Fruit Pouch Consumption Does Not Associate with Early Manifestations of Allergic Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection
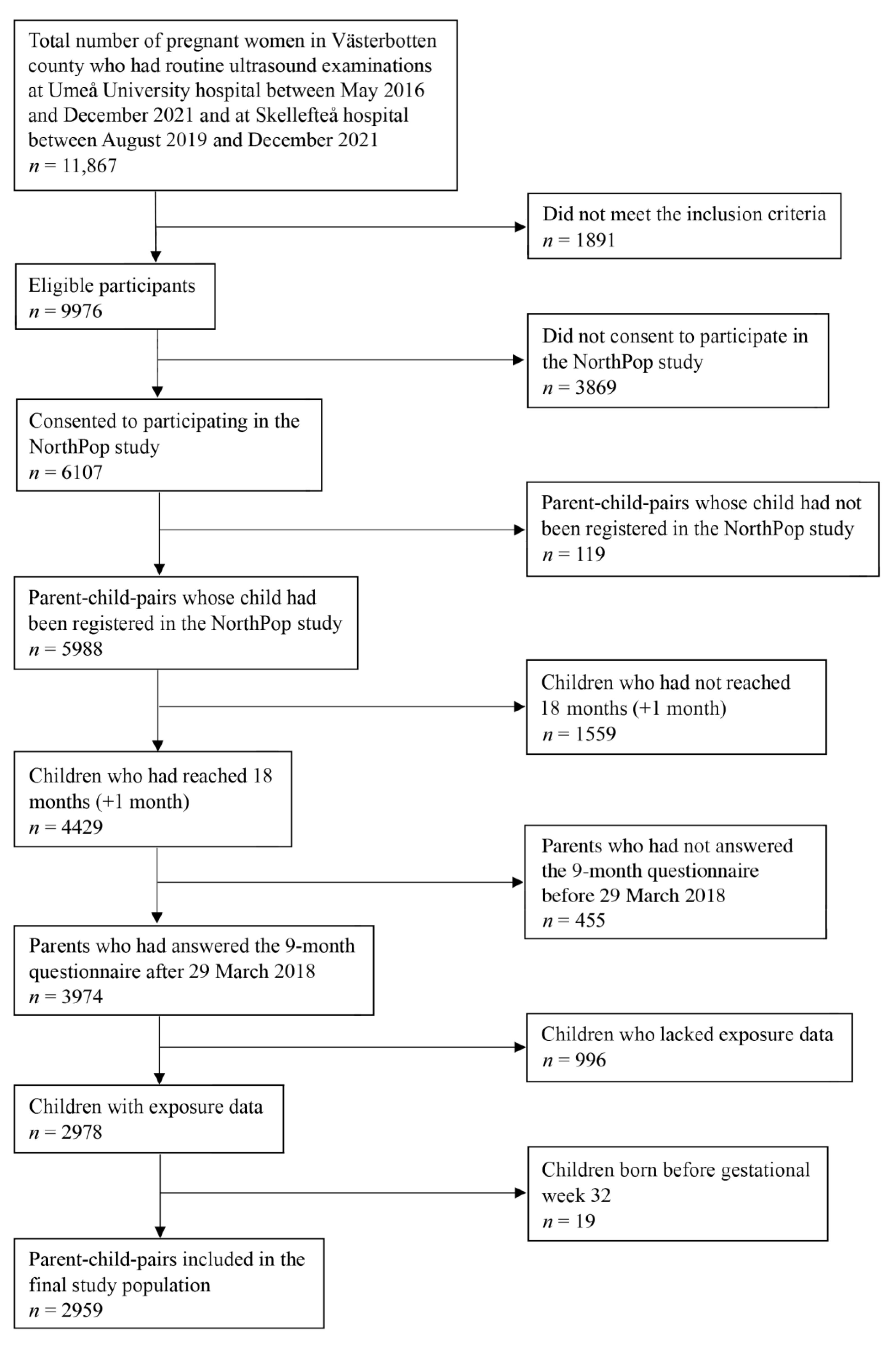
2.2. Study Population
2.3. Main Exposures
2.4. Primary Outcomes
2.5. Covariates
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
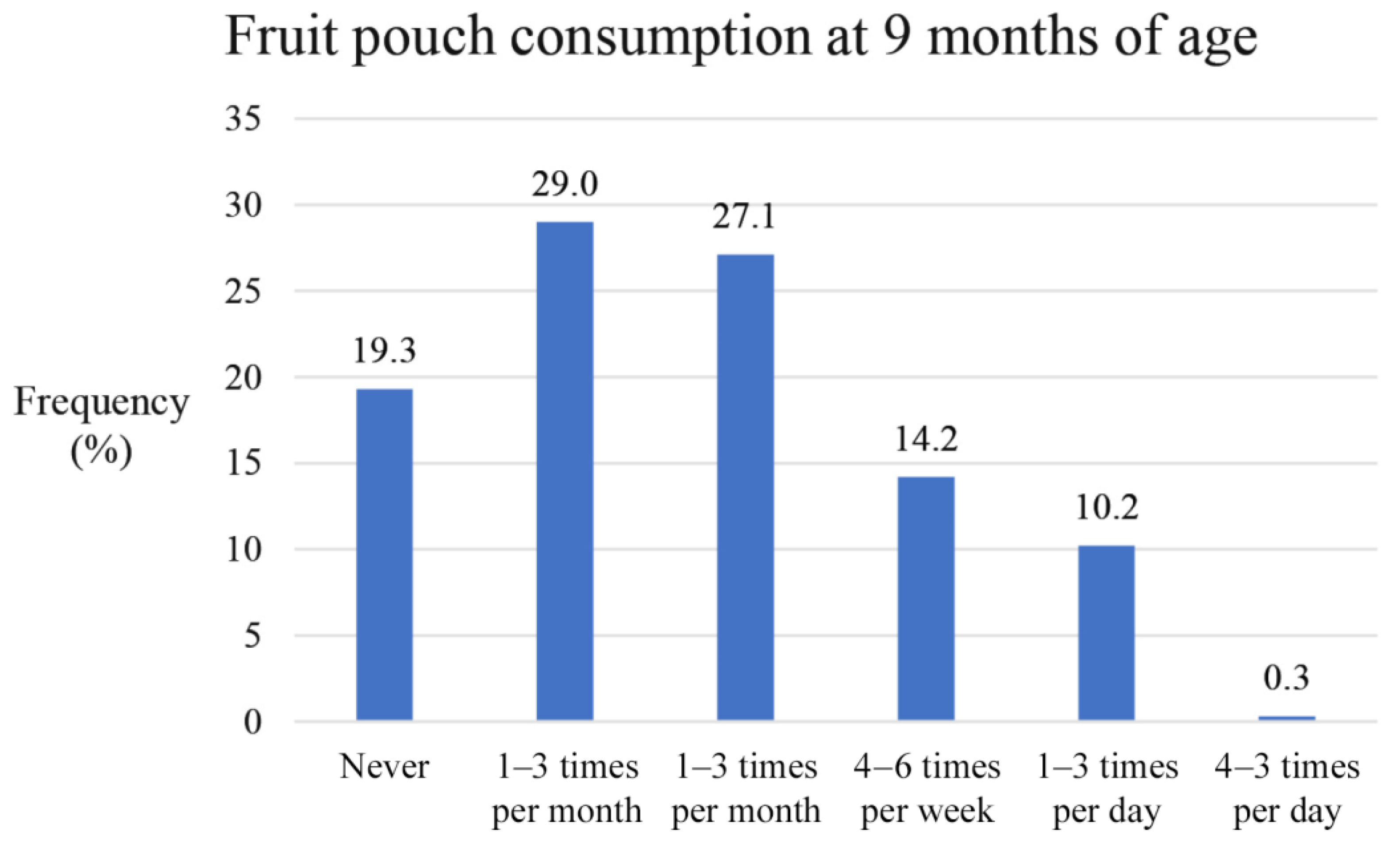
3.2. Fruit Pouch Consumption at 9 Months of Age
3.3. Prevalence of Allergic Diseases at 18 Months of Age
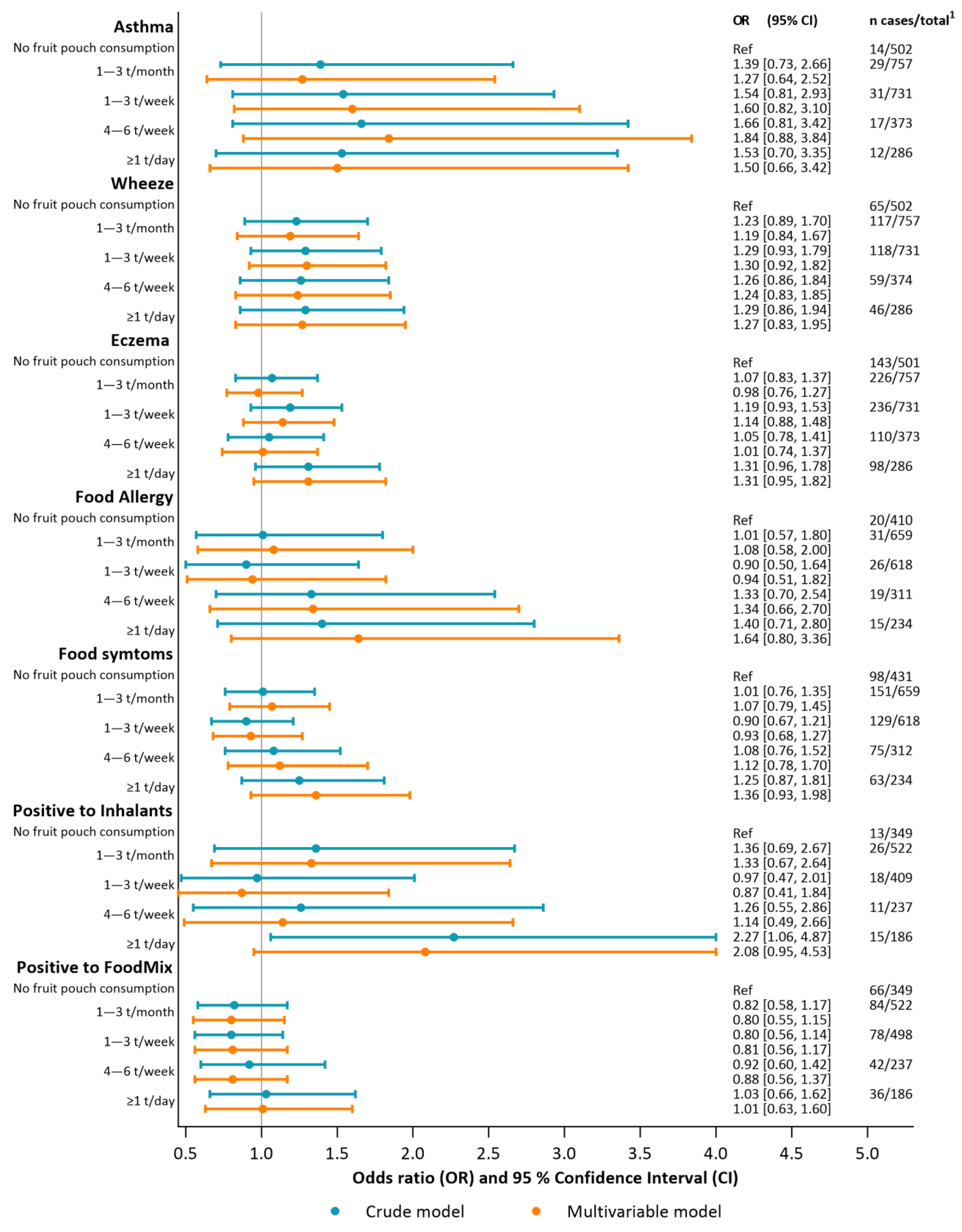
3.4. Associations between Fruit Pouch Consumption and Early Allergic Manifestations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B

References
- Pawankar, R. Allergic diseases and asthma: A global public health concern and a call to action. World Allergy Organ. J. 2014, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Schernhammer, E.S.; Vutuc, C.; Waldhör, T.; Haidinger, G. Time trends of the prevalence of asthma and allergic disease in Austrian children. Pediatr. Allergy Immunol. 2008, 19, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Backman, H.; Räisänen, P.; Hedman, L.; Stridsman, C.; Andersson, M.; Lindberg, A.; Lundbäck, B.; Rönmark, E. Increased prevalence of allergic asthma from 1996 to 2006 and further to 2016-results from three population surveys. Clin. Exp. Allergy 2017, 47, 1426–1435. [Google Scholar] [CrossRef]
- Borna, E.; Nwaru, B.I.; Bjerg, A.; Mincheva, R.; Rådinger, M.; Lundbäck, B.; Ekerljung, L. Changes in the prevalence of asthma and respiratory symptoms in western Sweden between 2008 and 2016. Allergy 2019, 74, 1703–1715. [Google Scholar] [CrossRef] [PubMed]
- Sterner, T.; Uldahl, A.; Svensson, Å.; Björk, J.; Svedman, C.; Nielsen, C.; Tunsäter, A.; Bruze, M.; Kiotseridis, H. The Southern Sweden Adolescent Allergy-Cohort: Prevalence of allergic diseases and cross-sectional associations with individual and social factors. J. Asthma 2019, 56, 227–235. [Google Scholar] [CrossRef]
