Propolis Has an Anticancer Effect on Early Stage Colorectal Cancer by Affecting Epithelial Differentiation and Gut Immunity in the Tumor Microenvironment
Abstract
:1. Introduction
2. Materials and Methods
3. Results
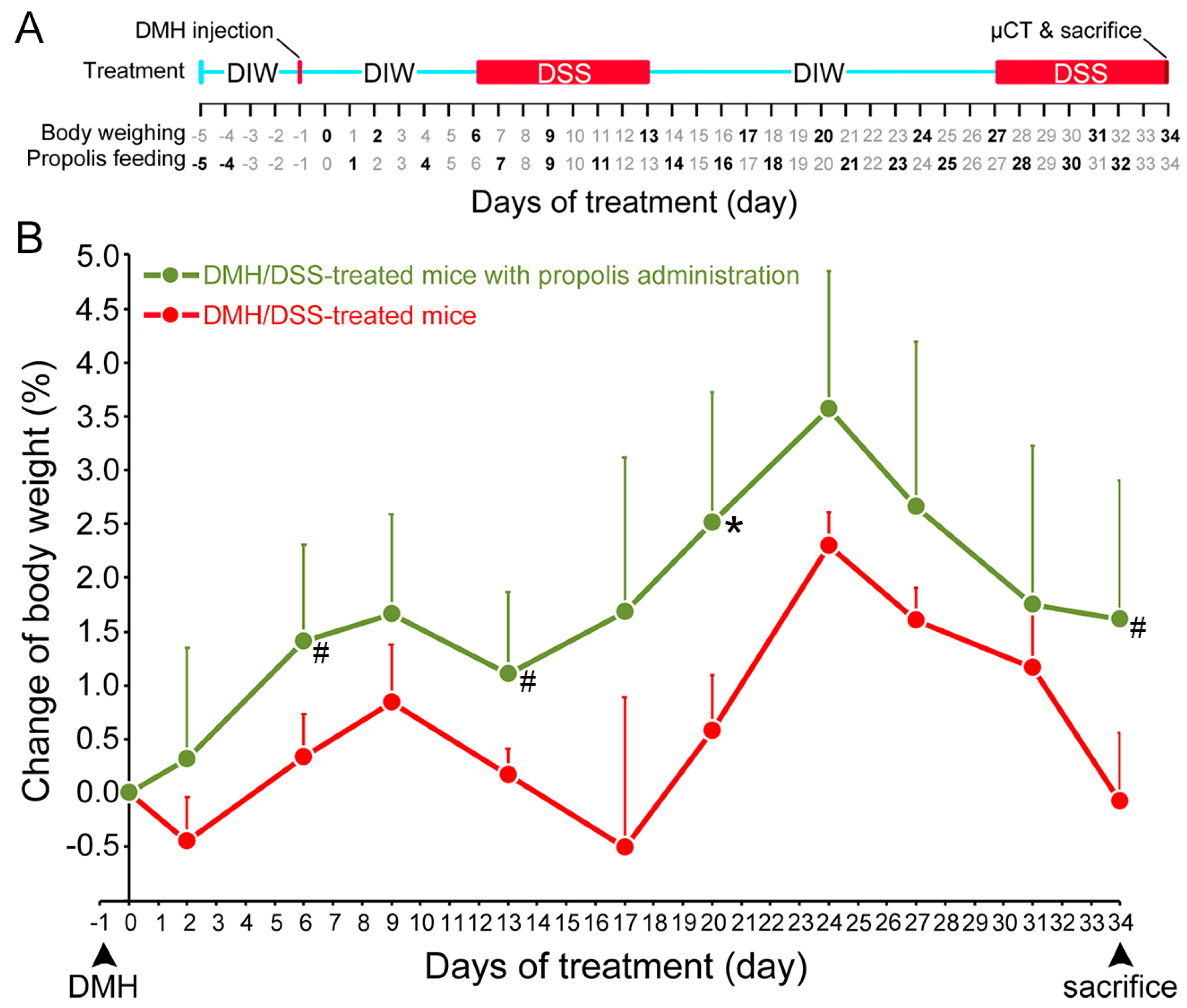
3.1. Propolis Administration Increased the Body Weight of DMH/DSS-Treated Mice
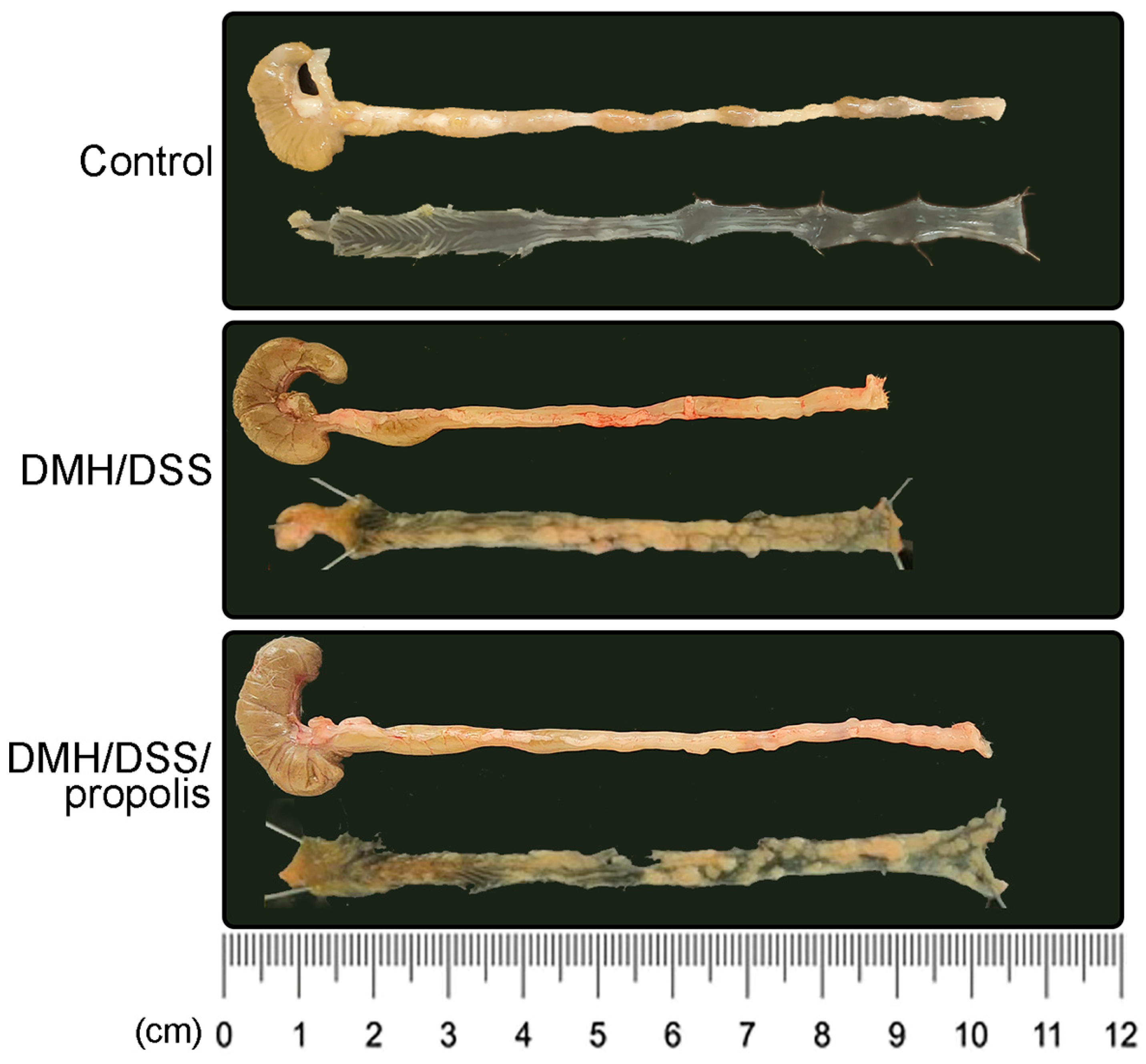
3.2. Lower Contrast of µCT Imaging and Longer Length of Mice Colons in CRC Bearing Mice with Propolis Administration
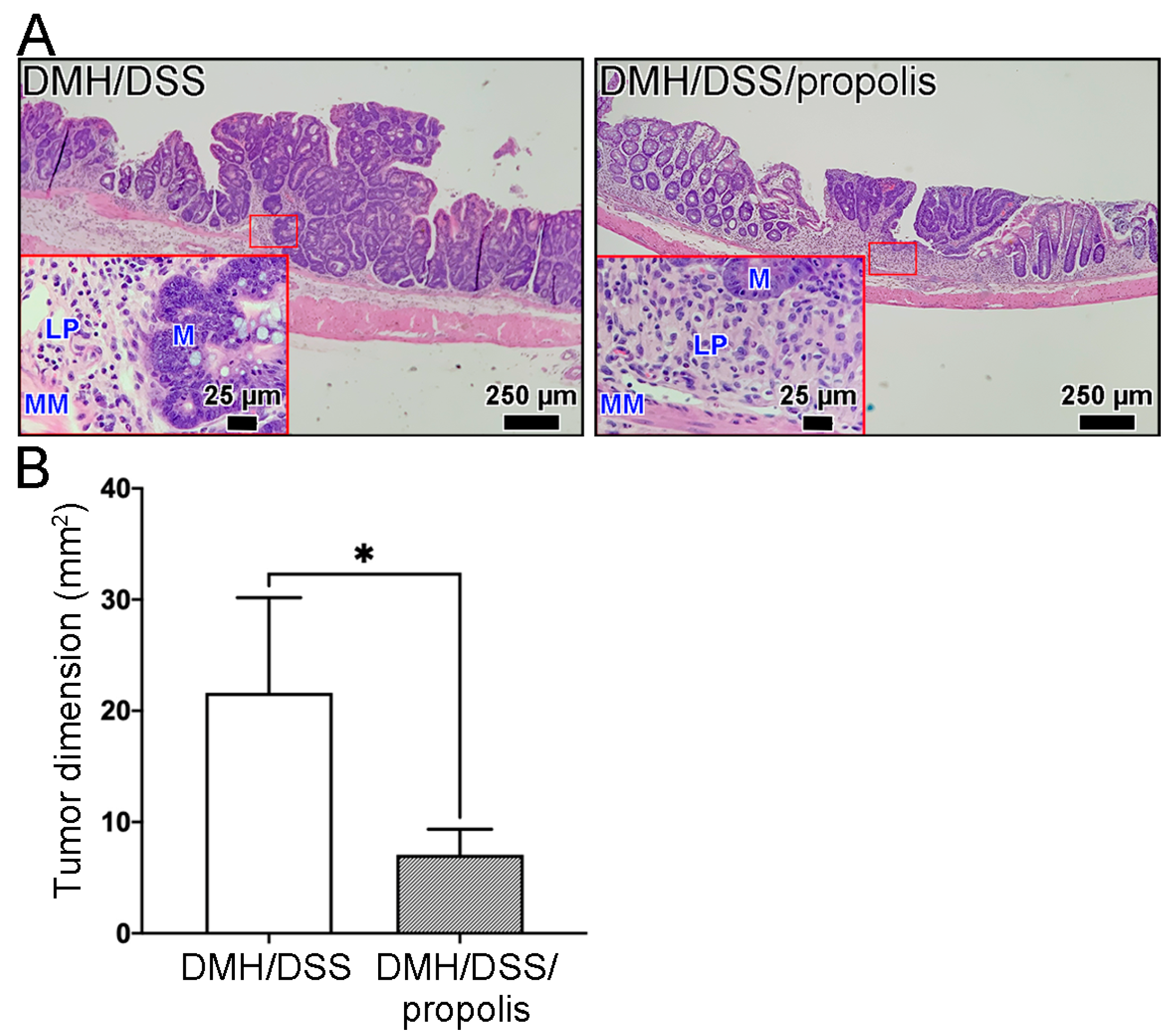
3.3. Changes of Total Tumor Dimension in Colon of CRC-Bearing Mice with Propolis Administration
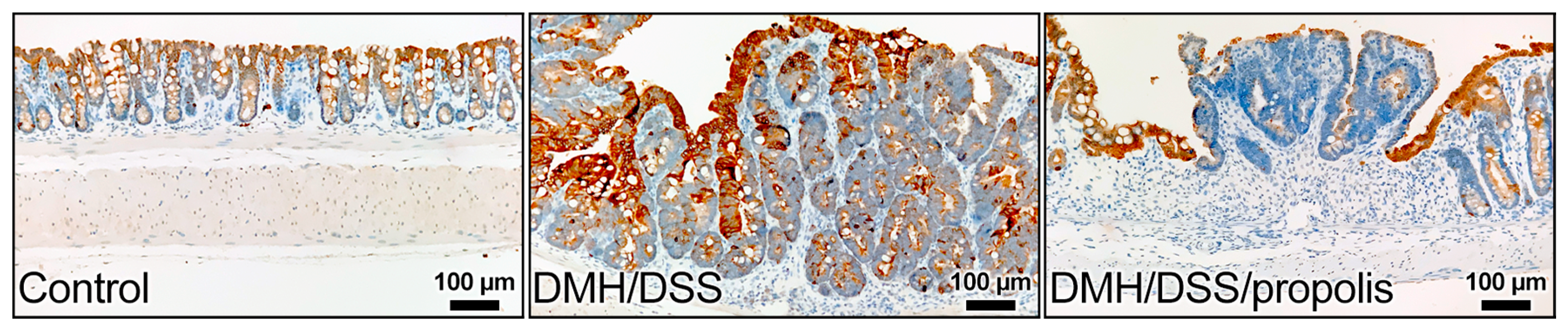
3.4. Effect of Propolis on the Level of KRT20 in CRC-Bearing Mice
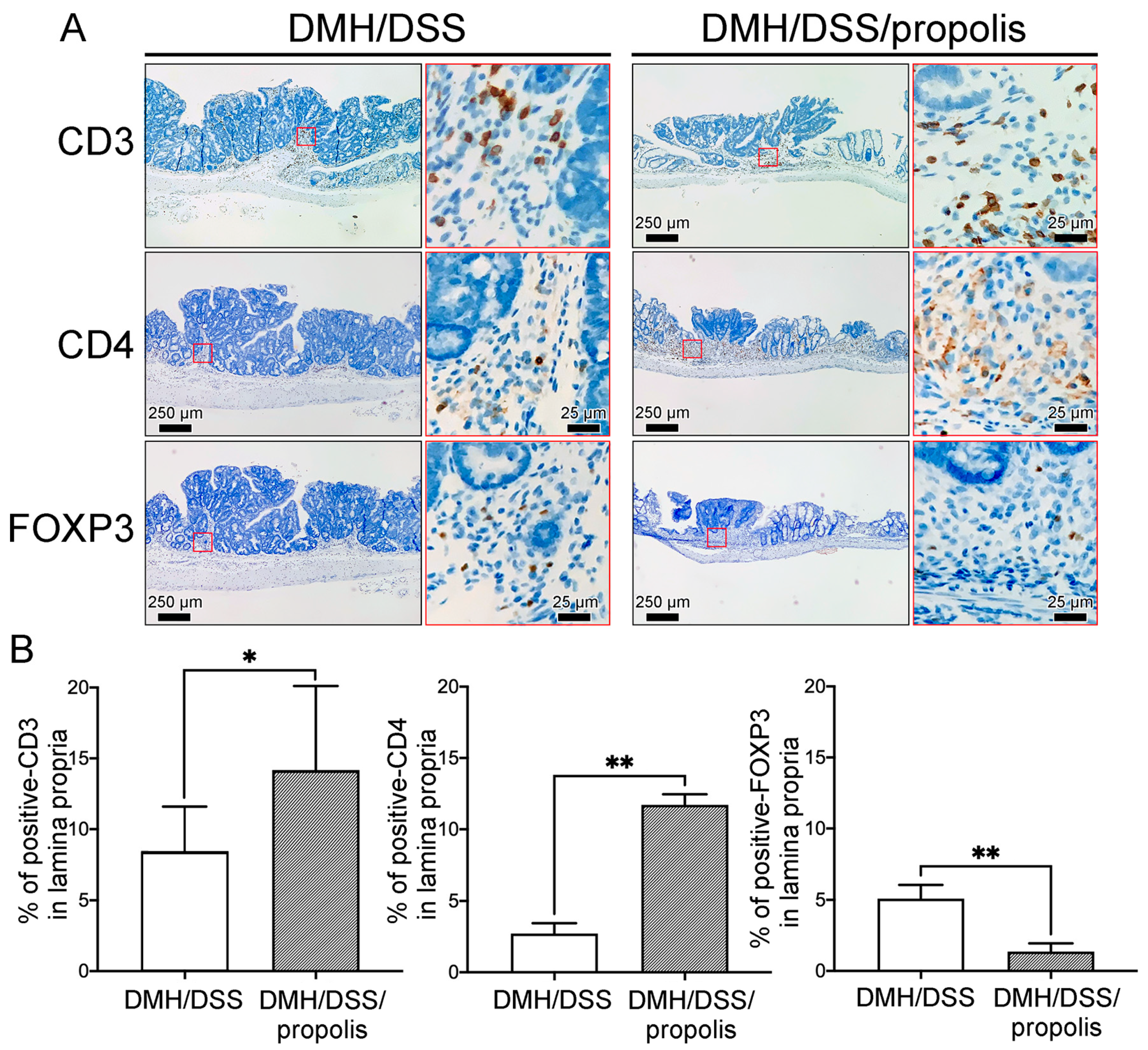
3.5. Associations between the Propolis Administration and the Density of Tumor-Infiltrating T Lymphocytes Subsets in Mice with DMH/DSS-Induced CRC
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Biller, L.H.; Schrag, D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA 2021, 325, 669–685. [Google Scholar] [CrossRef] [PubMed]
- Baidoun, F.; Elshiwy, K.; Elkeraie, Y.; Merjaneh, Z.; Khoudari, G.; Sarmini, M.T.; Gad, M.; Al-Husseini, M.; Saad, A. Colorectal Cancer Epidemiology: Recent Trends and Impact on Outcomes. Curr. Drug Targets 2021, 22, 998–1009. [Google Scholar] [PubMed]
- Onyoh, E.F.; Hsu, W.F.; Chang, L.C.; Lee, Y.C.; Wu, M.S.; Chiu, H.M. The Rise of Colorectal Cancer in Asia: Epidemiology, Screening, and Management. Curr. Gastroenterol. Rep. 2019, 21, 36. [Google Scholar] [CrossRef]
- Akimoto, N.; Ugai, T.; Zhong, R.; Hamada, T.; Fujiyoshi, K.; Giannakis, M.; Wu, K.; Cao, Y.; Ng, K.; Ogino, S. Rising incidence of early-onset colorectal cancer—A call to action. Nat. Rev. Clin. Oncol. 2020, 18, 230–243. [Google Scholar] [CrossRef] [PubMed]
- Hofseth, L.J.; Hebert, J.R.; Chanda, A.; Chen, H.; Love, B.L.; Pena, M.M.; Murphy, E.A.; Sajish, M.; Sheth, A.; Buckhaults, P.J.; et al. Early-onset colorectal cancer: Initial clues and current views. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 352–364, Correction in Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 517. [Google Scholar] [CrossRef]
- Patel, S.G.; Karlitz, J.J.; Yen, T.; Lieu, C.H.; Boland, C.R. The rising tide of early-onset colorectal cancer: A comprehensive review of epidemiology, clinical features, biology, risk factors, prevention, and early detection. Lancet Gastroenterol. Hepatol. 2022, 7, 262–274. [Google Scholar] [CrossRef]
