Geis, E.; Maldonado, J.; McDonough-Means, S.; et al. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: An open-label study. Microbiome 2017, 5, 10. [Google Scholar] [CrossRef]
- Santocchi, E.; Guiducci, L.; Prosperi, M.; Calderoni, S.; Gaggini, M.; Apicella, F.; Tancredi, R.; Billeci, L.; Mastromarino, P.; Grossi, E.; et al. Effects of probiotic supplementation on gastrointestinal, sensory and core symptoms in autism spectrum disorders: A randomized controlled trial. Front. Psychiatry 2020, 11, 550593. [Google Scholar] [CrossRef]
- Pratico, D.; Clark, C.M.; Liun, F.; Rokach, J.; Lee, V.Y.; Trojanowski, J.Q. Increase of brain oxidative stress in mild cognitive impairment: A possible predictor of Alzheimer disease. Arch. Neurol. 2002, 59, 972–976. [Google Scholar] [CrossRef]
- Torres, L.L.; Quaglio, N.B.; de Souza, G.T.; Garcia, R.T.; Dati, L.M.; Moreira, W.L.; Loureiro, A.P.; de Souza-Talarico, J.N.; Smid, J.; Porto, C.S.; et al. Peripheral oxidative stress biomarkers in mild cognitive impairment and Alzheimer’s disease. J. Alzheimer’s Dis. 2011, 26, 59–68. [Google Scholar] [CrossRef]
- Cai, Z.; Zhao, B.; Ratka, A. Oxidative stress and beta-amyloid protein in Alzheimer’s disease. Neuromol. Med. 2011, 13, 223–250. [Google Scholar] [CrossRef] [PubMed]
- Marangoni, F.; Colombo, C.; Martiello, A.; Poli, A.; Paoletti, R.; Galli, C. Levels of the n-3 fatty acid eicosapentaenoic acid in addition to those of alpha linolenic acid are significantly raised in blood lipids by the intake of four walnuts a day in humans. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Chung, S.H. Anti-inflammatory effect of alpha-linolenic acid and its mode of action through the inhibition of nitric oxide production and inducible nitric oxide synthase gene expression via NF-kappaB and mitogen-activated protein kinase pathways. J. Agric. Food Chem. 2007, 55, 5073–5080. [Google Scholar] [CrossRef] [PubMed]
- Erdinest, N.; Shmueli, O.; Grossman, Y.; Ovadia, H.; Solomon, A. Anti-inflammatory effects of alpha linolenic acid on human corneal epithelial cells. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4396–4406. [Google Scholar] [CrossRef] [PubMed]
- Reifen, R.; Karlinsky, A.; Stark, A.H.; Berkovich, Z.; Nyska, A. Alpha-Linolenic acid (ALA) is an anti-inflammatory agent in inflammatory bowel disease. J. Nutr. Biochem. 2015, 26, 1632–1640. [Google Scholar] [CrossRef]
- Chauhan, A.; Chauhan, V. Potential Beneficial Effects of a Diet with Walnuts in Aging and Alzheimer’s Disease; Thakur, M.K., Rattan, S.I.S., Eds.; Brain Aging and Therapeutic Interventions; Springer: Dordrecht, The Netherland, 2012; pp. 239–252. [Google Scholar]
- Julvez, J.; Gignac, F.; Fernández-Barrés, S.; Romaguera, D.; Sala-Vila, A.; Ranzani, O.T.; Persavento, C.; Delgado, A.; Carol, A.; Torrent, J.; et al. Walnuts, Long-Chain Polyunsaturated Fatty Acids, and Adolescent Brain Development: Protocol for the Walnuts Smart Snack Dietary Intervention Trial. Front. Pediatr. 2021, 9, 593847. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.J.; Liu, S.C.; Chen, Y.C.; Hsu, S.H.; Chang, Y.P.; Lin, J.T. Three pathways assess anti-inflammatory response of epicatechin with lipopolysaccharide-mediated macrophage RAW 264.7 Cells. J. Food Biochem. 2015, 39, 334–343. [Google Scholar] [CrossRef]
