Regulation of Tumor Apoptosis of Poriae cutis-Derived Lanostane Triterpenes by AKT/PI3K and MAPK Signaling Pathways In Vitro
Abstract
:1. Introduction
2. Materials and Methods
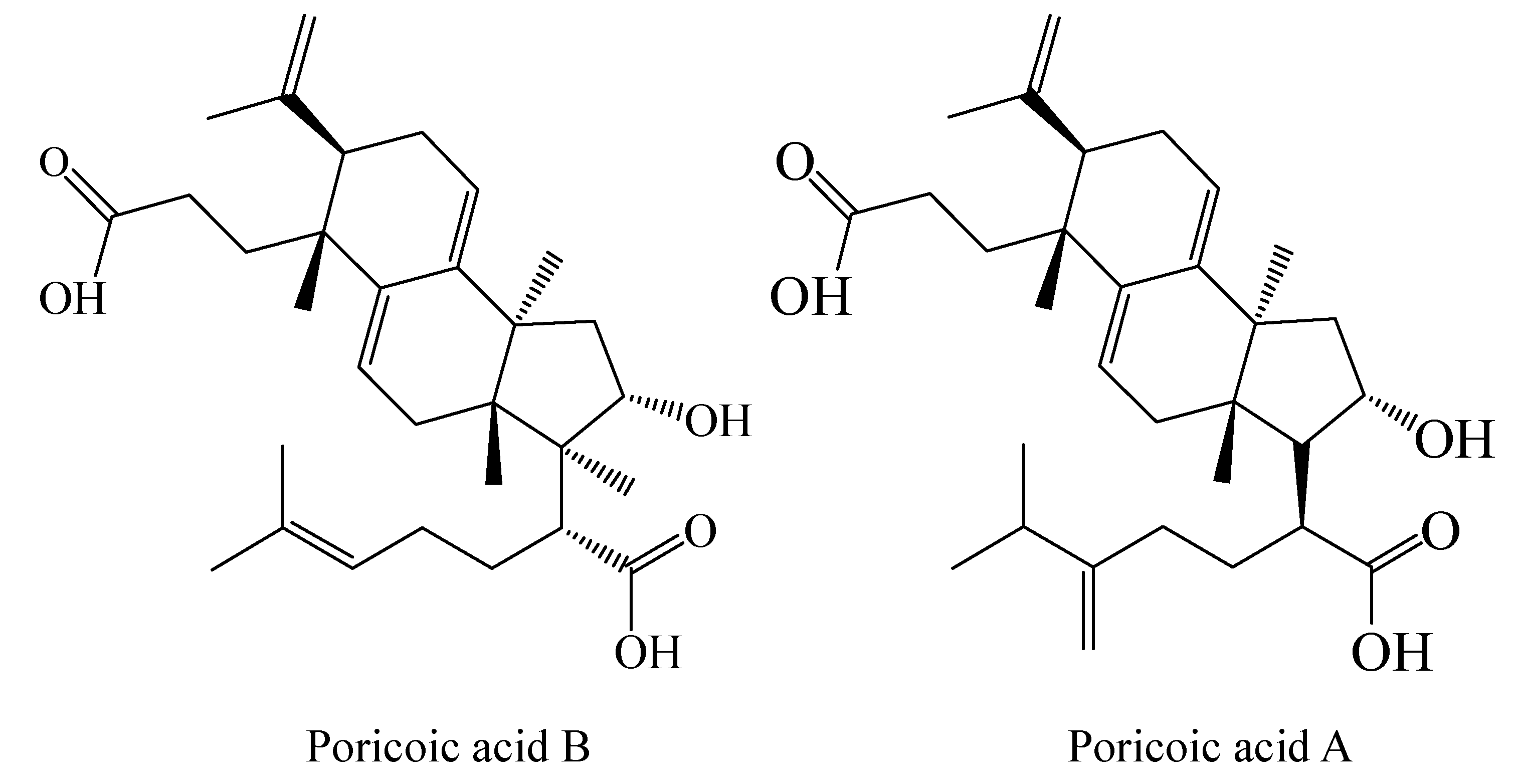
2.1. Extraction and Isolation of Triterpenes
2.2. Cell Culture
2.3. Antiproliferative MTT Assay
2.4. Acridine Orange/Ethidium Bromide (AO/EB) Staining
2.5. Apoptosis and Cell Cycle Assay
2.6. In Vitro Cell Migration/Invasion Assay
2.7. Intracellular ROS Generation
2.8. Real-Time Quantitative PCR
2.9. Molecular Docking
2.10. Statistics Analysis
3. Results and Discussion
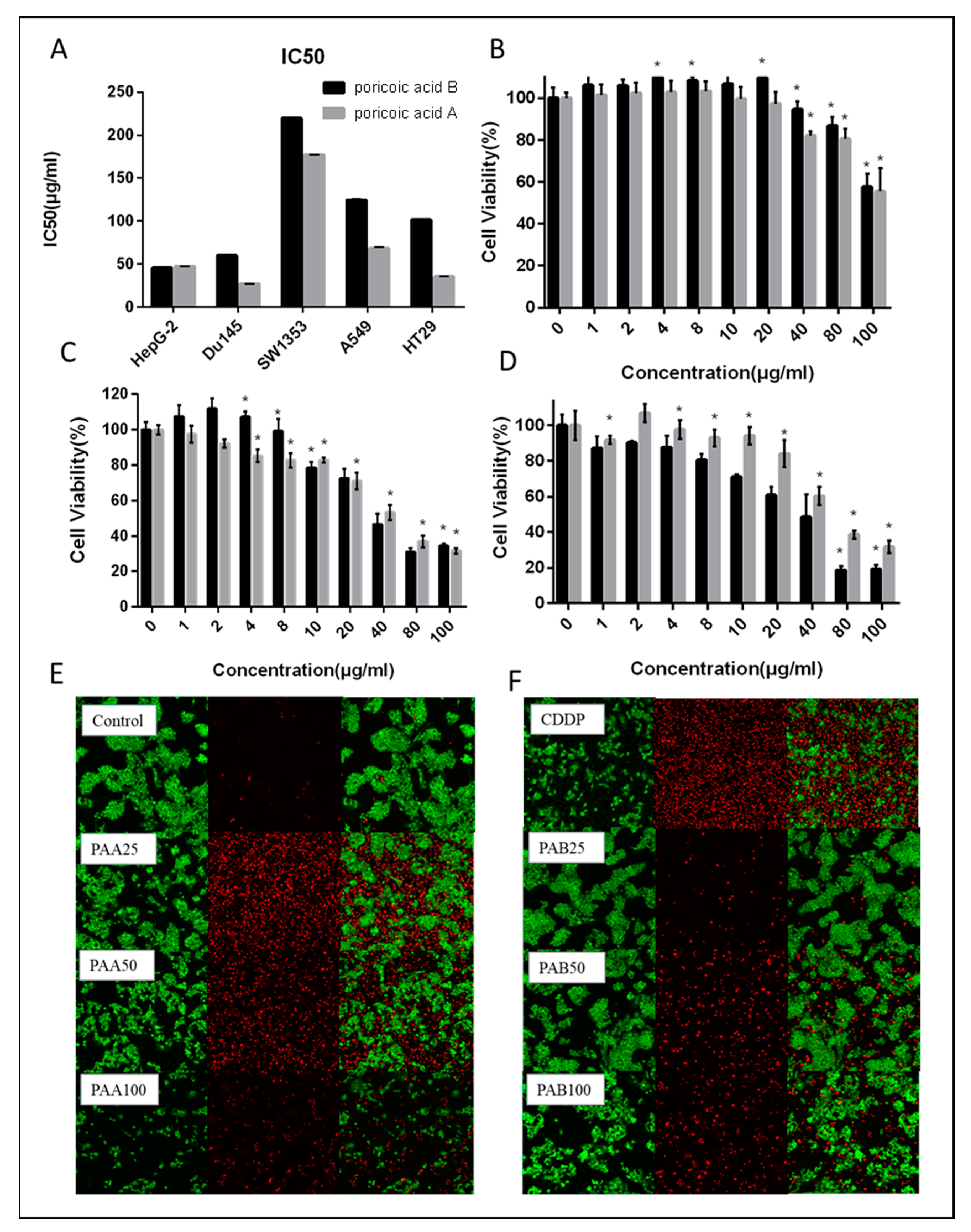
3.1. Antiproliferative Activity of PAA and PAB on Human Cancer Cells
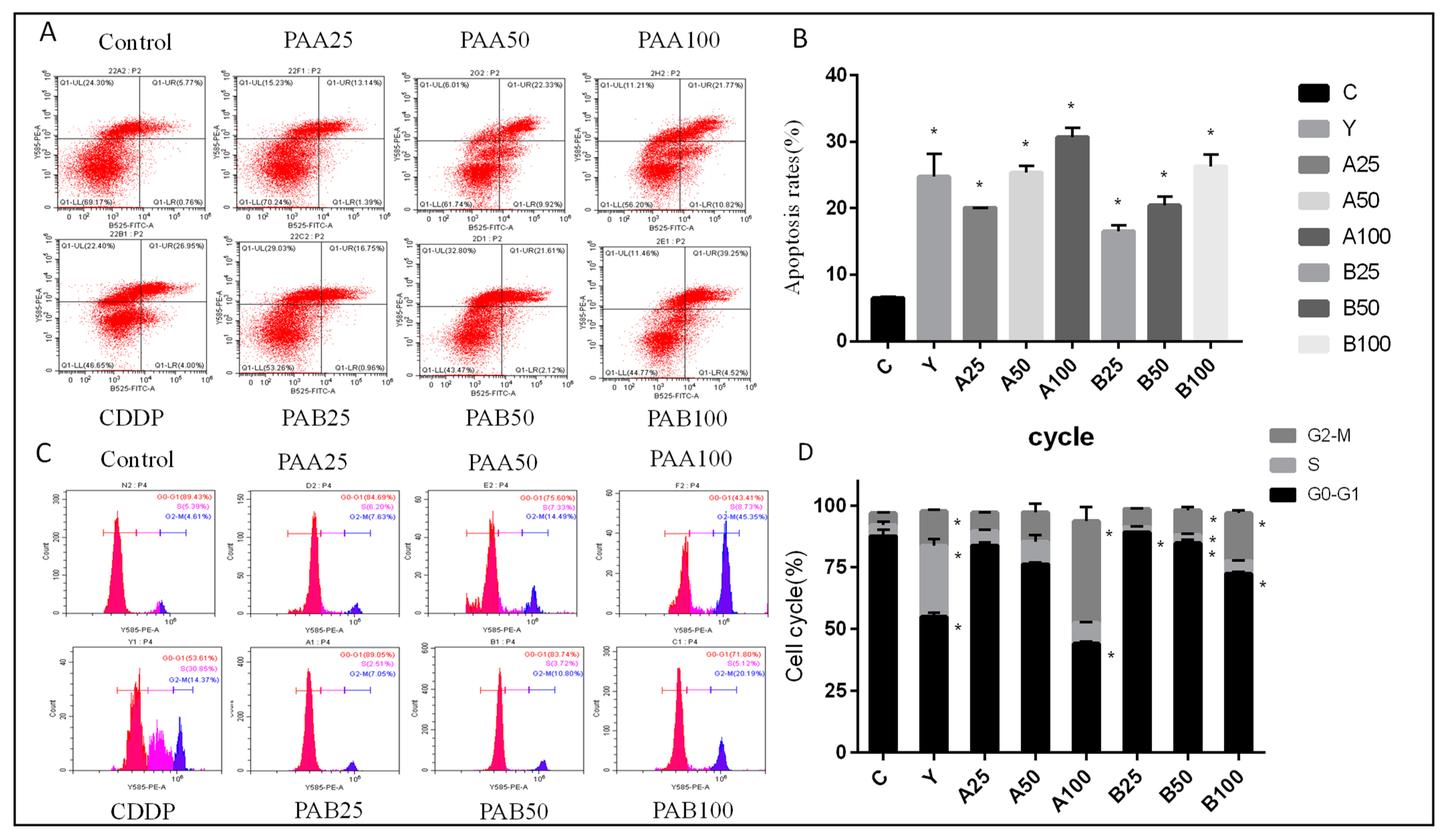
3.2. Effect of PAA and PAB on Cell Cycle Arrest and Apoptosis
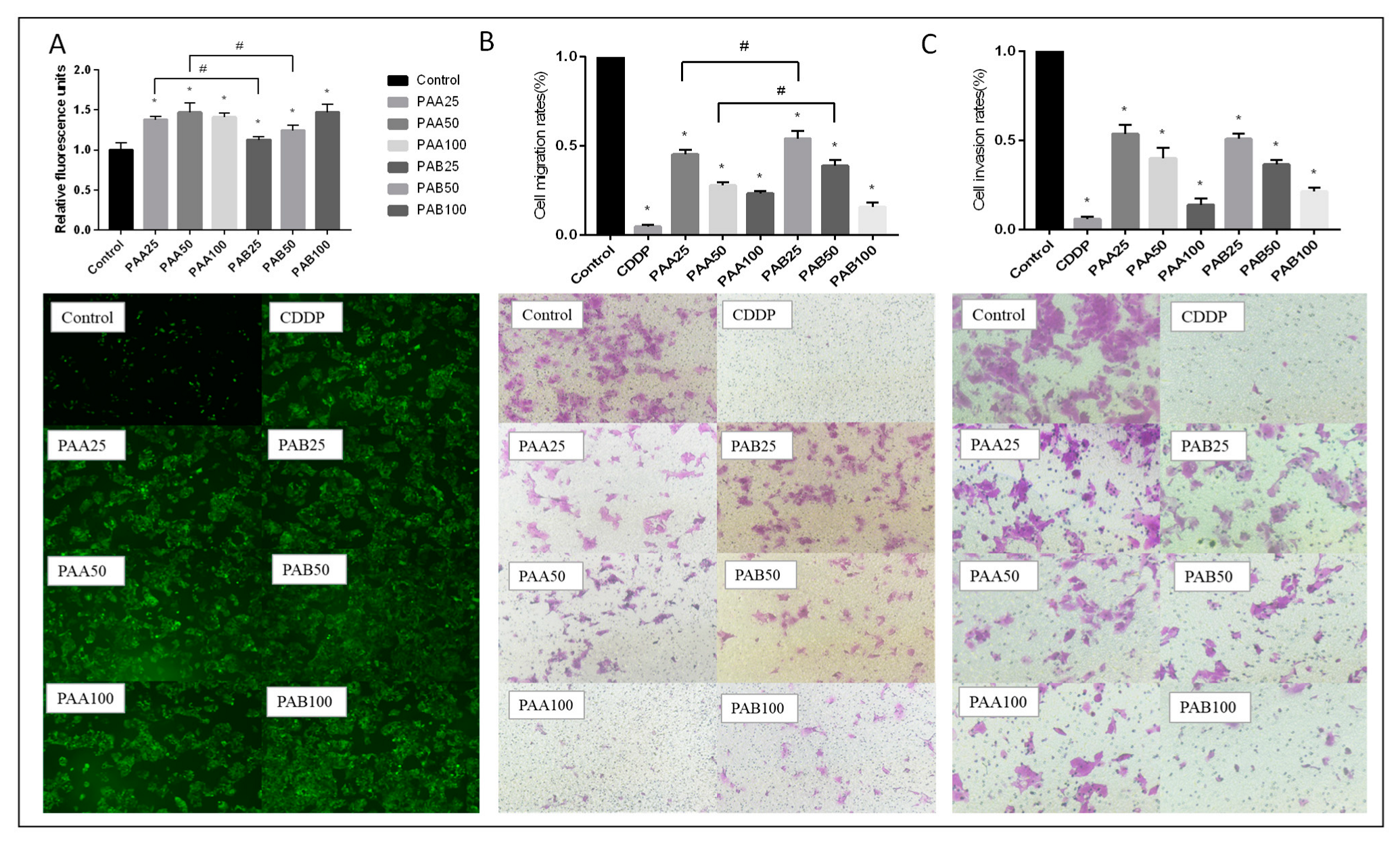
3.3. Effect of PAA and PAB on ROS Production in HepG2 Cells
3.4. Effect of PAA and PAB on Migration and Invasion in HepG2 Cells
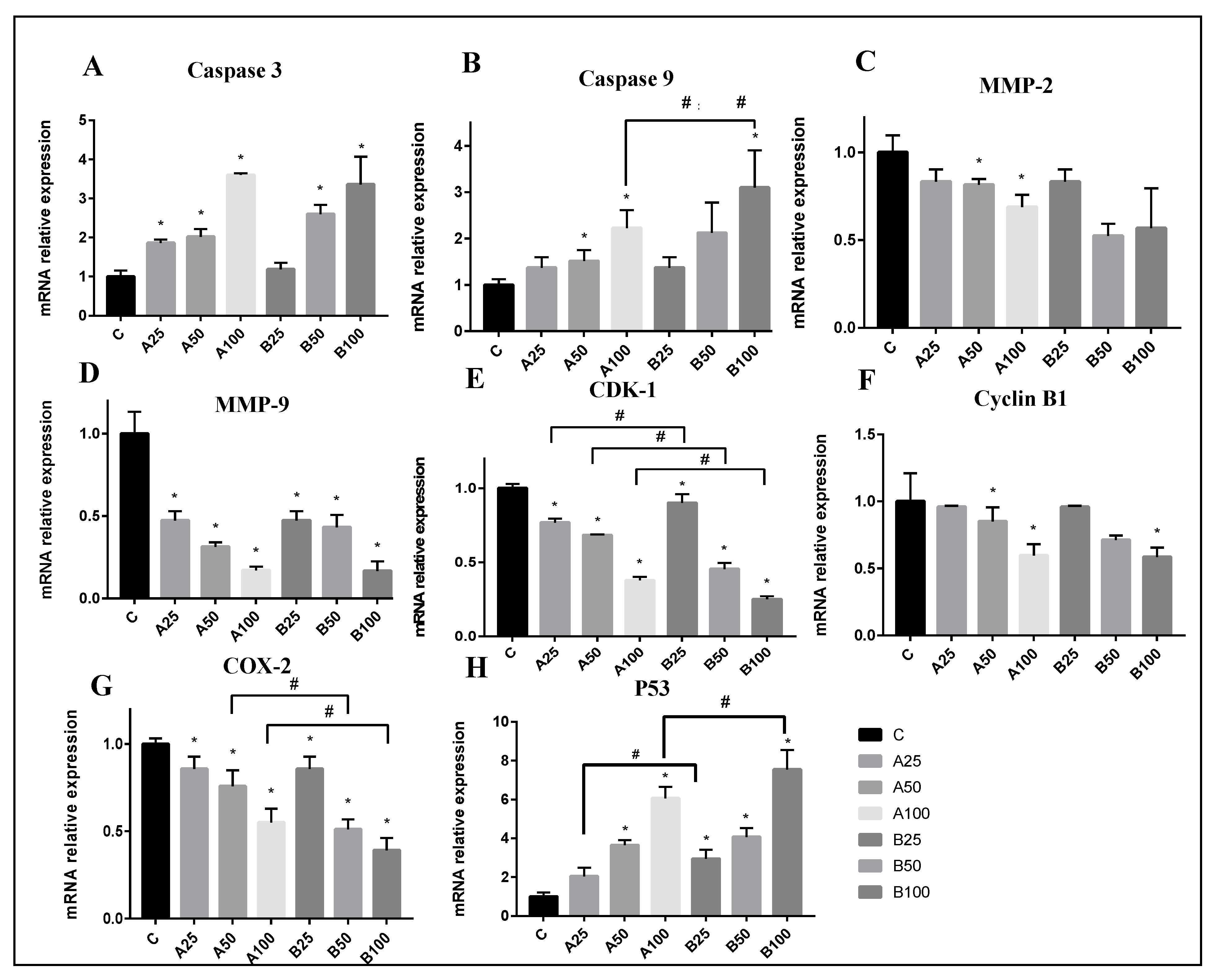
3.5. Mechanisms of Apoptosis Induced by PAA and PAB in HepG2 Cells
3.6. Screening by Molecular Docking Simulation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dong, H.-J.; Xue, Z.-Z.; Geng, Y.-L.; Wang, X.; Yang, B. Lanostane triterpenes isolated from epidermis of Poria cocos. Phytochem. Lett. 2017, 22, 102–106. [Google Scholar] [CrossRef]
- Bao, T.-R.; Long, G.-Q.; Wang, Y.; Wang, Q.; Liu, X.-L.; Hu, G.-S.; Gao, X.-X.; Wang, A.-H.; Jia, J.-M. New Lanostane-Type Triterpenes with Anti-Inflammatory Activity from the Epidermis of Wolfiporia cocos. J. Agric. Food Chem. 2022, 70, 4418–4433. [Google Scholar] [CrossRef] [PubMed]
- Ríos, J.-L. Chemical Constituents and Pharmacological Properties of Poria cocos. Planta Med. 2011, 77, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Z.; Zhang, J.; Zhao, Y.-L.; Li, T.; Shen, T.; Li, J.-Q.; Li, W.-Y.; Liu, H.-G. Mycology, cultivation, traditional uses, phytochemistry and pharmacology of Wolfiporia cocos (Schwein.) Ryvarden et Gilb.: A review. J. Ethnopharmacol. 2013, 147, 265–276. [Google Scholar] [CrossRef]
- Cheng, S.; Kristen, S.; Isaac, E.; Mcclintick, J.N.; Sandusky, G.E.; Daniel, S.; Guillermo, V. Pachymic Acid Inhibits Growth and Induces Apoptosis of Pancreatic Cancer In Vitro and In Vivo by Targeting ER Stress. PLoS ONE 2015, 10, e0122270. [Google Scholar] [CrossRef]
- Dong, H.; Wu, P.; Yan, R.; Xu, Q.; Li, H.; Zhang, F.; Li, J.; Yang, B. Enrichment and separation of antitumor triterpene acids from the epidermis of Poria cocos by pH-zone-refining counter-current chromatography and conventional high-speed counter-current chromatography. J. Sep. Sci. 2015, 38, 1977–1982. [Google Scholar] [CrossRef]
- Nie, A.; Chao, Y.; Zhang, X.; Jia, W.; Zhou, Z.; Zhu, C. Phytochemistry and Pharmacological Activities of Wolfiporia cocos (F.A. Wolf) Ryvarden & Gilb. Front. Pharmacol. 2020, 11, 505249. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, D.-Q.; Chen, L.; Liu, D.; Zhao, H.; Zhang, Z.-H.; Vaziri, N.D.; Guo, Y.; Zhao, Y.-Y.; Cao, G. Novel RAS Inhibitors Poricoic Acid ZG and Poricoic Acid ZH Attenuate Renal Fibrosis via a Wnt/β-Catenin Pathway and Targeted Phosphorylation of smad3 Signaling. J. Agric. Food Chem. 2018, 66, 1828–1842. [Google Scholar] [CrossRef]
