Kaempferitrin: A Flavonoid Marker to Distinguish Camellia oleifera Honey
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Honey and Nectar Samples’ Collection
2.3. Honey and Nectar Preparation
2.4. Analysis the Chemical Parameters of C. oleifera Honey
2.4.1. Analysis Methods for the Sugar Composition in C. oleifera Honey
2.4.2. Analysis Methods for the Water, Acidity, and 5-HMF Composition in C. oleifera Honey
2.5. LC-MS/MS Method for the Determination of Flavonoids in Honey and Nectar
2.6. LC-MS/MS Analysis
2.7. Conversion of Flavonoid Amounts in Honey and Nectar Samples
2.8. Data Processing
3. Results and Discussion
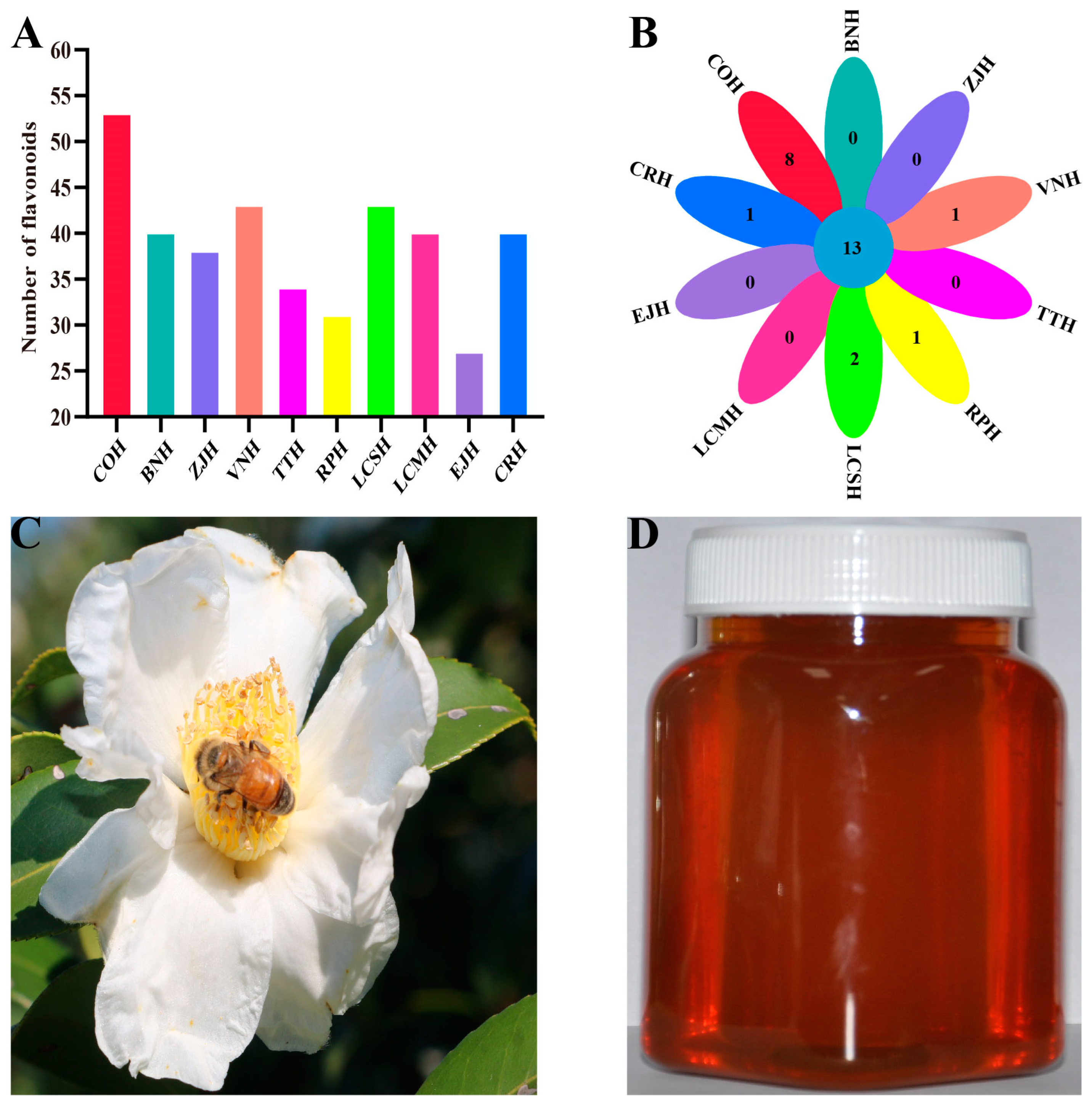
3.1. Differences in Flavonoid Species among C. oleifera Honey and Nine Kinds of Monofloral Honey
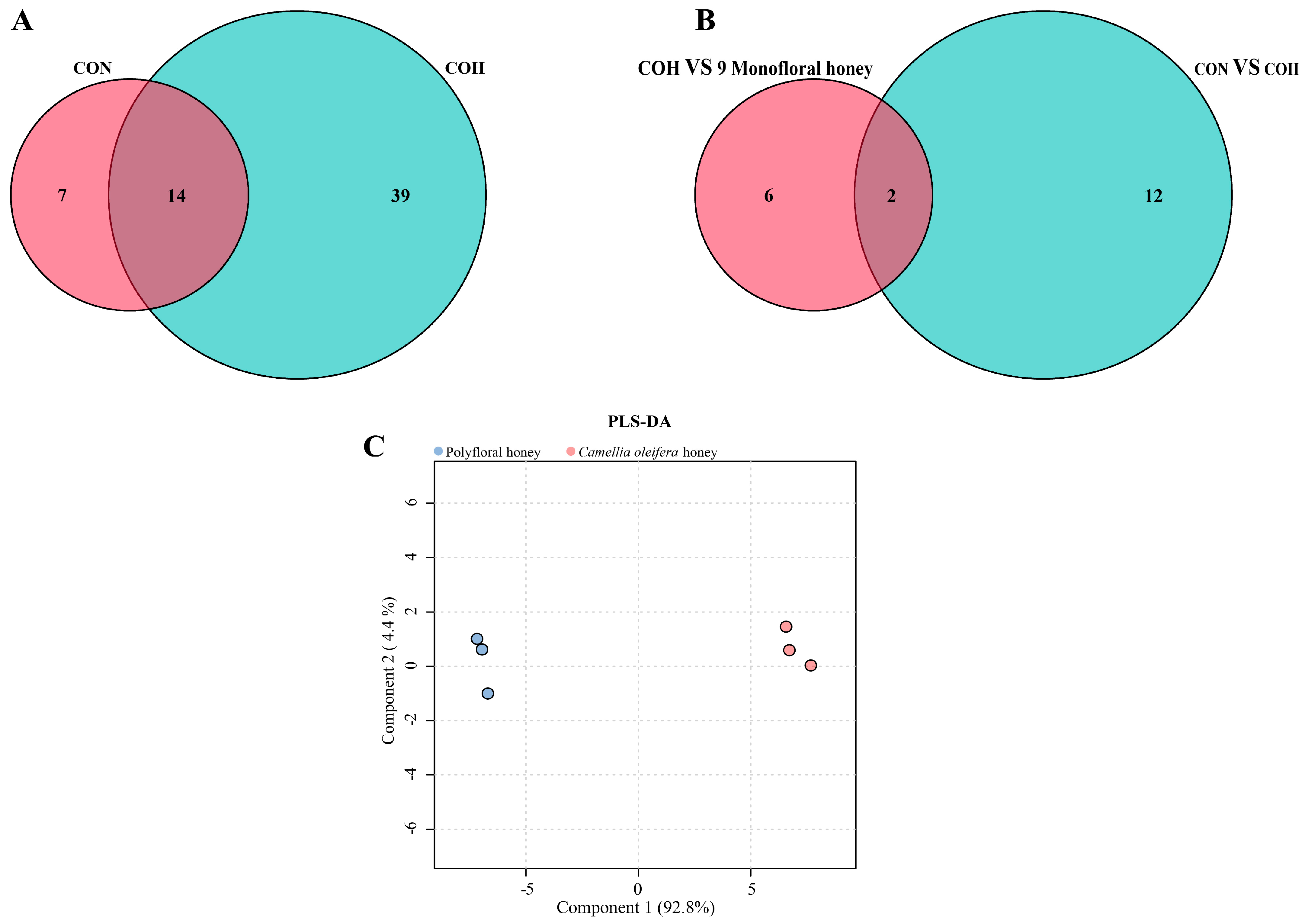
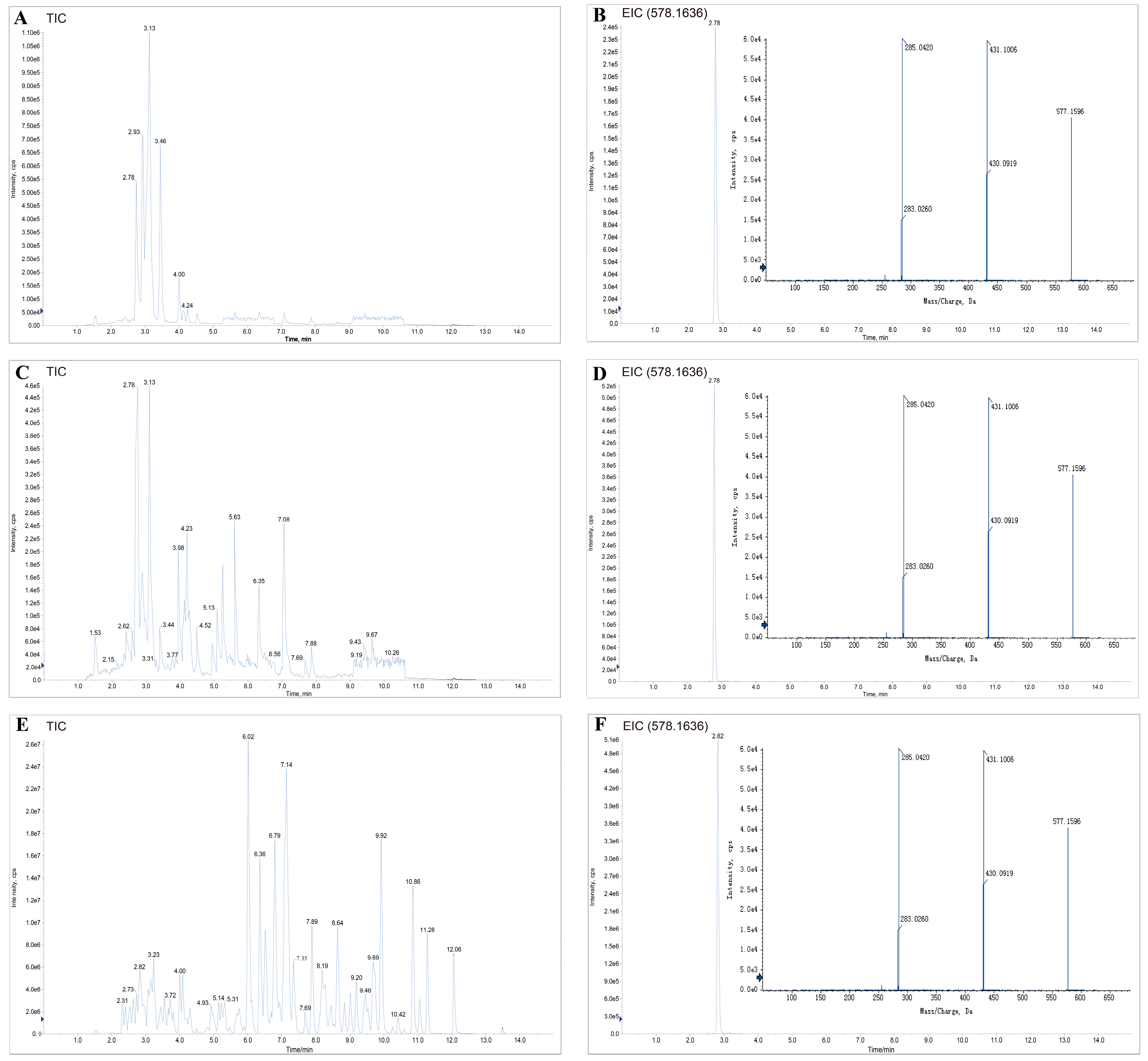
3.2. Identification of the Distinctive Flavonoid Marker in C. oleifera Honey
3.3. Kaempferitrin Quantification and Method Validation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Downey, G.; Hussey, K.; Kelly, J.D.; Walshe, T.F.; Martin, P. Preliminary