High-Fat Diet Enhances Platelet Activation and Is Associated with Proprotein Convertase Subtilisin Kexin 9: An Animal Study
Abstract
:1. Introduction
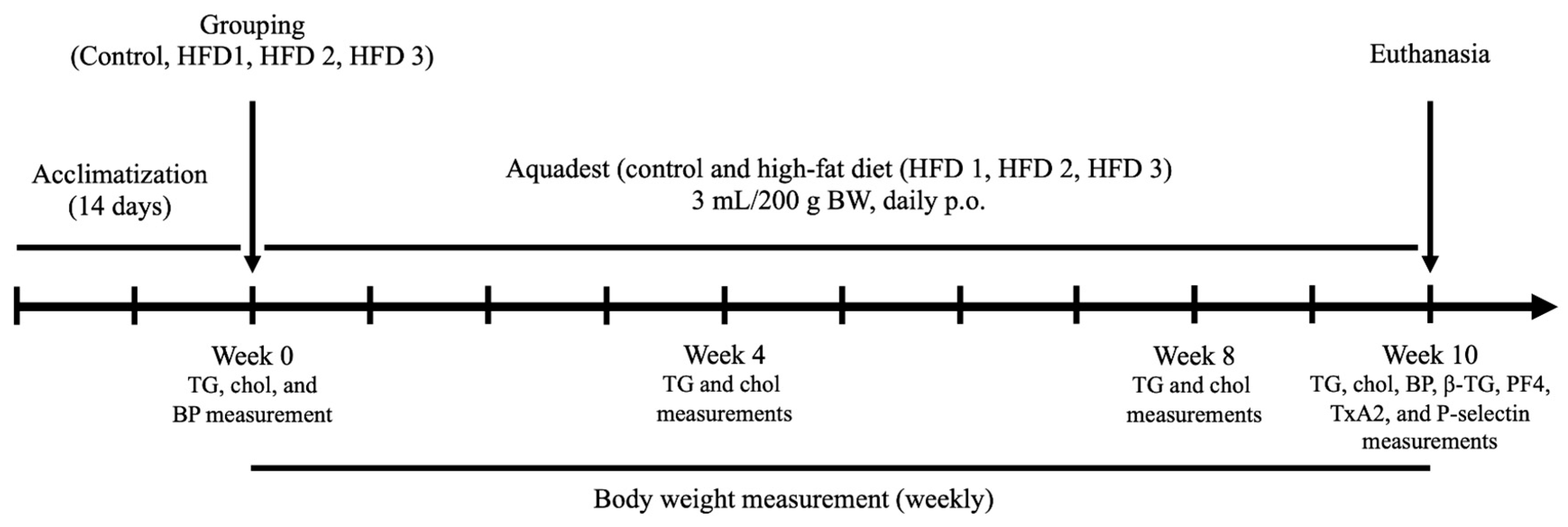
2. Materials and Methods
2.1. Animals and High-Fat Diet
2.2. Triglyceride and Cholesterol Measurement
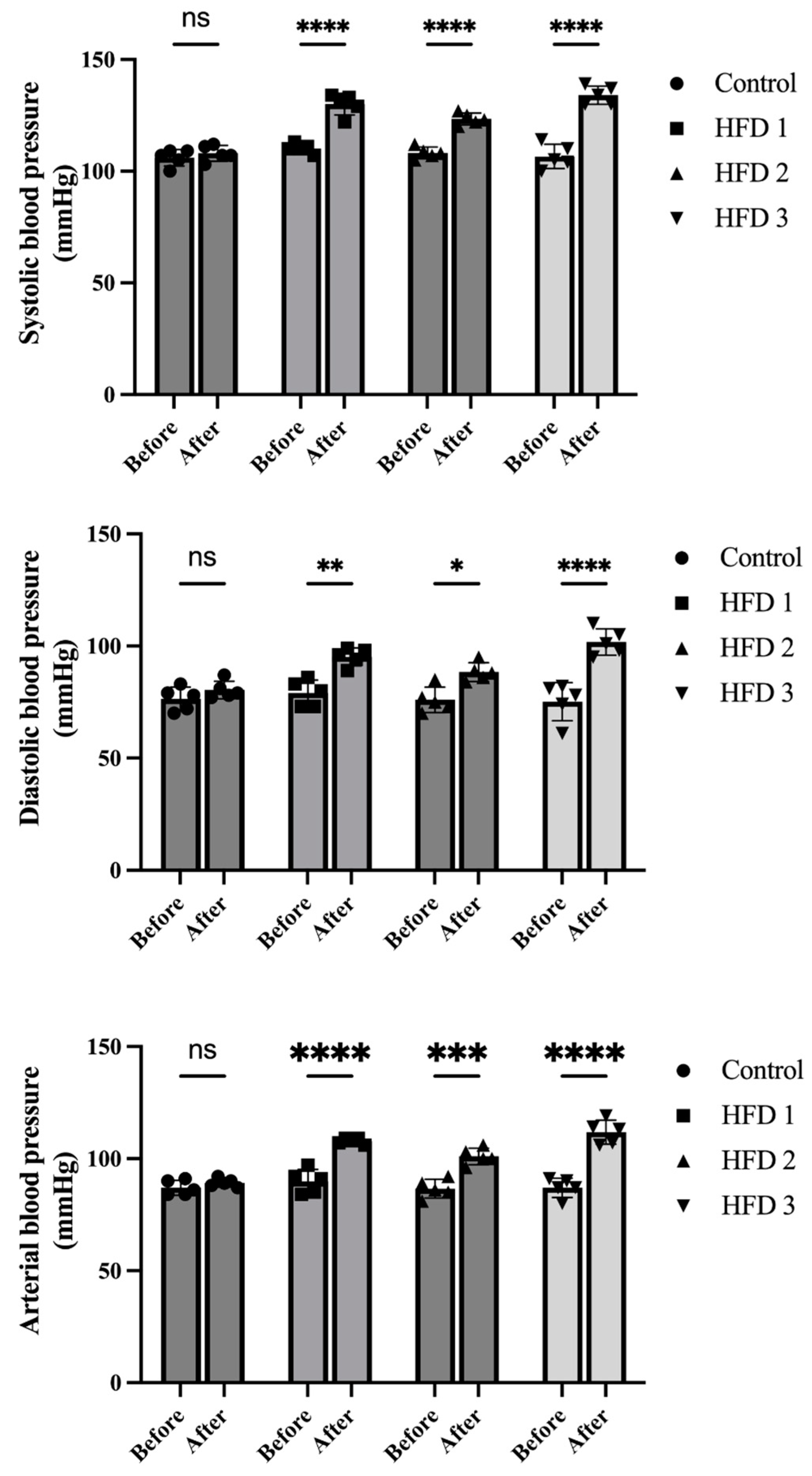
2.3. Systolic, Diastolic, and Arterial Blood Pressure Measurement
2.4. Blood Collection
2.5. Biochemical Parameters for Platelet Activation and PCSK9
2.6. Statistical Analysis
3. Results
3.1. High-Fat Diet Induces Hypercholesterolemia and Hypertriglyceridemia in Wistar Rats
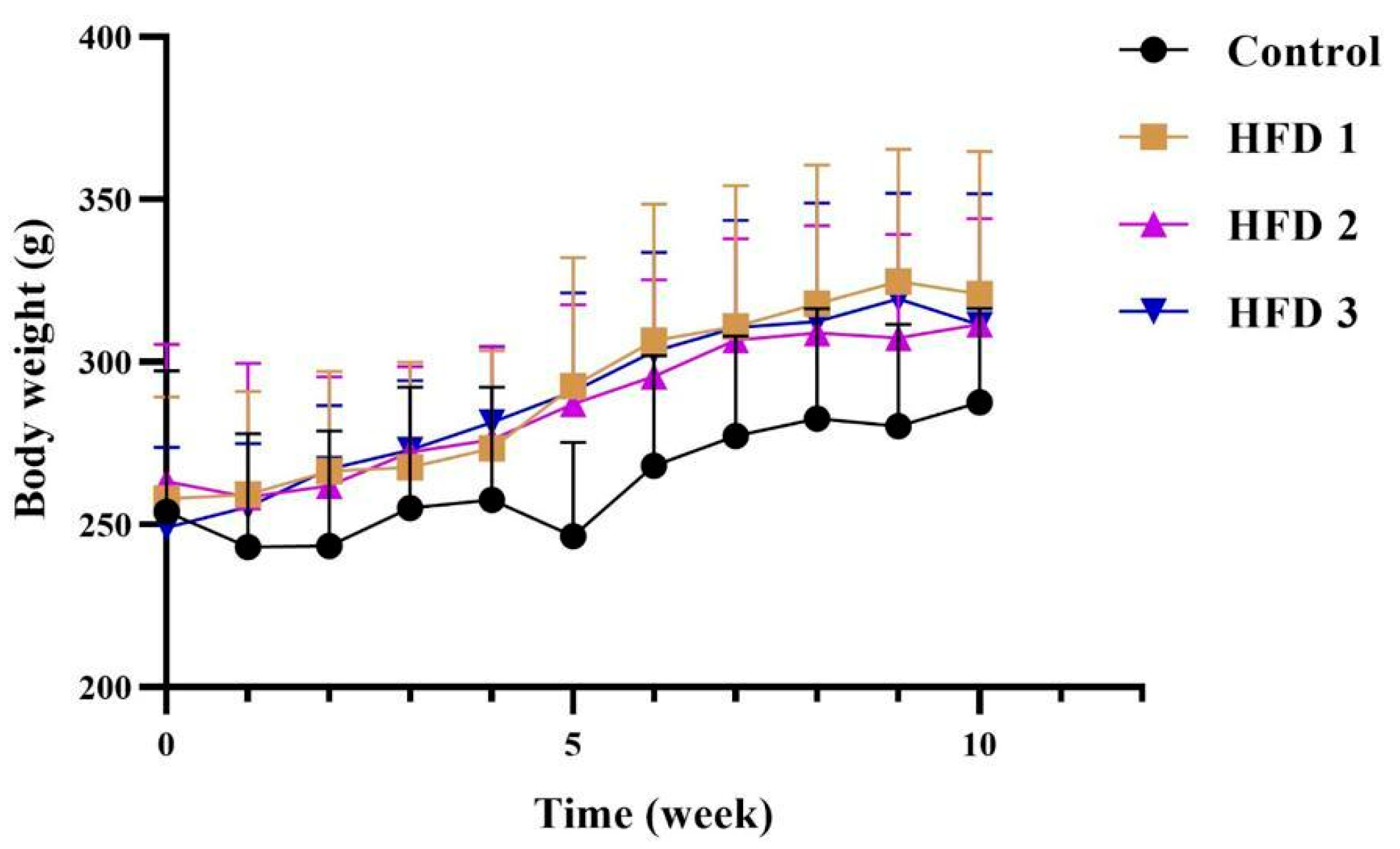
3.2. Body Weight and Blood Pressure Changes following High-Fat Diet Induction
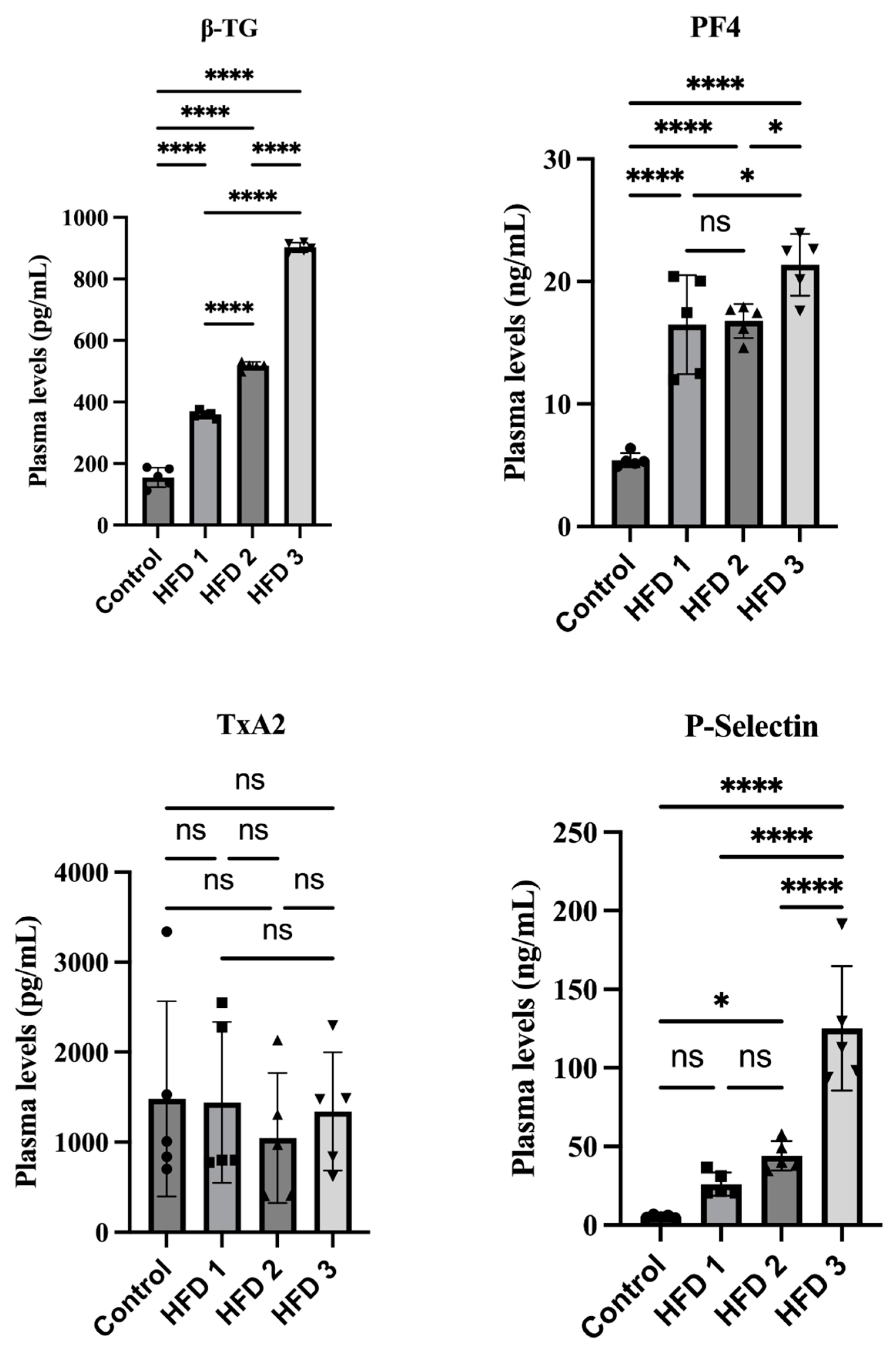
3.3. Hypercholesterolemia and Hypertriglyceridemia Conditions Increase β-TG, p-Selectin, and PF-4 in a Similar Pattern in Wistar Rats
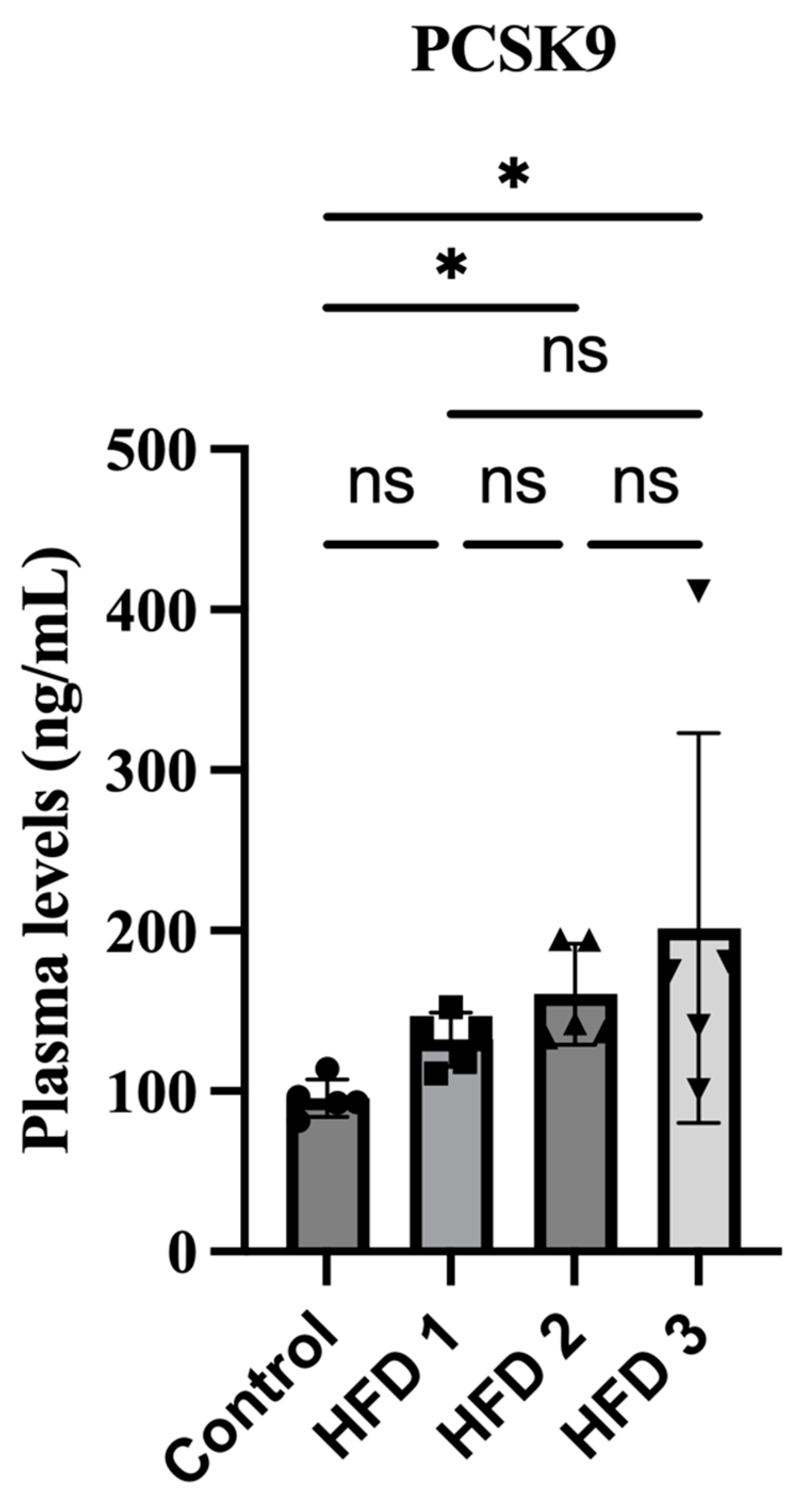
3.4. Plasma PCSK9 Levels Were Increased in High-Fat Diet Rats
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pawelczyk, M.; Kaczorowska, B.; Baj, Z. The impact of hyperglycemia and hyperlipidemia on plasma P-selectin and platelet markers after ischemic stroke. Arch. Med. Sci. 2017, 13, 1049–1056. [Google Scholar] [CrossRef]
- Libby, P.; Simon, D.I. Inflammation and thrombosis: The clot thickens. Circulation 2001, 103, 1718–1720. [Google Scholar] [CrossRef]
- Gawaz, M. Role of platelets in coronary thrombosis and reperfusion of ischemic myocardium. Cardiovasc. Res. 2004, 61, 498–511. [Google Scholar] [CrossRef]