- Sinicrope, F.A. Increasing Incidence of Early-Onset Colorectal Cancer. N. Engl. J. Med. 2022, 386, 1547–1558. [Google Scholar] [CrossRef]
- Mauri, G.; Sartore-Bianchi, A.; Russo, A.G.; Marsoni, S.; Bardelli, A.; Siena, S. Early-onset colorectal cancer in young individuals. Mol. Oncol. 2018, 13, 109–131. [Google Scholar] [CrossRef]
- Saraiva, M.R.; Rosa, I.; Claro, I. Early-onset colorectal cancer: A review of current knowledge. World J. Gastroenterol. 2023, 29, 1289–1303. [Google Scholar] [CrossRef]
- Guimaraes, D.P.; Mantuan, L.A.; de Oliveira, M.A.; Junior, R.L.; Costa, A.M.D.; Rossi, S.; Fava, G.; Taveira, L.N.; Giardina, K.M.; Talarico, T.; et al. The Performance of Colorectal Cancer Screening in Brazil: The First Two Years of the Implementation Program in Barretos Cancer Hospital. Cancer Prev. Res. 2021, 14, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Hur, J.; Nguyen, L.H.; Liu, J.; Song, M.; Wu, K.; Smith-Warner, S.A.; Ogino, S.; Willett, W.C.; Chan, A.T.; et al. Comprehensive Assessment of Diet Quality and Risk of Precursors of Early-Onset Colorectal Cancer. JNCI J. Natl. Cancer Inst. 2020, 113, 543–552. [Google Scholar] [CrossRef]
- Huang, X.M.; Yang, Z.J.; Xie, Q.; Zhang, Z.K.; Zhang, H.; Ma, J.Y. Natural products for treating colorectal cancer: A mechanistic review. Biomed. Pharmacother. 2019, 117, 109142. [Google Scholar] [CrossRef] [PubMed]
- Possamai, M.M.; Honorio-Franca, A.C.; Reinaque, A.P.; Franca, E.L.; Souto, P.C. Brazilian Propolis: A Natural Product That Improved the Fungicidal Activity by Blood Phagocytes. BioMed Res. Int. 2012, 2013, 541018. [Google Scholar] [CrossRef] [PubMed]
- Silva-Carvalho, R.; Baltazar, F.; Almeida-Aguiar, C. Propolis: A Complex Natural Product with a Plethora of Biological Activities That Can Be Explored for Drug Development. Evid.-Based Complement. Altern. Med. 2015, 2015, 206439. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, T.; Farooqui, A.A. Beneficial effects of propolis on human health and neurological diseases. Front. Biosci.-Elite 2012, 4, 779–793. [Google Scholar] [CrossRef]
- Memmedov, H.; Oktay, L.M.; Durmaz, B.; Gunel, N.S.; Yi Ldirim, H.K.; Sozmen, E.Y. Propolis prevents inhibition of apoptosis by potassium bromate in CCD 841 human colon cell. Cell Biochem. Funct. 2020, 38, 510–519. [Google Scholar] [CrossRef]
- Forma, E.; Bryś, M. Anticancer Activity of Propolis and Its Compounds. Nutrients 2021, 13, 2594. [Google Scholar] [CrossRef] [PubMed]