- Chen, W.; Fan, Z.; Huang, C.; Liu, J. Poricoic Acid A Inhibits the NF-κB/MAPK Pathway to Alleviate Renal Fibrosis in Rats with Cardiorenal Syndrome. Evid.-Based Complement. Altern. Med. 2022, 2022, 8644353. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Zhang, Z.; Xu, J.; Liang, X.; Zhao, Q. Poricoic acid A induces apoptosis and autophagy in ovarian cancer via modulating the mTOR/p70s6k signaling axis. Braz. J. Med Biol. Res. 2021, 54, e11183. [Google Scholar] [CrossRef]
- Chen, D.Q.; Feng, Y.L.; Chen, L.; Liu, J.R.; Wang, M.; Vaziri, N.D.; Zhao, Y.Y. Poricoic acid A enhances melatonin inhibition of AKI-to-CKD transition by regulating Gas6/Axl-NF-kappa B/Nrf2 axis. Free. Radic. Biol. Med. Off. J. Oxyg. Soc. 2019, 134, 484–497. [Google Scholar] [CrossRef]
- Zhang, L.; Yin, M.; Feng, X.; Ibrahim, S.A.; Liu, Y.; Huang, W. Anti-Inflammatory Activity of Four Triterpenoids Isolated from Poriae Cutis. Foods 2021, 10, 3155. [Google Scholar] [CrossRef]
- Lu, J.; Tian, J.; Zhou, L.; Meng, L.; Chen, S.; Ma, C.; Wang, J.; Liu, Z.; Li, C.; Kang, W. Phytochemistry and Biological Activities of Poria. J. Chem. 2021, 2021, 6659775. [Google Scholar] [CrossRef]
- Naqvi, A.A.T.; Mohammad, T.; Hasan, G.M.; Hassan, I. Advancements in Docking and Molecular Dynamics Simulations Towards Ligand-receptor Interactions and Structure-function Relationships. Curr. Top. Med. Chem. 2018, 18, 1755–1768. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, I.; Endo, K.; Yamamoto, E.; Hirano, Y.; Yasuoka, K. Differences in ligand-induced protein dynamics extracted from an unsupervised deep learning approach correlate with protein–ligand binding affinities. Commun. Biol. 2022, 5, 481. [Google Scholar] [CrossRef]
- Yin, M.; Liu, Y.; Zhang, L.; Wang, Y. Isolation and preparation of poricoic acid A and poricoic acid B from poriae cutis by high-speed counter current chromatography. Food Sci. 2020, 41, 179–184. [Google Scholar]
- Zhang, X.; He, H.; Xiang, J.; Li, B.; Zhao, M.; Hou, T. Selenium-containing soybean antioxidant peptides: Preparation and comprehensive comparison of different selenium supplements. Food Chem. 2021, 358, 129888. [Google Scholar] [CrossRef]
- Guo, D.; Liu, W.; Zhang, X.; Zhao, M.; Zhu, B.; Hou, T.; He, H. Duck Egg White–Derived Peptide VSEE (Val-Ser-Glu-Glu) Regulates Bone and Lipid Metabolisms by Wnt/β-Catenin Signaling Pathway and Intestinal Microbiota. Mol. Nutr. Food Res. 2019, 63, 1900525. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Liu, M.; Liu, Y.; Hu, H.; Pan, Y.; Zou, W.; Fan, X.; Hu, X. Transforming growth factor β1 promotes migration and invasion in HepG2 cells: Epithelial-to-mesenchymal transition via JAK/STAT3 signaling. Int. J. Mol. Med. 2017, 41, 129–136. [Google Scholar] [CrossRef]
- Feng, L.A.; Ying, L.A.; Xi, F.B.; Sai, C.; Wen, H.A. Structure characterization and in vitro immunomodulatory activities of carboxymethyl pachymaran. Int. J. Biol. Macromol. 2021, 178, 94–103. [Google Scholar]