- World Health Organization. The Top 10 Causes of Death—Factsheet. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 15 December 2020).
- Oliveira, G.B.; Avezum, A.; Roever, L. Cardiovascular Disease Burden: Evolving Knowledge of Risk Factors in Myocardial Infarction and Stroke through Population-Based Research and Perspectives in Global Prevention. Front. Cardiovasc. Med. 2015, 2, 32. [Google Scholar] [CrossRef]
- Tian, Z.; Li, K.; Fan, D.; Zhao, Y.; Gao, X.; Ma, X.; Xu, L.; Shi, Y.; Ya, F.; Zou, J.; et al. Dose-dependent effects of anthocyanin supplementation on platelet function in subjects with dyslipidemia: A randomized clinical trial. eBioMedicine 2021, 70, 103533. [Google Scholar] [CrossRef]
- Garg, R.; Aggarwal, S.; Kumar, R.; Sharma, G. Association of atherosclerosis with dyslipidemia and co-morbid conditions: A descriptive study. J. Nat. Sci. Biol. Med. 2015, 6, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Tall, A.R. Cholesterol in platelet biogenesis and activation. Blood 2016, 127, 1949–1953. [Google Scholar] [CrossRef] [PubMed]
- de Man, F.H.; Nieuwland, R.; van der Laarse, A.; Romijn, F.; Smelt, A.H.; Gevers Leuven, J.A.; Sturk, A. Activated platelets in patients with severe hypertriglyceridemia: Effects of triglyceride-lowering therapy. Atherosclerosis 2000, 152, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Coppinger, J.A.; Maguire, P.B. Insights into the platelet releasate. Curr. Pharm. Des. 2007, 13, 2640–2646. [Google Scholar] [CrossRef]