- Akdis, C.A. Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions? Nat. Rev. Immunol. 2021, 21, 739–751. [Google Scholar] [CrossRef]
- Finn, K.; Lenighan, Y.; Eldridge, A.; Kineman, B.; Pac, S. Pouch Use Among Infants Does Not Impact Exposure to Other Forms of Fruits and Vegetables: Data from the Feeding Infants and Toddlers Study (FITS) 2016. Curr. Dev. Nutr. 2020, 4 (Suppl. 2), 4140982. [Google Scholar] [CrossRef]
- Knight, T.; Smith, P.K.; Soutter, V.; Oswald, E.; Venter, C. Is the low pH of infant and toddler foods a concern? Pediatr. Allergy Immunol. 2021, 32, 1103–1106. [Google Scholar] [CrossRef]
- Northpop Research Infrastructure. Umu.se. 2023. Available online: https://www.umu.se/en/research/infrastructure/northpop/ (accessed on 21 August 2023).
- Charman, C.R.; Venn, A.J.; Ravenscroft, J.C.; Williams, H.C. Translating Patient-Oriented Eczema Measure (POEM) scores into clinical practice by suggesting severity strata derived using anchor-based methods. Br. J. Dermatol. 2013, 169, 1326–1332. [Google Scholar] [CrossRef]
- Kelderer, F.; Mogren, I.; Eriksson, C.; Silfverdal, S.A.; Domellöf, M.; West, C.E. Associations between pre- and postnatal antibiotic exposures and early allergic outcomes: A population-based birth cohort study. Pediatr. Allergy Immunol. 2022, 33, e13848. [Google Scholar] [CrossRef]
- Stephansson, O.; Petersson, K.; Björk, C.; Conner, P.; Wikström, A.K. The Swedish Pregnancy Register—For quality of care improvement and research. Acta Obstet. Gynecol. Scand. 2018, 97, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Textor, J.; van der Zander, B.; Gilthorpe, M.S.; Liskiewicz, M.; Ellison, G.T. Robust causal inference using directed acyclic graphs: The R package ‘dagitty’. Int. J. Epidemiol. 2016, 45, 1887–1894. [Google Scholar] [CrossRef] [PubMed]