- Guo, D.; He, H.; Hou, T. Purification and characterization of positive allosteric regulatory peptides of calcium sensing receptor (CaSR) from desalted duck egg white—ScienceDirect. Food Chem. 2020, 325, 126919. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Sun, J.; Hou, Z.; Luan, W.; Wang, S.; Cui, S.; Cheng, M.; Liu, Y. Novel antitumor compound optimized from natural saponin Albiziabioside A induced caspase-dependent apoptosis and ferroptosis as a p53 activator through the mitochondrial pathway. Eur. J. Med. Chem. 2018, 157, 759–772. [Google Scholar] [CrossRef] [PubMed]
- Ríos, J.-L.; Andújar, I. Lanostanoids from Fungi as Potential Medicinal Agents. In Fungal Metabolites; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–34. [Google Scholar] [CrossRef]
- Dong, H.; Yan, R.; Li, H.; Wang, W.; Wang, X.; Yang, B. Acid-Alkali Extraction of Triterpene Acids from Poria and Preparative Separation by High-Speed Counter-Current Chromatography. Sep. Sci. Technol. 2014, 49, 2765–2771. [Google Scholar] [CrossRef]
- Nie, J.; Qin, X.; Li, Z. Revealing the anti-melanoma mechanism of n-BuOH fraction from the red kidney bean coat extract based on network pharmacology and transcriptomic approach. Food Res. Int. 2021, 140, 109880. [Google Scholar] [CrossRef]
- Gao, L.; Cai, S.; Cai, A.; Zhao, Y.; Xu, T.; Ma, Y.; Xu, Y.; Wang, Y.; Wang, H.; Hu, Y. The improved antitumor efficacy of continuous intratumoral chemotherapy with cisplatin-loaded implants for the treatment of sarcoma 180 tumor-bearing mice. Drug Deliv. 2019, 26, 208–215. [Google Scholar] [CrossRef]
- Bata, N.; Cosford, N.D.P. Cell Survival and Cell Death at the Intersection of Autophagy and Apoptosis: Implications for Current and Future Cancer Therapeutics. ACS Pharmacol. Transl. Sci. 2021, 4, 1728–1746. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.-N.; Zhao, N.; Chen, J.-Y.; Ye, P.-P.; Nan, X.-W.; Zhou, H.-H.; Jiang, Q.-W.; Yang, Y.; Huang, J.-R.; Yuan, M.-L.; et al. Celastrol Inhibits the Growth of Ovarian Cancer Cells in vitro and in vivo. Front. Oncol. 2019, 9, 2. [Google Scholar] [CrossRef]
- Sachan, R.; Kundu, A.; Jeon, Y.; Choi, W.S.; Yoon, K.; Kim, I.S.; Kwak, J.H.; Kim, H.S. Afrocyclamin A, a triterpene saponin, induces apoptosis and autophagic cell death via the PI3K/Akt/mTOR pathway in human prostate cancer cells. Phytomedicine 2018, 51, 139–150. [Google Scholar] [CrossRef]
- Zhang, J.; Zuo, T.; Liang, X.; Xu, Y.; Yang, Y.; Fang, T.; Li, J.; Chen, D.; Shen, Q. Fenton-reaction-stimulative nanoparticles decorated with a reactive-oxygen-species (ROS)-responsive molecular switch for ROS amplification and triple negative breast cancer therapy. J. Mater. Chem. B 2019, 7, 7141–7151. [Google Scholar] [CrossRef]
- Liu, Y.; Guan, X.; Wang, M.; Wang, N.; Chen, Y.; Li, B.; Xu, Z.; Fu, F.; Du, C.; Zheng, Z. Disulfiram/Copper induces antitumor activity against gastric cancer via the ROS/MAPK and NPL4 pathways. Bioengineered 2022, 13, 6579–6589. [Google Scholar] [CrossRef]
- Inoue, T.; Suzuki-Karasaki, Y. Mitochondrial superoxide mediates mitochondrial and endoplasmic reticulum dysfunctions in TRAIL-induced apoptosis in Jurkat cells. Free. Radic. Biol. Med. 2013, 61, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Li-Weber, M. Targeting apoptosis pathways in cancer by Chinese medicine. Cancer Lett. 2013, 332, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Habtetsion, T.; Ding, Z.C.; Pi, W.; Li, T.; Lu, C.; Chen, T.; Xi, C.; Spartz, H.; Liu, K.; Hao, Z. Alteration of tumor metabolism by CD4+ T cells leads to Tnf-α-dependent intensification of oxidative stress and tumor cell death hhs public access. Cell Metab. 2019, 28, 228–242. [Google Scholar] [CrossRef]