- Baidildinova, G.; Nagy, M.; Jurk, K.; Wild, P.S.; Ten Cate, H.; van der Meijden, P.E.J. Soluble Platelet Release Factors as Biomarkers for Cardiovascular Disease. Front. Cardiovasc. Med. 2021, 8, 684920. [Google Scholar] [CrossRef]
- Barale, C.; Melchionda, E.; Morotti, A.; Russo, I. PCSK9 Biology and Its Role in Atherothrombosis. Int. J. Mol. Sci. 2021, 22, 5880. [Google Scholar] [CrossRef]
- Puteri, M.U.; Azmi, N.U.; Kato, M.; Saputri, F.C. PCSK9 Promotes Cardiovascular Diseases: Recent Evidence about Its Association with Platelet Activation-Induced Myocardial Infarction. Life 2022, 12, 190. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yan, B.; Tai, S.; Zhou, S.; Zheng, X.L. PCSK9: Associated with cardiac diseases and their risk factors? Arch. Biochem. Biophys. 2021, 704, 108717. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.R.; Sabatine, M.S. PCSK9 inhibition in patients with hypercholesterolemia. Trends Cardiovasc. Med. 2015, 25, 567–574. [Google Scholar] [CrossRef]
- Song, L.; Zhao, X.; Chen, R.; Li, J.; Zhou, J.; Liu, C.; Zhou, P.; Wang, Y.; Chen, Y.; Zhao, H.; et al. Association of PCSK9 with inflammation and platelet activation markers and recurrent cardiovascular risks in STEMI patients undergoing primary PCI with or without diabetes. Cardiovasc. Diabetol. 2022, 21, 80. [Google Scholar] [CrossRef]