- Carullo, G.; Cappello, A.R.; Frattaruolo, L.; Badolato, M.; Armentano, B.; Aiello, F. Quercetin and derivatives: Useful tools in inflammation and pain management. Future Med. Chem. 2017, 9, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Lin, W.; Deng, X.; Ba, X.; Han, L.; Chen, Z.; Qin, K.; Huang, Y.; Tu, S. Potential implications of quercetin in autoimmune diseases. Front. Immunol. 2021, 12, 689044. [Google Scholar] [CrossRef] [PubMed]
- Okoko, T.; Oruambo, I.F. Inhibitory activity of quercetin and its metabolite on lipopolysaccharide-induced activation of macrophage U937 cells. Food Chem. Toxicol. 2009, 47, 809–812. [Google Scholar] [CrossRef]
- Yui, K.; Sato, A.; Imataka, G. Mitochondrial Dysfunction and Its Relationship with mTOR Signaling and Oxidative Damage in Autism Spectrum Disorders. Mini Rev. Med. Chem. 2015, 15, 373–389. [Google Scholar] [CrossRef]
- Auerbach, B.D.; Osterweil, E.K.; Bear, M.F. Mutations causing syndromic autism define an axis of synaptic pathophysiology. Nature 2011, 480, 63–68. [Google Scholar] [CrossRef]
- Sato, A. mTOR, a potential target to treat autism spectrum disorder. CNS Neurological Disord. Drug Targets. Former. Curr. Drug Targets-CNS Neurol. Disord. 2016, 15, 533–543. [Google Scholar] [CrossRef]
- Russo, C.; Nastro, A.; Cicala, D.; De Liso, M.; Covelli, E.M.; Cinalli, G. Neuroimaging in tuberous sclerosis complex. Child’s Nerv. Syst. 2020, 36, 2497–2509. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, J.; Lu, H.; Fang, L.; Qin, H.; Liu, C.; Min, W. Neuroprotection by walnut-derived peptides through autophagy promotion via Akt/mTOR signaling pathway against oxidative stress in PC12 cells. J. Agric. Food Chem. 2020, 68, 3638–3648. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, M.; Lin, L.; Wang, J.; Sun-Waterhouse, D.; Dong, Y.; Su, G. Identification of antioxidative peptides from defatted walnut meal hydrolysate with potential for improving learning and memory. Food Res. Int. 2015, 78, 216–223. [Google Scholar] [CrossRef]
- Gu, M.U.; Chen, H.P.; Zhao, M.M.; Wang, X.I.; Yang, B.; Ren, J.Y.; Su, G.W. Identification of antioxidant peptides released from defatted walnut (Juglans Sigillata Dode) meal proteins with pancreatin. LWT-Food Sci. Technol. 2015, 60, 213–220. [Google Scholar] [CrossRef]
- Liu, C.; Guo, Y.; Zhao, F.; Qin, H.; Lu, H.; Fang, L.; Wang, J.; Min, W. Potential mechanisms mediating the protective effects of a peptide from walnut (Juglans mandshurica Maxim.) against hydrogen peroxide induced neurotoxicity in PC12 cells. Food Funct. 2019, 10, 3491–3501. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zheng, L.; Zhao, T.; Zhang, Q.; Liu, Y.; Sun, B.; Su, G.; Zhao, M. Inhibitory effects of walnut (Juglans regia) peptides on neuroinflammation and oxidative stress in lipopolysaccharide-induced cognitive impairment mice. J. Agric. Food Chem. 2020, 68, 2381–2392. [Google Scholar] [CrossRef]
- Li, Q.; Shi, C.; Wang, M.; Zhou, M.; Liang, M.; Zhang, T.; Yuan, E.; Wang, Z.; Yao, M.; Ren, J. Tryptophan residue enhances in vitro walnut protein-derived peptides exerting xanthine oxidase inhibition and antioxidant activities. J. Funct. Foods 2019, 53, 276–285. [Google Scholar] [CrossRef]
- Yang, Z.; Amrit, B.K.; Zhao, W.; Shi, L.; Wu, H.; Barrow, C.; Dunshea, F.; Hafiz, A.R. Suleria, Bioaccessibility and bioavailability changes of phenolic compounds in pumpkins (Cucurbita moschata): A review. Food Biosci. 2022, 47, 101753. [Google Scholar] [CrossRef]
- Umadevi, P.; Murugan, S.; Jennifer Suganthi, S.; Subakanmani, S. Evaluation of antidepressant like activity of Cucurbita pepo seed extracts in rats. Int. J. Curr. Pharm. Res. 2011, 3, 108–113. [Google Scholar]