- Hermansyah, D.; Zulhendri, F.; Perera, C.O.; Firsty, N.N.; Chandrasekaran, K.; Abdulah, R.; Herman, H.; Lesmana, R. The Potential Use of Propolis as an Adjunctive Therapy in Breast Cancers. Integr. Cancer Ther. 2022, 21, 15347354221096868. [Google Scholar] [CrossRef]
- Chiu, H.F.; Han, Y.C.; Shen, Y.C.; Golovinskaia, O.; Venkatakrishnan, K.; Wang, C.K. Chemopreventive and Chemotherapeutic Effect of Propolis and Its Constituents: A Mini-review. J. Cancer Prev. 2020, 25, 70–78. [Google Scholar] [CrossRef]
- de Lima, R.O.; Bazo, A.P.; Said, R.A.; Sforcin, J.M.; Bankova, V.; Darros, B.R.; Salvadori, D.M. Modifying effect of propolis on dimethylhydrazine-induced DNA damage but not colonic aberrant crypt foci in rats. Environ. Mol. Mutagen. 2005, 45, 8–16. [Google Scholar] [CrossRef]
- Kubina, R.; Kabala-Dzik, A.; Dziedzic, A.; Bielec, B.; Wojtyczka, R.D.; Buldak, R.J.; Wyszynska, M.; Stawiarska-Pieta, B.; Szaflarska-Stojko, E. The Ethanol Extract of Polish Propolis Exhibits Anti-Proliferative and/or Pro-Apoptotic Effect on HCT 116 Colon Cancer and Me45 Malignant Melanoma Cells In Vitro Conditions. Adv. Clin. Exp. Med. 2015, 24, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wen, D.; Li, X.; Yao, C.; Chong, W.; Chen, H. Identification of an Immune Signature Predicting Prognosis Risk and Lymphocyte Infiltration in Colon Cancer. Front. Immunol. 2020, 11, 1678. [Google Scholar] [CrossRef]
- Shankaran, V.; Ikeda, H.; Bruce, A.T.; White, J.M.; Swanson, P.E.; Old, L.J.; Schreiber, R.D. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 2001, 410, 1107–1111. [Google Scholar] [CrossRef]
- Dunn, G.P.; Bruce, A.T.; Ikeda, H.; Old, L.J.; Schreiber, R.D. Cancer immunoediting: From immunosurveillance to tumor escape. Nat. Immunol. 2002, 3, 991–998. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, X.; Zu, S.; Lu, Y. Characteristics of circulating adaptive immune cells in patients with colorectal cancer. Sci. Rep. 2022, 12, 18166. [Google Scholar] [CrossRef]
- Gardner, I.H.; Siddharthan, R.; Watson, K.; Dewey, E.; Ruhl, R.; Khou, S.; Guan, X.; Xia, Z.; Tsikitis, V.L.; Anand, S. A Distinct Innate Immune Signature of Early Onset Colorectal Cancer. ImmunoHorizons 2021, 5, 489–499. [Google Scholar] [CrossRef]
- Yao, D.; Dong, M.; Dai, C.; Wu, S. Inflammation and Inflammatory Cytokine Contribute to the Initiation and Development of Ulcerative Colitis and Its Associated Cancer. Inflamm. Bowel Dis. 2019, 25, 1595–1602. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Wang, L.; Yin, R.; Hudlikar, R.; Li, S.; Kuo, H.D.; Peter, R.; Sargsyan, D.; Guo, Y.; Liu, X.; et al. Epigenetics/epigenomics and prevention by curcumin of early stages of inflammatory-driven colon cancer. Mol. Carcinog. 2019, 59, 227–236. [Google Scholar] [CrossRef]