contribution to the characterisation of artisanal honey produced on the island of Ireland by palynological and physico-chemical data. Food Chem. 2005, 91, 347–354. [Google Scholar] [CrossRef]
- Ramsay, E.I.; Rao, S.; Madathil, L.; Hegde, S.K.; Baliga-Rao, M.P.; George, T.; Baliga, M.S. Honey in oral health and care: A mini review. J. Oral Sci. 2019, 61, 32–36. [Google Scholar] [CrossRef] [PubMed]
- El Sohaimy, S.A.; Masry, S.; Shehata, M. Physicochemical characteristics of honey from different origins. Ann. Agric. Sci. 2015, 60, 279–287. [Google Scholar] [CrossRef]
- Valenzuela, R.; Das, U.N.; Videla, L.A.; Llorente, C.G. Nutrients and diet: A relationship between oxidative stress, aging, obesity, and related noncommunicable diseases. Oxid. Med. Cell. Longev. 2018, 2018, 7460453. [Google Scholar] [CrossRef] [PubMed]
- da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Sulaiman, S.A.; Baig, A.A.; Ibrahim, M.; Liaqat, S.; Fatima, S.; Jabeen, S.; Shamim, N.; Othman, N.H. Honey as a potential natural antioxidant medicine: An insight into its molecular mechanisms of action. Oxid. Med. Cell. Longev. 2018, 2018, 8367846. [Google Scholar] [CrossRef]