- Petersen-Uribe, Á.; Kremser, M.; Rohlfing, A.K.; Castor, T.; Kolb, K.; Dicenta, V.; Emschermann, F.; Li, B.; Borst, O.; Rath, D.; et al. Platelet-Derived PCSK9 Is Associated with LDL Metabolism and Modulates Atherothrombotic Mechanisms in Coronary Artery Disease. Int. J. Mol. Sci. 2021, 22, 11179. [Google Scholar] [CrossRef] [PubMed]
- Navarese, E.P.; Kolodziejczak, M.; Winter, M.P.; Alimohammadi, A.; Lang, I.M.; Buffon, A.; Lip, G.Y.H.; Siller-Matula, J.M. Association of PCSK9 with platelet reactivity in patients with acute coronary syndrome treated with prasugrel or ticagrelor: The PCSK9-REACT study. Int. J. Cardiol. 2017, 227, 644–649. [Google Scholar] [CrossRef]
- Cammisotto, V.; Pastori, D.; Nocella, C.; Bartimoccia, S.; Castellani, V.; Marchese, C.; Sili Scavalli, A.; Ettorre, E.; Viceconte, N.; Violi, F.; et al. PCSK9 Regulates Nox2-Mediated Platelet Activation via CD36 Receptor in Patients with Atrial Fibrillation. Antioxidants 2020, 9, 296. [Google Scholar] [CrossRef]
- Qi, Z.; Hu, L.; Zhang, J.; Yang, W.; Liu, X.; Jia, D.; Yao, Z.; Chang, L.; Pan, G.; Zhong, H.; et al. PCSK9 (Proprotein Convertase Subtilisin/Kexin 9) Enhances Platelet Activation, Thrombosis, and Myocardial Infarct Expansion by Binding to Platelet CD36. Circulation 2021, 143, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Munshi, R.P.; Joshi, S.G.; Rane, B.N. Development of an experimental diet model in rats to study hyperlipidemia and insulin resistance, markers for coronary heart disease. Indian J. Pharmacol. 2014, 46, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, M.I.; Naheed, S.; Jalil, S.; Alam, J.M.; Attaur, R. Effects of ethanolic extract of Iris germanica on lipid profile of rats fed on a high-fat diet. J. Ethnopharmacol. 