- Chang, C.-C.; Kao, W.-Y.; Liu, C.-Y.; Su, H.-H.; Kan, Y.-A.; Lin, P.-Y.; Ku, W.-C.; Chang, K.-W.; Yang, R.-N.; Huang, C.-J. Butyrate supplementation regulates expression of chromosome segregation 1-like protein to reverse the genetic distortion caused by p53 mutations in colorectal cancer. Int. J. Oncol. 2022, 60, 64. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.C.; Shen, M.H.; Liu, C.Y.; Pu, C.M.; Hu, J.M.; Huang, C.J. A gut butyrate-producing bacterium Butyricicoccus pullicaecorum regulates short-chain fatty acid trans-porter and receptor to reduce the progression of 1,2-dimethylhydrazine-associated colorectal cancer. Oncol. Lett. 2020, 20, 327. [Google Scholar] [CrossRef]
- de Castro, S.; Higashi, K. Effect of different formulations of propolis on mice infected with Trypanosoma cruzi. J. Ethnopharmacol. 1995, 46, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Kohno, M.; Murakami, J.; Wu, L.; Chan, M.-L.; Yun, Z.; Cho, B.C.J.; de Perrot, M. Foxp3+ Regulatory T Cell Depletion after Nonablative Oligofractionated Irradiation Boosts the Abscopal Effects in Murine Malignant Mesothelioma. J. Immunol. 2020, 205, 2519–2531. [Google Scholar] [CrossRef] [PubMed]
- de Ruiter, E.J.; Bisheshar, S.K.; de Roest, R.H.; Wesseling, F.W.R.; Hoebers, F.J.P.; van den Hout, M.; Leemans, C.R.; Brakenhoff, R.H.; de Bree, R.; Terhaard, C.H.J.; et al. Assessing the prognostic value of tumor-infiltrating CD57+ cells in advanced stage head and neck cancer using QuPath digital image analysis. Virchows Arch. 2022, 481, 223–231. [Google Scholar] [CrossRef]
- Tran, T.D.; Ogbourne, S.M.; Brooks, P.R.; Sánchez-Cruz, N.; Medina-Franco, J.L.; Quinn, R.J. Lessons from Exploring Chemical Space and Chemical Diversity of Propolis Components. Int. J. Mol. Sci. 2020, 21, 4988. [Google Scholar] [CrossRef]
- Berretta, A.A.; Silveira, M.A.D.; Condor Capcha, J.M.; De Jong, D. Propolis and its potential against SARS-CoV-2 infection mechanisms and COVID-19 disease: Running title: Propolis against SARS-CoV-2 infection and COVID-19. Biomed. Pharmacother. 2020, 131, 110622. [Google Scholar] [CrossRef]
- Zulhendri, F.; Lesmana, R.; Tandean, S.; Christoper, A.; Chandrasekaran, K.; Irsyam, I.; Suwantika, A.A.; Abdulah, R.; Wathoni, N. Recent Update on the Anti-Inflammatory Activities of Propolis. Molecules 2022, 27, 8473. [Google Scholar] [CrossRef]