- Xu, H.; Zhao, H.; Ding, C.; Jiang, D.; Zhao, Z.; Li, Y.; Ding, X.; Gao, J.; Zhou, H.; Luo, C.; et al. Celastrol suppresses colorectal cancer via covalent targeting peroxiredoxin 1. Signal Transduct. Target. Ther. 2023, 8, 51. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, J.; Cao, N.; Gao, J.; Xie, Y.; Zhou, S.; Tang, X. ASIC1α up-regulates MMP-2/9 expression to enhance mobility and proliferation of liver cancer cells via the PI3K/AKT/mTOR pathway. BMC Cancer 2022, 22, 778. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhu, C.; An, B.; Chen, Y.; He, X.; Qian, L.; Lan, L.; Li, S. Indirubin inhibits cell proliferation, migration, invasion and angiogenesis in tumor-derived endothelial cells. OncoTargets Ther. 2017, 11, 2937–2944. [Google Scholar] [CrossRef]
- Kumar, N.; Drabu, S.; Mondal, S.C. NSAID’s and selectively COX-2 inhibitors as potential chemoprotective agents against cancer: 1st Cancer Update. Arab. J. Chem. 2013, 6, 1–23. [Google Scholar] [CrossRef]
- Tsapras, P.; Nezis, I.P. Caspase involvement in autophagy. Cell Death Differ. 2017, 24, 1369–1379. [Google Scholar] [CrossRef]
- Mcilwain, D.R.; Berger, T.; Mak, T.W. Caspase Functions in Cell Death and Disease. Cold Spring Harb. Perspect. Biol. 2013, 5, a008656. [Google Scholar] [CrossRef]
- Cho, D.H.; Jo, Y.K.; Hwang, J.J.; Lee, Y.M.; Roh, S.A.; Kim, J.C. Caspase-mediated cleavage of ATG6/Beclin-1 links apoptosis to autophagy in HeLa cells. Cancer Lett. 2009, 274, 95–100. [Google Scholar] [CrossRef]
- Nguyen, T.T.M.; Gillet, G.; Popgeorgiev, N. Caspases in the Developing Central Nervous System: Apoptosis and Beyond. Front. Cell Dev. Biol. 2021, 9, 702404. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Guo, Z.; Wu, K.; Zhang, Y.; Tian, Y.; Zhao, B.; Lu, H. Aconitine induces cell apoptosis via mitochondria and death receptor signaling pathways in hippocampus cell line. Res. Veter. Sci. 2022, 143, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Kesavardhana, S.; Kanneganti, T.D. Targeting Apoptosis Inhibition to Activate Antitumor Immunity. Trends Immunol. 2019, 40, 1073–1075. [Google Scholar] [CrossRef]
- Zhang, C.-H.; Xu, G.-L.; Jia, W.-D.; Li, J.-S.; Ma, J.-L.; Ren, W.-H.; Ge, Y.-S.; Yu, J.-H.; Liu, W.-B.; Wang, W. Activation of STAT3 Signal Pathway Correlates with Twist and E-Cadherin Expression in Hepatocellular Carcinoma and Their Clinical Significance. J. Surg. Res. 2012, 174, 120–129. [Google Scholar] [CrossRef]
- Rudolf, E.; Schröterová, L.; Ježková, A.; Caltová, K.; Králová, V.; Hanušová, V. Inositol hexaphosphate limits the migration and the invasiveness of colorectal carcinoma cells in vitro. Int. J. Oncol. 2018, 53, 1625–1632. [Google Scholar] [CrossRef]
- Wen, Y.; Cai, X.; Chen, S.; Fu, W.; Chai, D.; Zhang, H.; Zhang, Y. 7-Methoxy-1-Tetralone Induces Apoptosis, Suppresses Cell Proliferation and Migration in Hepatocellular Carcinoma via Regulating c-Met, p-AKT, NF-κB, MMP2, and MMP9 Expression. Front. Oncol. 2020, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Yufu, T.; Pengfei, L.; Zhongyi, S.; Lei, H.; Wenping, Z.; Eric, A. 14-3-3β Promotes Migration and Invasion of Human Hepatocellular Carcinoma Cells by Modulating Expression of MMP2 and MMP9 through PI3K/Akt/NF-κB Pathway. PLoS ONE 2016, 11, e0146070. [Google Scholar]