2005, 98, 217–220. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, M.; Zhang, L.; Li, S.; Zhai, J.; Shen, Y.; Chen, C.; Qin, J. Effect of a combination of calorie-restriction therapy and Lingguizhugan decoction on levels of fasting blood lipid and inflammatory cytokines in a high-fat diet induced hyperlipidemia rat model. J. Tradit. Chin. Med. 2015, 35, 218–221. [Google Scholar]
- Febriani, W. Efek pemberian simvastatin terhadap kadar kolesterol telur puyuh. BIOSFER J. Tadris Pendidik. Biol. 2017, 8, 158–170. [Google Scholar] [CrossRef]
- Polat, E.S.; Citil, O.B.; Garip, M. Fatty acid composition of yolk of nine poultry species kept in their natural environment. Animal Sci. Pap. Rep. 2013, 31, 363–368. [Google Scholar]
- Nutrition Data. Fat, Mutton Tallow Nutrition Facts & Calories. 3 December 2020. Available online: https://nutritiondata.self.com/facts/fats-and-oils/581/2 (accessed on 2 February 2021).
- Anchor. Anchor Unsalted Butter. 2 February 2021. Available online: http://anchorbutter.com/product/anchor-unsalted-butter (accessed on 2 February 2021).
- Charrondiere, R.U.; Haytowitz, D.; Stadlmayr, B. FAO/Infoods Density Database Version 2.0 (2012); FAO: Rome, Italy, 2012. [Google Scholar]
- Royal Society of Chemistry. Predicted Data Is Generated Using the Acd/Labs Percepta Platform—Physchem Module. 20 February 2021. Available online: http://www.chemspider.com/Chemical-Structure.192176.html (accessed on 2 February 2021).