- Celebi Sozener, Z.; Özbey Yücel, Ü.; Altiner, S.; Ozdel Oztürk, B.; Cerci, P.; Türk, M.; Gorgülü Akin, B.; Akdis, M.; Yilmaz, I.; Ozdemir, C.; et al. The External Exposome and Allergies: From the Perspective of the Epithelial Barrier Hypothesis. Front. Allergy 2022, 3, 887672. [Google Scholar] [CrossRef] [PubMed]
- West, C.E.; Renz, H.; Jenmalm, M.C.; Kozyrskyj, A.L.; Allen, K.J.; Vuillermin, P.; Prescott, S.L.; MacKay, C.; Salminen, S.; Wong, G.; et al. The gut microbiota and inflammatory noncommunicable diseases: Associations and potentials for gut microbiota therapies. J. Allergy Clin. Immunol. 2015, 135, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Lif Holgerson, P.; Esberg, A.; West, C.E.; Johansson, I. The breast milk and childhood gastrointestinal microbiotas and disease outcomes: A longitudinal study. Pediatr. Res. 2023, 93, 570–578. [Google Scholar] [CrossRef]
- Sjödin, K.S.; Hammarström, M.; Rydén, P.; Sjödin, A.; Hernell, O.; Engstrand, L.; West, C.E. Temporal and long-term gut microbiota variation in allergic disease: A prospective study from infancy to school age. Allergy 2019, 74, 176–185. [Google Scholar] [CrossRef]
- West, C.E.; Rydén, P.; Lundin, D.; Engstrand, L.; Tulic, M.K.; Prescott, S.L. Gut microbiome and innate immune response patterns in IgE-associated eczema. Clin. Exp. Allergy 2015, 45, 1419–1429. [Google Scholar] [CrossRef]
- Barber, D.; Díaz-Perales, A.; Villalba, M.; Chivato, T. Challenges for allergy diagnosis in regions with complex pollen exposures. Curr. Allergy Asthma Rep. 2015, 15, 496. [Google Scholar] [CrossRef]