- Hu, J.; Zhai, W.; Ma, J.; Zhou, X. Study of the expression of matrix metalloproteinase and its tissue inhibitor in hepatocellular carcinoma. Chin. J. Dig. 2001, 21, 461–464. [Google Scholar]
- Shen, J.; Zhu, X.; Wu, Z.; Shi, Y.; Wen, T. Uvangoletin, extracted from Sarcandra glabra, exerts anticancer activity by inducing autophagy and apoptosis and inhibiting invasion and migration on hepatocellular carcinoma cells. Phytomedicine 2021, 94, 153793. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; You, J.; Wu, Q.; Meng, W.; He, Q.; Yang, B.; Zhu, C.; Cao, J. Cyclin-dependent kinases-based synthetic lethality: Evidence, concept, and strategy. Acta Pharm. Sin. B 2021, 11, 2738–2748. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhang, B.; Lv, J.; Zhang, P.; Mao, Q.; Lin, F.; Zhao, J.; Fu, X.; Yang, Y.; Li, Z.; et al. Scaffold hopping of celastrol provides derivatives containing pepper ring, pyrazine and oxazole substructures as potent autophagy inducers against breast cancer cell line MCF-7. Eur. J. Med. Chem. 2022, 234, 114254. [Google Scholar] [CrossRef]
- Xu, K.; Wang, L.; Shu, H. COX-2 overexpression increases malignant potential of human glioma cells through Id1. Oncotarget 2014, 5, 1241. [Google Scholar] [CrossRef]
- Mota, N.S.; Kviecinski, M.R.; Zeferino, R.C.; de Oliveira, D.A.; Bretanha, L.C.; Ferreira, S.R.; Micke, G.A.; Filho, D.W.; Pedrosa, R.C.; Ourique, F. In vivo antitumor activity of by-products of Passiflora edulis f. flavicarpa Deg. Rich in medium and long chain fatty acids evaluated through oxidative stress markers, cell cycle arrest and apoptosis induction. Food Chem. Toxicol. 2018, 118, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Cebola, I.; Peinado, M.A. Epigenetic deregulation of the COX pathway in cancer. Prog. Lipid Res. 2012, 51, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Vosooghi, M.; Amini, M. The discovery and development of cyclooxygenase-2 inhibitors as potential anticancer therapies. Expert Opin. Drug Discov. 2014, 9, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Bin, W.; He, W.; Feng, Z.; Xiangdong, L.; Yong, C.; Lele, K.; Hongbin, Z.; Honglin, G. Prognostic relevance of cyclooxygenase-2 (COX-2) expression in Chinese patients with prostate cancer. Acta Histochem. 2011, 113, 131–136. [Google Scholar] [CrossRef]
- Chen, J.-H.; Wu, C.-W.; Kao, H.-L.; Chang, H.-M.; Li, A.F.-Y.; Liu, T.-Y.; Chi, C.-W. Effects of COX-2 inhibitor on growth of human gastric cancer cells and its relation to hepatocyte growth factor. Cancer Lett. 2006, 239, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Heravi, R.E.; Hadizadeh, F.; Sankian, M.; Afshari, J.T.; Taghdisi, S.M.; Jafarian, H.; Behravan, J. Novel selective Cox-2 inhibitors induce apoptosis in Caco-2 colorectal carcinoma cell line. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2011, 44, 479–486. [Google Scholar]