- Botella, M.B.; González, R.E.; Minguillón, C.; Della Gaspera, P.G.; Wuilloud, R.G.; Quintas, P.Y. Direct determination of tyrosine and tryptophane enantiomers in pumpkin (Cucurbita moschata) by HPLC-UV/Vis: Effect of cooking treatment on enantiomers profile. J. Food Compos. Anal. 2023, 122, 105469. [Google Scholar] [CrossRef]
- Muller, C.L.; Anacker, A.M.J.; Veenstra-VanderWeele, J. The serotonin system in autism spectrum disorder: From biomarker to animal models. Neuroscience 2016, 321, 24–41. [Google Scholar] [CrossRef]
- Pagan, C.; Goubran-Botros, H.; Delorme, R.; Benabou, M.; Lemière, N.; Murray, K.; Amsellem, F.; Callebert, J.; Chaste, P.; Jamain, S.; et al. Disruption of melatonin synthesis is associated with impaired 14-3-3 and miR-451 levels in patients with autism spectrum disorders. Sci. Rep. 2017, 7, 2096. [Google Scholar] [CrossRef]
- Maes, M.; Anderson, G.; Betancort Medina, S.R.; Seo, M.; Ojala, J.O. Integrating Autism Spectrum Disorder Pathophysiology: Mitochondria, Vitamin A, CD38, Oxytocin, Serotonin and Melatonergic Alterations in the Placenta and Gut. Curr. Pharm. Des. 2019, 25, 4405–4420. [Google Scholar] [CrossRef]
- Gil-Zamorano, J.; Cofán, M.; López de Las Hazas, M.C.; García-Blanco, T.; García-Ruiz, A.; Doménech, M.; Serra-Mir, M.; Roth, I.; Valls-Pedret, C.; Rajaram, S.; et al. Interplay of Walnut Consumption, Changes in Circulating miRNAs and Reduction in LDL-Cholesterol in Elders. Nutrients 2022, 14, 1473. [Google Scholar] [CrossRef]
- Lambertini, E.; Penolazzi, L.; Notarangelo, M.P.; Fiorito, S.; Epifano, F.; Pandolfi, A.; Piva, R. Pro-differentiating compounds for human intervertebral disc cells are present in Violina pumpkin leaf extracts. Int. J. Mol. Med. 2023, 51, 39. [Google Scholar] [CrossRef]
- Alonso-Garrido, M.; Pallarés, N.; Font, G.; Tedeschi, P.; Manyes, L.; Lozano, M. The role of pumpkin pulp extract carotenoids against mycotoxin damage in the blood brain barrier in vitro. Arch. Ind. Hyg. Toxicol. 2021, 72, 173–181. [Google Scholar] [CrossRef]
- Balgoon, M.J.; Al-Zahrani, M.H.; Jaouni, S.A.; Ayuob, N. Combined Oral and Topical Application of Pumpkin (Cucurbita pepo L.) Alleviates Contact Dermatitis Associated with Depression through Downregulation Pro-Inflammatory Cytokines. Front. Pharmacol. 2021, 12, 663417. [Google Scholar] [CrossRef] [PubMed]
- Wibrand, K.; Berge, K.; Messaoudi, M.; Duffaud, A.; Panja, D.; Bramham, C.R.; Burri, L. Enhanced cognitive function and antidepressant-like effects after krill oil supplementation in rats. Lipids Health Dis. 2013, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Wang, X.; Xiang, Q.; Meng, X.; Peng, Y.; Du, N.; Liu, Z.; Sun, Q.; Wang, C.; Liu, X. Astaxanthin alleviates brain aging in rats by attenuating oxidative stress and increasing BDNF levels. Food Funct. 2014, 5, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Yook, J.S.; Okamoto, M.; Rakwal, R.; Shibato, J.; Lee, M.C.; Matsui, T.; Chang, H.; Chang, J.Y.; Soya, H. Astaxanthin supplementation enhances adult hippocampal neurogenesis and spatial memory in mice. Mol. Nutr. Food Res. 2016, 60, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Al-Amin, M.M.; Rahman, M.M.; Khan, F.R.; Zaman, F.; Mahmud Reza, H. Astaxanthin improves behavioral disorder and oxidative stress in prenatal valproic acid-induced mice model of autism. Behav. Brain Res. 2015, 286, 112–121. [Google Scholar] [CrossRef]