- Saputri, F.C.; Hutahaean, I.; Mun’im, A. Peperomia pellucida (L.) Kunth as an angiotensin-converting enzyme inhibitor in two-kidney, one-clip Goldblatt hypertensive rats. Saudi J. Biol. Sci. 2021, 28, 6191–6197. [Google Scholar] [CrossRef]
- Gage, G.J.; Kipke, D.R.; Shain, W. Whole animal perfusion fixation for rodents. J. Vis. Exp. 2012, 65, e3564. [Google Scholar]
- Hirunpanich, V.; Utaipat, A.; Morales, N.P.; Bunyapraphatsara, N.; Sato, H.; Herunsale, A.; Suthisisang, C. Hypocholesterolemic and antioxidant effects of aqueous extracts from the dried calyx of Hibiscus sabdariffa L. in hypercholesterolemic rats. J. Ethnopharmacol. 2006, 103, 252–260. [Google Scholar] [CrossRef]
- Andreadou, I.; Schulz, R.; Badimon, L.; Adameová, A.; Kleinbongard, P.; Lecour, S.; Nikolaou, P.-E.; Falcão-Pires, I.; Vilahur, G.; Woudberg, N.; et al. Hyperlipidaemia and cardioprotection: Animal models for translational studies. Br. J. Pharmacol. 2020, 177, 5287–5311. [Google Scholar] [CrossRef]
- Silva, C.; Perris, P.D.; Fernandez, I.; Godoy, M.F.; Mambrin, C.; Slobodianik, N.H.; Feliu, S.M. Effects of high-fat diets from different sources on serum and thymus lipid profile: Study in an experimental model. Endocr. Metab. Immune Disord. Drug Targets 2014, 14, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Lasker, S.; Rahman, M.M.; Parvez, F.; Zamila, M.; Miah, P.; Nahar, K.; Kabir, F.; Sharmin, S.B.; Subhan, N.; Ahsan, G.U.; et al. High-fat diet-induced metabolic syndrome and oxidative stress in obese rats are ameliorated by yogurt supplementation. Sci. Rep. 2019, 9, 20026. [Google Scholar] [CrossRef]
- Od-Ek, P.; Deenin, W.; Malakul, W.; Phoungpetchara, I.; Tunsophon, S. Anti-obesity effect of Carica papaya in high-fat diet fed rats. Biomed. Rep. 2020, 13, 30. [Google Scholar]
- Panchal, S.K.; Brown, L. Rodent models for metabolic syndrome research. J. Biomed. Biotechnol. 2011, 2011, 351982. [Google Scholar] [CrossRef] [PubMed]
- Camelo, L.; Marinho, T.S.; Águila, M.B.; Souza-Mello, V.; Barbosa-da-Silva, S. Intermittent fasting exerts beneficial metabolic effects on blood pressure and cardiac structure by modulating local renin-angiotensin system in the heart of mice fed high-fat or high-fructose diets. Nutr. Res. 2019, 63, 51–62. [Google Scholar] [CrossRef]
- Maneesai, P.; Bunbupha, S.; Kukongviriyapan, U.; Prachaney, P.; Tangsucharit, P.; Kukongviriyapan, V.; Pakdeechote, P. Asiatic acid attenuates renin-angiotensin system activation and improves vascular function in high-carbohydrate, high-fat diet fed rats. BMC Complement. Altern. Med. 2016, 16, 123. [Google Scholar] [CrossRef]
- Borghi, C.; Urso, R.; Cicero, A.F. Renin-angiotensin system at the crossroad of hypertension and hypercholesterolemia. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Neaton, J.D.; Wentworth, D. Serum cholesterol, blood pressure, cigarette smoking, and death from coronary heart disease. Overall findings and differences by age for 316,099 white men. Multiple Risk Factor Intervention Trial Research Group. Arch. Intern. Med. 1992, 152, 56–64. [Google Scholar] [CrossRef]
- Ariyanti, R.; Besral, B. Dyslipidemia Associated with Hypertension Increases the Risks for Coronary Heart Disease: A Case-Control Study in Harapan Kita Hospital, National Cardiovascular Center, Jakarta. J. Lipids 2019, 2019, 2517013. [Google Scholar] [CrossRef]
- Davì, G.; Romano, M.; Mezzetti, A.; Procopio, A.; Iacobelli, S.; Antidormi, T.; Bucciarelli, T.; Alessandrini, P.; Cuccurullo, F.; Bon, G.B. Increased levels of soluble P-selectin in hypercholesterolemic patients. Circulation 1998, 97, 953–957. [Google Scholar] [CrossRef]
- Wang, L.; Tang, C. Targeting Platelet in Atherosclerosis Plaque Formation: Current Knowledge and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 9760. [Google Scholar] [CrossRef]
- Pawelczyk, M.; Baj, Z.; Chmielewski, H.; Kaczorowska, B.; Klimek, A. The influence of hyperlipidemia on platelet activity markers in patients after ischemic stroke. Cerebrovasc. Dis. 2009, 27, 131–137. [Google Scholar] [CrossRef]