- Zhang, H.; Li, X.; Ding, J.; Xu, H.; Dai, X.; Hou, Z.; Zhang, K.; Sun, K.; Sun, W. Delivery of ursolic acid (UA) in polymeric nanoparticles effectively promotes the apoptosis of gastric cancer cells through enhanced inhibition of cyclooxygenase 2 (COX-2). Int. J. Pharm. 2013, 441, 261–268. [Google Scholar] [CrossRef]
- Bieging, K.T.; Mello, S.S.; Attardi, L.D. Unravelling mechanisms of p53-mediated tumour suppression. Nat. Rev. Cancer 2014, 14, 359–370. [Google Scholar] [CrossRef]
- Valente, L.J.; Gray, D.H.D.; Michalak, E.M.; Pinon-Hofbauer, J.; Egle, A.; Scott, C.L.; Janic, A.; Strasser, A. p53 Efficiently Suppresses Tumor Development in the Complete Absence of Its Cell-Cycle Inhibitory and Proapoptotic Effectors p21, Puma, and Noxa. Cell Rep. 2013, 3, 1339–1345. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Wei, T.; Li, Z.; Zhu, J. p53-dependent apoptosis is essential for the antitumor effect of paclitaxel response to DNA damage in papillary thyroid carcinoma. Int. J. Med. Sci. 2021, 18, 3197–3205. [Google Scholar] [CrossRef] [PubMed]
- Roos, W.P.; Thomas, A.D.; Kaina, B. DNA damage and the balance between survival and death in cancer biology. Nat. Rev. Cancer 2015, 16, 20–33. [Google Scholar] [CrossRef]
- Thangavel, N.; Albratty, M. Pharmacophore model-aided virtual screening combined with comparative molecular docking and molecular dynamics for identification of marine natural products as SARS-CoV-2 papain-like protease inhibitors. Arab. J. Chem. 2022, 15, 104334. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.S.; Kang, O.; Mai, C.W.; Tiong, K.H.; Khoo, A.S.-B.; Pichika, M.R.; Bradshaw, T.D.; Leong, C.-O. 6-Shogaol inhibits breast and colon cancer cell proliferation through activation of peroxisomal proliferator activated receptor γ (PPARγ). Cancer Lett. 2013, 336, 127–139. [Google Scholar] [CrossRef]
- Tsubaki, M.; Satou, T.; Itoh, T.; Imano, M.; Ogaki, M.; Yanae, M.; Nishida, S. Reduction of metastasis, cell invasion, and adhesion in mouse osteosarcoma by YM529/ONO-5920-induced blockade of the Ras/MEK/ERK and Ras/PI3K/Akt pathway. Toxicol. Appl. Pharmacol. 2012, 259, 402–410. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, L.; Zhang, C.; Deng, Y.; Zhao, B.; Ren, Y.; Fu, Y.; Meng, X. Inhibitor of growth 3 induces cell death by regulating cell proliferation, apoptosis and cell cycle arrest by blocking the PI3K/AKT pathway. Cancer Gene Ther. 2018, 25, 240–247. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, W.; Li, B.; Zhang, K.; Wu, Y.; Xu, H.; Wang, J.; Zhang, J.; Fan, R.; Wei, J. Curcumin Promotes Cell Cycle Arrest and Inhibits Survival of Human Renal Cancer Cells by Negative Modulation of the PI3K/AKT Signaling Pathway. Cell Biochem. Biophys. 2015, 73, 681–686. [Google Scholar] [CrossRef]
- Bonel-Pérez, G.C.; Pérez-Jiménez, A.; Gris-Cárdenas, I.; Parra-Pérez, A.M.; Lupiáñez, J.A.; Reyes-Zurita, F.J.; Siles, E.; Csuk, R.; Peragón, J.; Rufino-Palomares, E.E. Antiproliferative and Pro-Apoptotic Effect of Uvaol in Human Hepatocarcinoma HepG2 Cells by Affecting G0/G1 Cell Cycle Arrest, ROS Production and AKT/PI3K Signaling Pathway. Molecules 2020, 25, 4254. [Google Scholar] [CrossRef]
- Dai, X.; Xu, X.; Zhang, J.; Wu, G. Effect of Butein on the Proliferation of Gastric Cancer Cells. Genom. Appl. Biol. 2019, 38, 4715–4719. [Google Scholar]