- Rosace, D.; Gomez-Casado, C.; Fernandez, P.; Perez-Gordo, M.; Dominguez, M.d.C.; Vega, A.; Belver, M.T.; Ramos, T.; Vega, F.; Marco, G.; et al. Profilin-mediated food-induced allergic reactions are associated with oral epithelial remodeling. J. Allergy Clin. Immunol. 2019, 143, 681–690.e1. [Google Scholar] [CrossRef]
- Hosseini, B.; Berthon, B.S.; Wark, P.; Wood, L.G. Effects of Fruit and Vegetable Consumption on Risk of Asthma, Wheezing and Immune Responses: A Systematic Review and Meta-Analysis. Nutrients 2017, 9, 341. [Google Scholar] [CrossRef]
- West, C.E.; Videky, D.J.; Prescott, S.L. Role of diet in the development of immune tolerance in the context of allergic disease. Curr. Opin. Pediatr. 2010, 22, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Leong, C.; Haszard, J.J.; Lawley, B.; Otal, A.; Taylor, R.W.; Szymlek-Gay, E.A.; Fleming, E.A.; Daniels, L.; Fangupo, L.J.; Tannock, G.W.; et al. Mediation Analysis as a Means of Identifying Dietary Components That Differentially Affect the Fecal Microbiota of Infants Weaned by Modified Baby-Led and Traditional Approaches. Appl. Environ. Microbiol. 2018, 84, e00914-18. [Google Scholar] [CrossRef] [PubMed]
- Di Profio, E.; Magenes, V.C.; Fiore, G.; Agostinelli, M.; La Mendola, A.; Acunzo, M.; Francavilla, R.; Indrio, F.; Bosetti, A.; D’auria, E.; et al. Special Diets in Infants and Children and Impact on Gut Microbioma. Nutrients 2022, 14, 3198. [Google Scholar] [CrossRef]
- Laursen, M.F. Gut Microbiota Development: Influence of Diet from Infancy to Toddlerhood. Ann. Nutr. Metab. 2021, 77, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-T.; Li, C.-Y.; Chang, Y.-T.; Bai, Y.-M.; Tsai, S.-J.; Chen, T.-J.; Chiou, S.-H.; Chen, M.-H. Proton-pump inhibitors are associated with an increased risk of asthma: A nationwide nested case-control study. Allergy Asthma Proc. 2023, 44, 345–353. [Google Scholar] [CrossRef]