- Koyama, H.; Maeno, T.; Fukumoto, S.; Shoji, T.; Yamane, T.; Yokoyama, H.; Emoto, M.; Shoji, T.; Tahara, H.; Inaba, M.; et al. Platelet P-selectin expression is associated with atherosclerotic wall thickness in carotid artery in humans. Circulation 2003, 108, 524–529. [Google Scholar] [CrossRef]
- Wen, S.; Jadhav, K.S.; Williamson, D.L.; Rideout, T.C. Treadmill Exercise Training Modulates Hepatic Cholesterol Metabolism and Circulating PCSK9 Concentration in High-Fat-Fed Mice. J. Lipids 2013, 2013, 908048. [Google Scholar] [CrossRef]
- Arunsak, B.; Pratchayasakul, W.; Amput, P.; Chattipakorn, K.; Tosukhowong, T.; Kerdphoo, S.; Jaiwongkum, T.; Thonusin, C.; Palee, S.; Chattipakorn, N.; et al. Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor exerts greater efficacy than atorvastatin on improvement of brain function and cognition in obese rats. Arch. Biochem. Biophys. 2020, 689, 108470. [Google Scholar] [CrossRef]
- Krysa, J.A.; Ooi, T.C.; Proctor, S.D.; Vine, D.F. Nutritional and Lipid Modulation of PCSK9: Effects on Cardiometabolic Risk Factors. J. Nutr. 2017, 147, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.J.; Liu, J.; Guo, Y.L.; Xu, R.X.; Sun, J.; Li, J.J. Dyslipidemia in rat fed with high-fat diet is not associated with PCSK9-LDL-receptor pathway but ageing. J. Geriatr. Cardiol. 2013, 10, 361–368. [Google Scholar]
- Seidah, N.G.; Garçon, D. Expanding Biology of PCSK9: Roles in Atherosclerosis and Beyond. Curr. Atheroscler. Rep. 2022, 24, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, M.D.; Tavori, H.; Fazio, S. PCSK9: From Basic Science Discoveries to Clinical Trials. Circ. Res. 2018, 122, 1420–1438. [Google Scholar] [CrossRef] [PubMed]
- Nishikido, T.; Ray, K.K. Non-antibody Approaches to Proprotein Convertase Subtilisin Kexin 9 Inhibition: siRNA, Antisense Oligonucleotides, Adnectins, Vaccination, and New Attempts at Small-Molecule Inhibitors Based on New Discoveries. Front. Cardiovasc. Med. 2018, 5, 199. [Google Scholar] [CrossRef]
- Guardiola, M.; Plana, N.; Ibarretxe, D.; Cabré, A.; González, M.; Ribalta, J.; Masana, L. Circulating PCSK9 levels are positively correlated with NMR-assessed atherogenic dyslipidaemia in patients with high cardiovascular risk. Clin. Sci. 2015, 128, 877–882. [Google Scholar] [CrossRef]
- Druce, I.; Abujrad, H.; Ooi, T.C. PCSK9 and triglyceride-rich lipoprotein metabolism. J. Biomed. Res. 2015, 29, 429–436. [Google Scholar]
- Raal, F.; Panz, V.; Immelman, A.; Pilcher, G. Elevated PCSK9 levels in untreated patients with heterozygous or homozygous familial hypercholesterolemia and the response to high-dose statin therapy. J. Am. Heart Assoc. 2013, 2, e000028. [Google Scholar] [CrossRef]
- Timmers, S.; de Vogel-van den Bosch, J.; de Wit, N.; Schaart, G.; van Beurden, D.; Hesselink, M.; van der Meer, R.; Schrauwen, P. Differential effects of saturated versus unsaturated dietary fatty acids on weight gain and myocellular lipid profiles in mice. Nutr. Diabetes 2011, 1, e11. [Google Scholar] [CrossRef]
- Tubagus, T.A. Kadar Kolesterol Plasma Tikus Wistar pada Pemberiak Ekstrak Etanol dan Heksana dari Daun Gedi Merah (Abelmoschus manihot L.). J. MIPA 2015, 4, 63. [Google Scholar] [CrossRef]
- Mumtazah, D.F.; Busman, H.; Pratami, G.D.; Dewantara, A.P.; Amanda, F. Modification of atherogenic diet causes atherosclerosis, increase total cholesterol, and showing hepar damage in mice as alternative animal model in atherosclerosis research. GSC Biol. Pharm. Sci. 2021, 14, 001–006. [Google Scholar] [CrossRef]
- Mensink, R.P. Effects of Saturated Fatty Acids on Serum Lipids and Lipoproteins: A Systematic Review and Regression Analysis; World Health Organization: Geneva, Switzerland, 2016; pp. 1–63. [Google Scholar]
- Puteri, M.U.; Azmi, N.U.; Ridwan, S.; Iqbal, M.; Fatimah, T.; Rini, T.D.P.; Kato, M.; Saputri, F.C. Recent Update on PCSK9 and Platelet Activation Experimental Research Methods: In Vitro and In Vivo Studies. J. Cardiovasc. Dev. Dis. 2022, 9, 258. [Google Scholar] [CrossRef] [PubMed]
| Ingredients | HFD 1 (w/v) | HFD 2 (w/v) | HFD 3 (w/v) |
|---|---|---|---|