- Chen, J.; Li, L.; Su, J.; Li, B.; Chen, T.; Ling, F.; Zhang, X. Enhancing effect of natural borneol on the cellular uptake of demethoxycurcumin and their combined induction of G2/M arrest in HepG2 cells via ROS generation. J. Funct. Foods 2015, 17, 103–114. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, G.; Zhang, H.; Zhang, F.; Zhou, B.; Ning, F.; Wang, H.S.; Cai, S.H.; Du, J. Acquisition of epithelial-mesenchymal transition phenotype and cancer stem cell-like properties in cisplatin-resistant lung cancer cells through AKT/p-catenin/Snail signaling pathway. Eur. J. Pharmacol. Int. J. 2014, 723, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Yip, W.K.; Seow, H.F. Activation of phosphatidylinositol 3-kinase/Akt signaling by EGF downregulates membranous E-cadherin and β-catenin and enhances invasion in nasopharyngeal carcinoma cells. Cancer Lett. 2012, 318, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Kolch, W. Meaningful relationships: The regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem. J. 2000, 351, 289–305. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; Qi, Y.; Zhong, S.; Zeng, J.; Chen, X.; Yao, J. Mitogen-activated protein kinase inhibition enhances the antitumor effects of sporamin in human pancreatic cancer cells. Oncol. Lett. 2018, 16, 1237–1242. [Google Scholar] [CrossRef] [PubMed]
- Peti, W.; Page, R. Molecular basis of MAP kinase regulation. Protein Sci. Publ. Protein Soc. 2013, 22, 1698–1710. [Google Scholar] [CrossRef]
- Johnson, G.L.; Lapadat, R. Mitogen-Activated Protein Kinase Pathways Mediated by ERK, JNK, and p38 Protein Kinases. Science 2002, 298, 1911–1912. [Google Scholar] [CrossRef]
- Xu, H.; Liu, L.; Ding, M.; Fan, W.; Song, H. Effect of Ganoderma applanatum polysaccharides on MAPK/ERK pathway affecting autophagy in breast cancer MCF-7 cells. Int. J. Biol. Macromol. 2020, 146, 353–362. [Google Scholar]
- Min, L.; He, B.; Hui, L. Mitogen-activated protein kinases in hepatocellular carcinoma development. Semin. Cancer Biol. 2011, 21, 10–20. [Google Scholar] [CrossRef]
- Wang, J.; Yuan, L.; Xiao, H.; Wang, C.; Xiao, C.; Wang, Y.; Liu, X. A novel mechanism for momordin Ic-induced HepG2 apoptosis: Involvement of PI3K- and MAPK-dependent PPARg activation. Food Funct. 2014, 5, 859–868. [Google Scholar] [CrossRef]
- Hao, D.; Sarfaraz, M.O.; Farshidfar, F.; Bebb, D.G.; Lee, C.Y.; Card, C.M.; David, M.; Weljie, A.M. Temporal characterization of serum metabolite signatures in lung cancer patients undergoing treatment. Metabolomics 2016, 12, 58. [Google Scholar] [CrossRef]
- Cong, F.-S.; Zhang, Y.-R.; Sheng, H.-C.; Ao, Z.-H.; Zhang, S.-Y.; Wang, J.-Y. Deuterium-depleted water inhibits human lung carcinoma cell growth by apoptosis. Exp. Ther. Med. 2010, 1, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Arima, K.; Lau, M.C.; Zhao, M.; Haruki, K.; Kosumi, K.; Mima, K.; Gu, M.; Väyrynen, J.P.; Twombly, T.S.; Baba, Y.; et al. Metabolic Profiling of Formalin-Fixed Paraffin-Embedded Tissues Discriminates Normal Colon from Colorectal Cancer. Mol. Cancer Res. 2020, 18, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Sabtu, S.N.; Sani, S.F.A.; Looi, L.M.; Chiew, S.F.; Pathmanathan, D.; Bradley, D.A.; Osman, Z. Indication of high lipid content in epithelial-mesenchymal transitions of breast tissues. Sci. Rep. 2021, 11, 3250. [Google Scholar] [CrossRef]