- Wang, Y.H.; Wintzell, V.; Ludvigsson, J.F.; Svanström, H.; Pasternak, B. Association Between Proton Pump Inhibitor Use and Risk of Asthma in Children. JAMA Pediatr. 2021, 175, 394–403. [Google Scholar] [CrossRef]
- Mitre, E.; Susi, A.; Kropp, L.E.; Schwartz, D.J.; Gorman, G.H.; Nylund, C.M. Associations Between Use of Acid-Suppressive Medications and Antibiotics During Infancy and Allergic Diseases in Early Childhood. JAMA Pediatr. 2018, 172, e180315. [Google Scholar] [CrossRef]
- Robinson, L.B.; Camargo, C.A., Jr. Acid suppressant medications and the risk of allergic diseases. Expert. Rev. Clin. Immunol. 2018, 14, 771–780. [Google Scholar] [CrossRef]
- Taylor, R.W.; A Conlon, C.; Beck, K.L.; von Hurst, P.R.; Morenga, L.A.T.; Daniels, L.; Haszard, J.J.; Meldrum, A.M.; McLean, N.H.; Cox, A.M.; et al. Nutritional Implications of Baby-Led Weaning and Baby Food Pouches as Novel Methods of Infant Feeding: Protocol for an Observational Study. JMIR Res. Protoc. 2021, 10, e29048. [Google Scholar] [CrossRef]
- Beauregard, J.L.; Bates, M.; Cogswell, M.E.; Nelson, J.M.; Hamner, H.C. Nutrient Content of Squeeze Pouch Foods for Infants and Toddlers Sold in the United States in 2015. Nutrients 2019, 11, 1689. [Google Scholar] [CrossRef]
- Moding, K.J.; Ferrante, M.J.; Bellows, L.L.; Bakke, A.J.; Hayes, J.E.; Johnson, S.L. Nutritional Content and Ingredients of Commercial Infant and Toddler Food Pouches Compared With Other Packages Available in the United States. Nutr. Today 2019, 54, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Lehmann Hirsch, N.; Jewell, J.M.; Caroli, M.; Rodrigues Da Silva Breda, J.; Weber, M. Pureed Fruit Pouches for Babies: Child Health Under Squeeze. J. Pediatr. Gastroenterol. Nutr. 2018, 67, 561–563. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Bührer, C.; Ensenauer, R.; Jochum, F.; Kalhoff, H.; Lawrenz, B.; Körner, A.; Mihatsch, W.; Rudloff, S.; Zimmer, K.-P. Complementary foods in baby food pouches: Position statement from the Nutrition Commission of the German Society for Pediatrics and Adolescent Medicine (DGKJ, e.V.). Mol. Cell Pediatr. 2019, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Lundkvist, E.; Stoltz Sjöström, E.; Lundberg, R.; Silfverdal, S.-A.; West, C.E.; Domellöf, M. Fruit Pouch Consumption and Dietary Patterns Related to BMIz at 18 Months of Age. Nutrients 2021, 13, 2265. [Google Scholar] [CrossRef]
- Sly, P.D.; Boner, A.L.; Björksten, B.; Bush, A.; Custovic, A.; A Eigenmann, P.; E Gern, J.; Gerritsen, J.; Hamelmann, E.; Helms, P.J.; et al. Early identification of atopy in the prediction of persistent asthma in children. Lancet 2008, 372, 1100–1106. [Google Scholar] [CrossRef]

| Child Characteristics | ||||
| Child, n = 2959 | Missing Data, Child | |||
| N (%) 1/Mean (SD) 2 | N (%) 1 | |||
| Girls | 1441 (48.7) 1 | 0 (0) 1 | ||
| Birth weight | 3536.0 (522.9) 2 | 0 (0) 1 | ||
| Birth length | 50.11 (2.5) 2 | 0 (0) 1 | ||
| Delivery method Vaginal Caesarean section Obstetrical vacuum extraction | 2303 (77.8) 1 515 (17.4) 1 135 (4.6) 1 | 0 (0) 1 | ||
| Gestational week | 39.33 (1.6) 2 | 0 (0) 1 | ||
| Siblings (yes) | 1165 (39.9) 1 | 150 (5.1) 1 | ||
| Feeding at 4 months Breastfeeding Breastfeeding and formula Formula | 1883 (63.6) 1 450 (15.2) 1 489 (16.5) 1 | 137 (4.6) 1 | ||
| Pets in first 9 months of life (yes) | 1576 (53.2) 1 | 145 (4.9) 1 | ||
| Blood sample taken at 18 months | 1792 (60.6) 1 | 1167 (39.4) 1 | ||
| Parental characteristics | ||||
| Mother, n = 2959 | Missing data, mother | Partner, n = 2922 | Missing data, partner | |
| N (%) 1/Mean (SD) 2 | N (%) 1 | N (%)1/Mean (SD) 2 | N (%) 1 | |