- da Silva, L.M.; de Souza, P.; Jaouni, S.K.A.; Harakeh, S.; Golbabapour, S.; de Andrade, S.F. Propolis and Its Potential to Treat Gastrointestinal Disorders. Evid.-Based Complement. Altern. Med. 2018, 2018, 2035820. [Google Scholar] [CrossRef]
- Soleimani, D.; Miryan, M.; Tutunchi, H.; Navashenaq, J.G.; Sadeghi, E.; Ghayour-Mobarhan, M.; Ferns, G.A.; Ostadrahimi, A. A systematic review of preclinical studies on the efficacy of propolis for the treatment of inflammatory bowel disease. Phytotherapy Res. 2020, 35, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Frión-Herrera, Y.; Gabbia, D.; Scaffidi, M.; Zagni, L.; Cuesta-Rubio, O.; De Martin, S.; Carrara, M. The Cuban Propolis Component Nemorosone Inhibits Proliferation and Metastatic Properties of Human Colorectal Cancer Cells. Int. J. Mol. Sci. 2020, 21, 1827. [Google Scholar] [CrossRef]
- Sameni, H.R.; Yosefi, S.; Alipour, M.; Pakdel, A.; Torabizadeh, N.; Semnani, V.; Bandegi, A.R. Co-administration of 5FU and propolis on AOM/DSS induced colorectal cancer in BALB-c mice. Life Sci. 2021, 276, 119390. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.-J.; Chiang, J.-H.; Lin, W.-C.; Tiu, C.-M.; Chang, C.-Y. Tumor and tumor-like lesions of duodenum: CT and barium imaging features. Clin. Imaging 2003, 27, 89–96. [Google Scholar] [CrossRef]
- Chen, F.; Ni, Y.-C.; Zheng, K.-E.; Ju, S.-H.; Sun, J.; Ou, X.-L.; Xu, M.-H.; Zhang, H.; Marchal, G. Spiral CT in gastric carcinoma: Comparison with barium study, fiberoptic gastroscopy and histopathology. World J. Gastroenterol. 2003, 9, 1404–1408. [Google Scholar] [CrossRef]
- Halligan, S.; Wooldrage, K.; Dadswell, E.; Kralj-Hans, I.; von Wagner, C.; Edwards, R.; Yao, G.; Kay, C.; Burling, D.; Faiz, O.; et al. Computed tomographic colonography versus barium enema for diagnosis of colorectal cancer or large polyps in symptomatic patients (SIGGAR): A multicentre randomised trial. Lancet 2013, 381, 1185–1193. [Google Scholar] [CrossRef]
- Chan, C.W.M.; Wong, N.A.; Liu, Y.; Bicknell, D.; Turley, H.; Hollins, L.; Miller, C.J.; Wilding, J.L.; Bodmer, W.F. Gastrointestinal differentiation marker Cytokeratin 20 is regulated by homeobox gene CDX1. Proc. Natl. Acad. Sci. USA 2009, 106, 1936–1941. [Google Scholar] [CrossRef] [PubMed]
- Michel, J.; Schonhaar, K.; Schledzewski, K.; Gkaniatsou, C.; Sticht, C.; Kellert, B.; Lasitschka, F.; Geraud, C.; Goerdt, S.; Schmieder, A. Identification of the novel differentiation marker MS4A8B and its murine homolog MS4A8A in colonic epi-thelial cells lost during neoplastic transformation in human colon. Cell Death Dis. 2013, 4, e469. [Google Scholar] [CrossRef]