- Santaliz-Casiano, A.; Mehta, D.; Danciu, O.C.; Patel, H.; Banks, L.; Zaidi, A.; Buckley, J.; Rauscher, G.H.; Schulte, L.; Weller, L.R.; et al. Identification of metabolic pathways contributing to ER+ breast cancer disparities using a machine-learning pipeline. Sci. Rep. 2023, 13, 12136. [Google Scholar] [CrossRef] [PubMed]
- Somlyai, G.; Nagy, L.I.; Puskás, L.G.; Papp, A.; Kovács, B.Z.; Fórizs, I.; Czuppon, G.; Somlyai, I. Deuterium Content of the Organic Compounds in Food Has an Impact on Tumor Growth in Mice. Curr. Issues Mol. Biol. 2023, 45, 66–77. [Google Scholar] [CrossRef] [PubMed]
| Proteins | Poricoic Acid A (PAA) | Poricoic Acid B (PAB) | ||||||
|---|---|---|---|---|---|---|---|---|
| Total Score | Crash | Polar | Binding Energy (kcal/mol) | Total Score | Crash | Polar | Binding Energy (kcal/mol) | |
| AKT | 4.4734 | −1.6060 | 5.5532 | −7.6 | 4.3085 | −1.4271 | 4.8327 | −8.0 |
| PI3K | 4.2297 | −2.8787 | 4.7772 | −5.1 | 4.1559 | −1.4352 | 3.7563 | −6.7 |
| ERK | 4.4894 | −3.2933 | 3.7209 | −7.4 | 4.0913 | −1.6300 | 2.6628 | −6.5 |
| JNK | 4.1819 | −1.8209 | 2.3171 | −8.8 | 4.8064 | −1.3607 | 1.9971 | −8.2 |
| P38 | 5.6784 | −2.1307 | 4.0457 | −8.0 | 5.9058 | −2.2057 | 3.3674 | −6.9 |
| P53 | 4.5928 | −3.1685 | 3.1603 | −6.5 | 7.1757 | −2.8259 | 4.3213 | −7.2 |
| Bcl-2 | 2.3230 | −1.5790 | 2.7255 | −6.9 | 3.2755 | −0.8705 | 3.9976 | −7.5 |
| Bax | 1.4245 | −1.5416 | 1.7694 | −6.7 | 4.4059 | −0.8569 | 4.6240 | −6.2 |
| Caspase-3 | 5.1799 | −2.2216 | 5.6010 | −6.9 | 4.5097 | −1.6615 | 5.0136 | −7.1 |
| E-cadherin | 3.0189 | −2.0251 | 3.9197 | −6.4 | 3.3511 | −0.9367 | 4.9135 | −6.2 |
| ICAM-1 | 3.0536 | −2.7535 | 4.3288 | −6.1 | 3.1307 | −0.9398 | 2.4113 | −5.9 |
| MMP-9 | 5.4450 | −3.4685 | 3.3047 | −7.6 | 6.4213 | −1.8768 | 2.5850 | −6.5 |
| COX-2 | 3.7997 | −2.4828 | 1.5334 | −7.1 | 4.9986 | −2.0211 | 2.3840 | −8.3 |
| Cyclin D | 4.6249 | −1.8272 | 2.9440 | −6.5 | 5.5773 | −1.3391 | 2.8638 | −6.5 |
| CDK1 | 3.9919 | −1.9891 | 3.9244 | −7.8 | 5.5295 | −1.8477 | 4.5974 | −7.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, S.; Feng, X.; Cai, Y.; Ibrahim, S.A.; Liu, Y.; Huang, W. Regulation of Tumor Apoptosis of Poriae cutis-Derived Lanostane Triterpenes by AKT/PI3K and MAPK Signaling Pathways In Vitro. Nutrients 2023, 15, 4360. https://doi.org/10.3390/nu15204360
Yue S, Feng X, Cai Y, Ibrahim SA, Liu Y, Huang W. Regulation of Tumor Apoptosis of Poriae cutis-Derived Lanostane Triterpenes by AKT/PI3K and MAPK Signaling Pathways In Vitro. Nutrients. 2023; 15(20):4360. https://doi.org/10.3390/nu15204360
Chicago/Turabian StyleYue, Shuai, Xi Feng, Yousheng Cai, Salam A. Ibrahim, Ying Liu, and Wen Huang. 2023. "Regulation of Tumor Apoptosis of Poriae cutis-Derived Lanostane Triterpenes by AKT/PI3K and MAPK Signaling Pathways In Vitro" Nutrients 15, no. 20: 4360. https://doi.org/10.3390/nu15204360
APA StyleYue, S., Feng, X., Cai, Y., Ibrahim, S. A., Liu, Y., & Huang, W. (2023). Regulation of Tumor Apoptosis of Poriae cutis-Derived Lanostane Triterpenes by AKT/PI3K and MAPK Signaling Pathways In Vitro. Nutrients, 15(20), 4360. https://doi.org/10.3390/nu15204360