| Age | 31.0 (4.3) 2 | 0 (0) 1 | 32.9 (6.6) 2 | 0 (0) 1 |
| Education <9 years High school University | 74 (2.5) 1 802 (27.1) 1 2029 (68.6) 1 | 54 (1.8) 1 | 64 (2.2) 1 1144 (38.7) 1 1316 (44.5) 1 | 435 (14.7) 1 |
| Restricted diet 3 | 407 (13.7) 1 | 94 (3.2) 1 | 182 (6.1) 1 | 532 (18.0) 1 |
| BMI | 24.9 (4.6) 2 | 72 (2.4) 1 | - | - |
| Smoking No Last month before pregnancy During pregnancy | 2783 (94.1) 1 70 (2.4) 1 38 (1.3) 1 | 68 (2.3) 1 | - | - |
| Asthma (yes) | 543 (18.4) 1 | 61 (2.1) 1 | 426 (14.4) 1 | 467 (15.8) 1 |
| Hay fever (yes) | 788 (26.6) 1 | 61 (2.1) 1 | 732 (24.7) 1 | 467 (15.8) 1 |
| Fur allergy (yes) | 598 (20.2) 1 | 61 (2.1) 1 | 645 (21.8) 1 | 467 (15.8) 1 |
| Food allergy (yes) | 490 (16.6) 1 | 61 (2.1) 1 | 253 (8.6) 1 | 467 (15.8) 1 |
| Eczema (yes) | 477 (16.1) 1 | 61 (2.1) 1 | 180 (6.1) 1 | 467 (15.8) 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fredriksson, E.; Bodén, S.; Domellöf, M.; West, C.E. Fruit Pouch Consumption Does Not Associate with Early Manifestations of Allergic Disease. Nutrients 2023, 15, 4318. https://doi.org/10.3390/nu15204318
Fredriksson E, Bodén S, Domellöf M, West CE. Fruit Pouch Consumption Does Not Associate with Early Manifestations of Allergic Disease. Nutrients. 2023; 15(20):4318. https://doi.org/10.3390/nu15204318
Chicago/Turabian StyleFredriksson, Emmy, Stina Bodén, Magnus Domellöf, and Christina E. West. 2023. "Fruit Pouch Consumption Does Not Associate with Early Manifestations of Allergic Disease" Nutrients 15, no. 20: 4318. https://doi.org/10.3390/nu15204318
APA StyleFredriksson, E., Bodén, S., Domellöf, M., & West, C. E. (2023). Fruit Pouch Consumption Does Not Associate with Early Manifestations of Allergic Disease. Nutrients, 15(20), 4318. https://doi.org/10.3390/nu15204318