- Kawakami, Z.; Kanno, H.; Ikarashi, Y.; Kase, Y. Yokukansan, a kampo medicine, protects against glutamate cytotoxicity due to oxidative stress in PC12 cells. J. Ethnopharmacol. 2011, 134, 74–81. [Google Scholar] [CrossRef]
- Ma, S.; Liu, H.; Jiao, H.; Wang, L.; Chen, L.; Liang, J.; Zhao, M.; Zhang, X. Neuroprotective effect of ginkgolide K on glutamate-induced cytotoxicity in PC 12 cells via inhibition of ROS generation and Ca2+ influx. NeuroToxicology 2012, 33, 59–69. [Google Scholar] [CrossRef]
- Cassano, T.; Pace, L.; Bedse, G.; Lavecchia, A.M.; De Marco, F.; Gaetani, S.; Serviddio, G. Glutamate and Mitochondria: Two Prominent Players in the Oxidative Stress-Induced Neurodegeneration. Curr. Alzheimer Res. 2016, 13, 185–197. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J.; John, A.; Jiang, Y.; Zhu, H.; Yang, B.; Wen, L. Structure identification of walnut peptides and evaluation of cellular antioxidant activity. Food Chem. 2022, 388, 132943. [Google Scholar] [CrossRef] [PubMed]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Kassab, A.A.; Moustafa, K.A.; Abd-El-Hafez, A.A. The possible protective role of pumpkin seed oil in ameliorating tongue mucosal damage induced by orlistat in adult male albino rats: A light and scanning electron microscopic study. Egypt. J. Histol. 2020, 43, 975–987. [Google Scholar] [CrossRef]
- Wu, H.; Niu, H.; Shao, A.; Wu, C.; Dixon, B.J.; Zhang, J.; Yang, S.; Wang, Y. Astaxanthin as a potential neuroprotective agent for neurological diseases. Mar. Drugs 2015, 13, 5750–5766. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, H. Inhibitory effect of astaxanthin on oxidative stress-induced mitochondrial dysfunction-a mini-review. Nutrients 2018, 10, 1137. [Google Scholar] [CrossRef] [PubMed]
- Baburina, Y.; Krestinin, R.; Odinokova, I.; Sotnikova, L.; Kruglov, A.; Krestinina, O. Astaxanthin inhibits mitochondrial permeability transition pore opening in rat heart mitochondria. Antioxidants 2019, 8, 576. [Google Scholar] [CrossRef]
- Brasil, F.B.; de Almeida FJ, S.; Luckachaki, M.D.; Dall’Oglio, E.L.; de Oliveira, M.R. Astaxanthin prevents mitochondrial impairment in the dopaminergic SH-SY5Y cell line exposed to glutamate-mediated excitotoxicity: Role for the Nrf2/HO-1/CO-BR axis. Eur. J. Pharmacol. 2021, 908, 174336. [Google Scholar] [CrossRef]
- Taheri, F.; Sattari, E.; Hormozi, M.; Ahmadvand, H.; Bigdeli, M.R.; Kordestani-Moghadam, P.; Moghaddasi, M. Dose-dependent effects of astaxanthin on ischemia/reperfusion induced brain injury in mcao model rat. Neurochem. Res. 2022, 47, 1736–1750. [Google Scholar] [CrossRef]
- García, F.; Lobos, P.; Ponce, A.; Cataldo, K.; Meza, D.; Farías, P.; Estay, C.; Oyarzun-Ampuero, F.; Herrera-Molina, R.; Paula-Lima, A.; et al. Astaxanthin counteracts excitotoxicity and reduces the ensuing increases in calcium levels and mitochondrial reactive oxygen species generation. Mar. Drugs 2020, 18, 335. [Google Scholar] [CrossRef]
- Kandy, S.K.; Nimonkar, M.M.; Dash, S.S.; Mehta, B.; Markandeya, Y.S. Astaxanthin Protection against Neuronal Excitotoxicity via Glutamate Receptor Inhibition and Improvement of Mitochondrial Function. Mar. Drugs 2022, 20, 645. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.Y.; Lu, C.W.; Wang, S.J. Astaxanthin inhibits glutamate release in rat cerebral cortex nerve terminals via suppression of voltage-dependent Ca2+ entry and mitogen-activated protein kinase signaling pathway. J. Agric. Food Chem. 2010, 58, 8271–8278. [Google Scholar] [CrossRef] [PubMed]