| Quail egg yolk | 40% | 30% | - |
| Goat fat | 25% | - | 50% |
| Beef fat | - | 35% | - |
| Butter | - | - | 15% |
| Cholesterol (Dyets, Bethlehem) | 2% | 2% | 2% |
| Cholic acid (Wako, Japan) | 0.5% | 0.5% | 0.5% |
| Fructose | 20% | 20% | 20% |
| Coconut oil | 12.5% | 12.5% | 12.5% |
| Ingredients | Weight | Carbohydrates | Protein | Fat (%) | Cholesterol | |||
|---|---|---|---|---|---|---|---|---|
| (g) | (%) | (%) | SFA | MUFA | PUFA | TFA | (%) | |
| Quail egg yolk | 100 [24] | 0.20–1.00 [24] | 15.70–16.60 [24] | 29.90 ± 3.54 [25] | 45.02 ± 5.16 [25] | 25.09 ± 6.58 [25] | N/A | 2.14 [24] |
| Goat Fat | 100 [26] | N/A | N/A | 47.32 [27] | 40.59 [27] | 7.80 [27] | N/A | 0.10 [27] |
| Beef fat | 100 [26] | N/A | N/A | 49.76 [27] | 41.80 [27] | 4 [27] | N/A | 0.11 [27] |
| Butter | 15 [27] # | 0 [27] # | 0 [27] # | 53.33 [27] # | 16.67 [27] # | 0 # | 3.33 [27] # | 0.20 # |
| Fructose | 10 # | 80 # | 0 [27] # | 0 # | 0 # | 0 # | N/A | N/A |
| Cholesterol | 500 # | N/A | N/A | N/A | N/A | N/A | N/A | 100 # |
| Cholic acid | 100 # | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Coconut oil | 14 # | 0 [27] # | 0 [27] # | 78.57 # | 7.14 # | 0 # | 0 # | 0 [27] # |
| Ingredients | HFD 1 | HFD 2 | HFD 3 | |||
|---|---|---|---|---|---|---|
| Weight (g) | Calories (kcal) | Weight (g) | Calories (kcal) | Weight (g) | Calories (kcal) | |
| Quail egg yolk | 1.55 | 4.99 | 1.16 | 3.74 | - | - |
| Goat fat | 0.66 | 5.95 | - | - | 1.31 | 11.82 |
| Beef fat | - | - | 0.92 | 8.30 | - | - |
| Butter | - | - | - | - | 0.51 | 3.74 |
| Fructose | 1.19 | 4.17 | 1.19 | 4.17 | 1.19 | 4.17 |
| Cholesterol | 0.08 | N/A | 0.08 | N/A | 0.08 | N/A |
| Cholic acid | 0.02 | N/A | 0.02 | N/A | 0.02 | N/A |
| Coconut oil | 0.42 | 3.30 | 0.42 | 3.30 | 0.42 | 3.30 |
| Total calories (kcal) | 18.41 | 19.51 | 23.03 | |||
| Control | HFD 1 | HFD 2 | HFD 3 | ||
|---|---|---|---|---|---|
| Plasma cholesterol (mg/dL) | Week 0 | 61.22 ± 8.31 | 60.62 ± 2.88 | 55.27 ± 6.46 | 69.70 ± 8.56 |
| Week 4 | 68.2 ± 9.54 | 97.42 ± 13.5 *# | 94.59 ± 12.19 **# | 152.61 ± 11.37 **### | |
| Week 8 | 68.56 ± 6.39 | 102.07 ± 14.33 *# | 133.61 ± 14.75 ***### | 186.99 ± 14.10 ***### | |
| Week 10 | 69.74 ± 5.66 | 139.68 ± 11.88 ***### | 161.14 ± 14.42 ***### | 248.80 ± 7.48 ***### | |
| Plasma triglyceride (mg/dL) | Week 0 | 71.20 ± 7.58 | 68.14 ± 9.33 | 70.36 ± 10.04 | 68.34 ± 13.51 |
| Week 4 | 68.00 ± 13.37 | 86.75 ± 11.42 | 106.94 ± 6.70 *## | 134.39 ± 14.73 **### | |
| Week 8 | 72.40 ± 14.99 | 117.99 ± 9.59 **## | 133.89 ± 13.27 **### | 199.22 ± 1.80 ***### | |
| Week 10 | 79.22 ± 14.03 | 142.06 ± 11.76 ***### | 185.94 ± 13.36 ***### | 236.85 ± 7.53 ***### |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saputri, F.C.; Azmi, N.U.; Puteri, M.U.; Damayanti; Novita, V.; Marisi, G.; Oktavira, E.; Sari, A.N.; Ronaningtyas, K.; Herawati, E. High-Fat Diet Enhances Platelet Activation and Is Associated with Proprotein Convertase Subtilisin Kexin 9: An Animal Study. Nutrients 2023, 15, 4463. https://doi.org/10.3390/nu15204463
Saputri FC, Azmi NU, Puteri MU, Damayanti, Novita V, Marisi G, Oktavira E, Sari AN, Ronaningtyas K, Herawati E. High-Fat Diet Enhances Platelet Activation and Is Associated with Proprotein Convertase Subtilisin Kexin 9: An Animal Study. Nutrients. 2023; 15(20):4463. https://doi.org/10.3390/nu15204463
Chicago/Turabian StyleSaputri, Fadlina Chany, Nuriza Ulul Azmi, Meidi Utami Puteri, Damayanti, Vivi Novita, Gracia Marisi, Elin Oktavira, Aninda Novika Sari, Khairunisa Ronaningtyas, and Enny Herawati. 2023. "High-Fat Diet Enhances Platelet Activation and Is Associated with Proprotein Convertase Subtilisin Kexin 9: An Animal Study" Nutrients 15, no. 20: 4463. https://doi.org/10.3390/nu15204463
APA StyleSaputri, F. C., Azmi, N. U., Puteri, M. U., Damayanti, Novita, V., Marisi, G., Oktavira, E., Sari, A. N., Ronaningtyas, K., & Herawati, E. (2023). High-Fat Diet Enhances Platelet Activation and Is Associated with Proprotein Convertase Subtilisin Kexin 9: An Animal Study. Nutrients, 15(20), 4463. https://doi.org/10.3390/nu15204463