- Imai, Y.; Yamagishi, H.; Fukuda, K.; Okamura, T.; Ono, Y.; Ban, S.; Inoue, T.; Ueda, Y. Expression of cytokeratin 20 indicates invasive histological phenotype in poorly differentiated colorectal ade-nocarcinoma. Anticancer Res. 2014, 34, 159–167. [Google Scholar] [PubMed]
- Ouyang, S.; Kang, W.M. Research Advances in the Role of Keratins in Gastrointestinal Cancer. Chin. Med. Sci. J. 2022, 37, 73–78. [Google Scholar]
- Al-Maghrabi, J.; Emam, E.; Gomaa, W. Immunohistochemical staining of cytokeratin 20 and cytokeratin 7 in colorectal carcinomas: Four different immunostaining profiles. Saudi J. Gastroenterol. 2018, 24, 129–134. [Google Scholar] [CrossRef]
- O’Rourke, K.P.; Loizou, E.; Livshits, G.; Schatoff, E.M.; Baslan, T.; Manchado, E.; Simon, J.; Romesser, P.B.; Leach, B.; Han, T.; et al. Transplantation of engineered organoids enables rapid generation of metastatic mouse models of colo-rectal cancer. Nat. Biotechnol. 2017, 35, 577–582. [Google Scholar] [CrossRef]
- Tunca, B.; Tezcan, G.; Cecener, G.; Egeli, U.; Zorluoglu, A.; Yilmazlar, T.; Ak, S.; Yerci, O.; Ozturk, E.; Umut, G.; et al. Overexpression of CK20, MAP3K8 and EIF5A correlates with poor prognosis in early-onset colorectal cancer patients. J. Cancer Res. Clin. Oncol. 2013, 139, 691–702. [Google Scholar] [CrossRef]
- Wagh, V.D. Propolis: A Wonder Bees Product and Its Pharmacological Potentials. Adv. Pharmacol. Sci. 2013, 2013, 308249. [Google Scholar] [CrossRef]
- Hossain, R.; Quispe, C.; Khan, R.A.; Saikat, A.S.M.; Ray, P.; Ongalbek, D.; Yeskaliyeva, B.; Jain, D.; Smeriglio, A.; Trombetta, D.; et al. Propolis: An update on its chemistry and pharmacological applications. Chin. Med. 2022, 17, 100. [Google Scholar] [CrossRef]
- Garzarella, E.U.; Navajas-Porras, B.; Pérez-Burillo, S.; Ullah, H.; Esposito, C.; Santarcangelo, C.; Hinojosa-Nogueira, D.; Pastoriza, S.; Zaccaria, V.; Xiao, J.; et al. Evaluating the effects of a standardized polyphenol mixture extracted from poplar-type propolis on healthy and diseased human gut microbiota. Biomed. Pharmacother. 2022, 148, 112759. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.C.; Guerra, G.R.; Pham, T.; Mitchell, C.; Lynch, A.C.; Warrier, S.K.; Ramsay, R.G.; Heriot, A.G. Prognostic Impact of Tumor-Infiltrating Lymphocytes in Primary and Metastatic Colorectal Cancer: A Sys-tematic Review and Meta-analysis. Dis. Colon Rectum 2019, 62, 498–508. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, T.; Hazama, S.; Suzuki, N.; Yoshida, S.; Tomochika, S.; Nakagami, Y.; Matsui, H.; Shindo, Y.; Kanekiyo, S.; Tokumitsu, Y.; et al. Intratumoural-infiltrating CD4+ and FOXP3+ T cells as strong positive predictive markers for the prognosis of resectable colorectal cancer. Br. J. Cancer 2019, 121, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Salama, P.; Phillips, M.; Grieu, F.; Morris, M.; Zeps, N.; Joseph, D.; Platell, C.; Iacopetta, B. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J. Clin. Oncol. 2009, 27, 186–192. [Google Scholar] [CrossRef]
- Sinicrope, F.A.; Rego, R.L.; Ansell, S.M.; Knutson, K.L.; Foster, N.R.; Sargent, D.J. Intraepithelial effector (CD3+)/regulatory (FoxP3+) T-cell ratio predicts a clinical outcome of human colon carcinoma. Gastroenterology 2009, 137, 1270–1279. [Google Scholar] [CrossRef] [PubMed]
- Ohue, Y.; Nishikawa, H. Regulatory T (Treg) cells in cancer: Can Treg cells be a new therapeutic target? Cancer Sci. 2019, 110, 2080–2089. [Google Scholar] [CrossRef] [PubMed]
- Fridman, W.H.; Pagès, F.; Sautès-Fridman, C.; Galon, J. The immune contexture in human tumours: Impact on clinical outcome. Nat. Rev. Cancer 2012, 12, 298–306. [Google Scholar] [CrossRef]
- Betts, G.; Jones, E.; Junaid, S.; El-Shanawany, T.; Scurr, M.; Mizen, P.; Kumar, M.; Jones, S.; Rees, B.; Williams, G.; et al. Suppression of tumour-specific CD4+T cells by regulatory T cells is associated with progression of human colorectal cancer. Gut 2012, 61, 1163–1171. [Google Scholar] [CrossRef]
- Saleh, R.; Elkord, E. FoxP3+ T regulatory cells in cancer: Prognostic biomarkers and therapeutic targets. Cancer Lett. 2020, 490, 174–185. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, T.; Kang, Z.; Guo, G.; Sun, Y.; Lin, K.; Huang, Q.; Shi, X.; Ni, Z.; Ding, N.; et al. Tumor-Infiltrating Immune Cells Act as a Marker for Prognosis in Colorectal Cancer. Front. Immunol. 2019, 10, 2368. [Google Scholar] [CrossRef] [PubMed]
- Terzic, J.; Grivennikov, S.; Karin, E.; Karin, M. Inflammation and colon cancer. Gastroenterology 2010, 138, 2101–2114 e5. [Google Scholar] [CrossRef]
- Soler, A.P.; Miller, R.; Laughlin, K.V.; Carp, N.Z.; Klurfeld, D.M.; Mullin, J.M. Increased tight junctional permeability is associated with the development of colon cancer. Carcinogenesis 1999, 20, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Fan, W.; Zhang, Z.; Wang, J.; Wang, P.; Li, Y.; Yu, M. The Clinicopathological and Prognostic Implications of FoxP3+ Regulatory T Cells in Patients with Colorectal Cancer: A Meta-Analysis. Front. Physiol. 2017, 8, 950. [Google Scholar] [CrossRef] [PubMed]