- Altunrende, M.E.; Gezen-Ak, D.; Atasoy, I.L.; Candaş, E.; Dursun, E. The role of astaxanthin on transcriptional regulation of nmda receptors voltage sensitive calcium channels and calcium binding proteins in primary cortical neurons. Arch. Neuropsychiatry 2018, 55, 295. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.W.; Hung, C.F.; Jean, W.H.; Lin, T.Y.; Huang, S.K.; Wang, S.J. Lycopene depresses glutamate release through inhibition of voltage-dependent Ca2+ entry and protein kinase C in rat cerebrocortical nerve terminals. Can. J. Physiol. Pharmacol. 2018, 96, 479–484. [Google Scholar] [CrossRef]
- Murase, T.; Misawa, K.; Minegishi, Y.; Aoki, M.; Ominami, H.; Suzuki, Y.; Shibuya, Y.; Hase, T. Coffee polyphenols suppress diet-induced body fat accumulation by down-regulating SREBP-1c and related molecules in C57BL/6J mice. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E122–E133. [Google Scholar] [CrossRef]
- Muthaiyah, B.; Essa, M.M.; Chauhan, V.; Chauhan, A. Protective effects of walnut extract against amyloid beta peptide-induced cell death and oxidative stress in PC12 cells. Neurochem. Res. 2011, 36, 2096–2103. [Google Scholar] [CrossRef]
- Nagel, J.M.; Brinkoetter, M.; Magkos, F.; Liu, X.; Chamberland, J.P.; Shah, S.; Zhou, Z.; Blackburn, G.; Mantzoros, C.S. Dietary walnuts inhibit colorectal cancer growth in mice by suppressing angiogenesis. Nutrition 2012, 28, 67–75. [Google Scholar] [CrossRef]
- Naiki-Ito, A.; Chewonarin, T.; Tang, M.; Pitchakarn, P.; Kuno, T.; Ogawa, K.; Asamoto, M.; Shirai, T.; Takahashi, S. Ellagic acid, a component of pomegranate fruit juice, suppresses androgen-dependent prostate carcinogenesis via induction of apoptosis. Prostate 2015, 75, 151–160. [Google Scholar] [CrossRef]
- Strati, A.; Papoutsi, Z.; Lianidou, E.; Moutsatsou, P. Effect of ellagic acid on the expression of human telomerase reverse transcriptase (hTERT) alpha+beta+ transcript in estrogen receptor-positive MCF-7 breast cancer cells. Clin. Biochem. 2009, 42, 1358–1362. [Google Scholar] [CrossRef]
- Sudheer, A.R.; Muthukumaran, S.; Devipriya, N.; Menon, V.P. Ellagic acid, a natural polyphenol protects rat peripheral blood lymphocytes against nicotine-induced cellular and DNA damage in vitro: With the comparison of N-acetylcysteine. Toxicology 2007, 230, 11–21. [Google Scholar] [CrossRef]
- Taghi Mansouri, M.; Naghizadeh, B.; Ghorbanzadeh, B.; Farbood, Y. Central and peripheral antinociceptive effects of ellagic acid in different animal models of pain. Eur. J. Pharmacol. 2013, 707, 46–53. [Google Scholar] [CrossRef]
- Jayatunga, D.P.W.; Hone, E.; Khaira, H.; Lunelli, T.; Singh, H.; Guillemin, G.J.; Fernando, B.; Garg, M.L.; Verdile, G.; Martins, R.N. Therapeutic Potential of Mitophagy-Inducing Microflora Metabolite, Urolithin A for Alzheimer’s Disease. Nutrients 2021, 13, 3744. [Google Scholar] [CrossRef]
- Wojciechowska, O.; Kujawska, M. Urolithin A in Health and Diseases: Prospects for Parkinson’s Disease Management. Antioxidants 2023, 12, 1479. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.D.; Zhao, Z.; Montagne, A.; Nelson, A.R.; Zlokovic, B.V. Blood-Brain Barrier: From Physiology to Disease and Back. Physiol Rev. 2019, 99, 21–78. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.S.; Rai, S.N.; Birla, H.; Zahra, W.; Rathore, A.S.; Dilnashin, H.; Singh, R.; Singh, S.P. Neuroprotective Effect of Chlorogenic Acid on Mitochondrial Dysfunction-Mediated Apoptotic Death of DA Neurons in a Parkinsonian Mouse Model. Oxid. Med. Cell. Longev. 2020, 2020, 6571484. [Google Scholar] [CrossRef] [PubMed]
- Bamberger, C.; Rossmeier, A.; Lechner, K.; Wu, L.; Waldmann, E.; Fischer, S.; Stark, R.G.; Altenhofer, J.; Henze, K.; Parhofer, K.G. A Walnut-Enriched Diet Affects Gut Microbiome in Healthy Caucasian Subjects: A Randomized, Controlled Trial. Nutrients 2018, 10, 244. [Google Scholar] [CrossRef]
- Byerley, L.O.; Samuelson, D.; Blanchard, E.; Luo, M.; Lorenzen, B.N.; Banks, S.; Ponder, M.A.; Welsh, D.A.; Taylor, C.M. Changes in the gut microbial communities following addition of walnuts to the diet. J. Nutr. Biochem. 2017, 48, 94–102. [Google Scholar] [CrossRef]
- Zhi, T.; Hong, D.; Zhang, Z.; Li, S.; Xia, J.; Wang, C.; Wu, Y.; Jia, Y.; Ma, A. Anti-inflammatory and gut microbiota regulatory effects of walnut protein derived peptide LPF in vivo. Food Res. Int. 2022, 152, 110875. [Google Scholar] [CrossRef]
- Miao, F.; Shan, C.; Shah SA, H.; Akhtar, R.W.; Wang, X.; Ning, D. Effect of walnut (Juglans sigillata) oil on intestinal antioxidant, anti-inflammatory, immunity, and gut microbiota modulation in mice. J. Food Biochem. 2021, 45, e13567. [Google Scholar] [CrossRef]
- Tan, B.; Wang, Y.; Zhang, X.; Sun, X. Recent Studies on Protective Effects of Walnuts against Neuroinflammation. Nutrients 2022, 14, 4360. [Google Scholar] [CrossRef]
- Liu, G.; Liang, L.; Yu, G.; Li, Q. Pumpkin polysaccharide modifies the gut microbiota during alleviation of type 2 diabetes in rats. Int. J. Biol. Macromol. 2018, 115, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.W.; Park, J.G.; Ilhan, Z.E.; Wallstrom, G.; Labaer, J.; Adams, J.B.; Krajmalnik-Brown, R. Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PLoS ONE 2013, 8, e68322. [Google Scholar] [CrossRef] [PubMed]
- Tamana, S.K.; Tun, H.M.; Konya, T.; Chari, R.S.; Field, C.J.; Guttman, D.S.; Becker, A.B.; Moraes, T.J.; Turvey, S.E.; Subbarao, P.; et al. Bacteroides-dominant gut microbiome of late infancy is associated with enhanced neurodevelopment. Gut Microbes 2021, 13, 1930875. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Saunders, C.; Sanossian, N. Food, gut barrier dysfunction, and related diseases: A new target for future individualized disease prevention and management. Food Sci. Nutr. 2023, 11, 1671–1704. [Google Scholar] [CrossRef] [PubMed]





| Age | CARS |
|---|---|
| 2 years and 2 months | 36 |
| 4 years and 6 months | 27 |
| 6 years | 22 |
| 13 years (now) | 17 (most recent) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Ansary, A.; Al-Ayadhi, L. Effects of Walnut and Pumpkin on Selective Neurophenotypes of Autism Spectrum Disorders: A Case Study. Nutrients 2023, 15, 4564. https://doi.org/10.3390/nu15214564
El-Ansary A, Al-Ayadhi L. Effects of Walnut and Pumpkin on Selective Neurophenotypes of Autism Spectrum Disorders: A Case Study. Nutrients. 2023; 15(21):4564. https://doi.org/10.3390/nu15214564
Chicago/Turabian StyleEl-Ansary, Afaf, and Laila Al-Ayadhi. 2023. "Effects of Walnut and Pumpkin on Selective Neurophenotypes of Autism Spectrum Disorders: A Case Study" Nutrients 15, no. 21: 4564. https://doi.org/10.3390/nu15214564
APA StyleEl-Ansary, A., & Al-Ayadhi, L. (2023). Effects of Walnut and Pumpkin on Selective Neurophenotypes of Autism Spectrum Disorders: A Case Study. Nutrients, 15(21), 4564. https://doi.org/10.3390/nu15214564