- Ladabaum, U. Fulfilling the promise of colorectal cancer screening. Lancet Gastroenterol. Hepatol. 2022, 7, 690–691. [Google Scholar] [CrossRef]
- Islam, M.R.; Akash, S.; Rahman, M.M.; Nowrin, F.T.; Akter, T.; Shohag, S.; Rauf, A.; Aljohani, A.S.M.; Simal-Gandara, J. Colon cancer and colorectal cancer: Prevention and treatment by potential natural products. Chem. Biol. Interact. 2022, 368, 110170. [Google Scholar] [CrossRef]
- Braakhuis, A. Evidence on the Health Benefits of Supplemental Propolis. Nutrients 2019, 11, 2705. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, D.W.; Giardina, C.; Tanaka, T. Mouse models for the study of colon carcinogenesis. Carcinogenesis 2008, 30, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Bürtin, F.; Mullins, C.S.; Linnebacher, M. Mouse models of colorectal cancer: Past, present and future perspectives. World J. Gastroenterol. 2020, 26, 1394–1426. [Google Scholar] [CrossRef] [PubMed]
- Trusheva, B.; Trunkova, D.; Bankova, V. Different extraction methods of biologically active components from propolis: A preliminary study. Chem. Central J. 2007, 1, 13. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, M.-H.; Liu, C.-Y.; Chang, K.-W.; Lai, C.-L.; Chang, S.-C.; Huang, C.-J. Propolis Has an Anticancer Effect on Early Stage Colorectal Cancer by Affecting Epithelial Differentiation and Gut Immunity in the Tumor Microenvironment. Nutrients 2023, 15, 4494. https://doi.org/10.3390/nu15214494
Shen M-H, Liu C-Y, Chang K-W, Lai C-L, Chang S-C, Huang C-J. Propolis Has an Anticancer Effect on Early Stage Colorectal Cancer by Affecting Epithelial Differentiation and Gut Immunity in the Tumor Microenvironment. Nutrients. 2023; 15(21):4494. https://doi.org/10.3390/nu15214494
Chicago/Turabian StyleShen, Ming-Hung, Chih-Yi Liu, Kang-Wei Chang, Ching-Long Lai, Shih-Chang Chang, and Chi-Jung Huang. 2023. "Propolis Has an Anticancer Effect on Early Stage Colorectal Cancer by Affecting Epithelial Differentiation and Gut Immunity in the Tumor Microenvironment" Nutrients 15, no. 21: 4494. https://doi.org/10.3390/nu15214494
APA StyleShen, M.-H., Liu, C.-Y., Chang, K.-W., Lai, C.-L., Chang, S.-C., & Huang, C.-J. (2023). Propolis Has an Anticancer Effect on Early Stage Colorectal Cancer by Affecting Epithelial Differentiation and Gut Immunity in the Tumor Microenvironment. Nutrients, 15(21), 4494. https://doi.org/10.3390/nu15214494