- Zhao, L.; Ren, C.; Xue, X.; Lu, H.; Wang, K.; Wu, L. Safflomin A: A novel chemical marker for Carthamus tinctorius L. (Safflower) monofloral honey. Food Chem. 2022, 366, 130584. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Ji, K.; Yu, X.; Chen, L.; Wang, L.; Gong, W.; Yuan, D. Dynamic metabolic and transcriptomic profiling reveal synthetic characters and regulators of flavonoid biosynthesis in Camellia oleifera seeds. Ind. Crops Prod. 2022, 186, 115295. [Google Scholar] [CrossRef]
- Lee, C.P.; Yen, G.C. Antioxidant activity and bioactive compounds of tea seed (Camellia oleifera Abel.) oil. J. Agric. Food Chem. 2006, 54, 779–784. [Google Scholar] [CrossRef]
- Li, H.; Zhou, G.Y.; Zhang, H.Y.; Liu, J.A. Research progress on the health function of tea oil. J. Med. Plant 2011, 5, 485–489. [Google Scholar]
- Cheng, Y.T.; Lu, C.C.; Yen, G.C. Beneficial effects of camellia oil (Camellia oleifera Abel.) on hepatoprotective and gastroprotective activities. J. Nutr. Sci. Vitaminol. 2015, 61, S100–S102. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Dong, Y.; Dong, K.; He, S. The toxic honey plant Camellia oleifera. J. Apic. Res. 2012, 51, 277–279. [Google Scholar] [CrossRef]
- Li, Z.; Huang, Q.; Zheng, Y.; Zhang, Y.; Li, X.; Zhong, S.; Zeng, Z. Identification of the toxic compounds in Camellia oleifera honey and pollen to honey bees (Apis mellifera). J. Agric. Food Chem. 2022, 70, 13176–13185. [Google Scholar] [CrossRef]
- He, L.; Zhang, F.; Jian, Z.; Sun, J.; Chen, J.; Liapao, V.; He, Q. Stachyose modulates gut microbiota and alleviates dextran sulfate sodium-induced acute colitis in mice. Saudi J. Gastroenterol. 2020, 26, 153. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Bei, J.; Liang, L.; Yu, G.; Li, L.; Li, Q. Stachyose improves inflammation through modulating gut microbiota of high-fat diet/streptozotocin-induced type 2 diabetes in rats. Mol. Nutr. Food Res. 2018, 62, 1700954. [Google Scholar] [CrossRef] [PubMed]
- Xi, M.; Zhao, S.; Ge, W.; Chen, Y.; Cui, X.; Sun, Q. Effects of stachyose on the intestinal microbiota and barrier in antibiotic-treated mice. J. Funct. Foods 2021, 83, 104493. [Google Scholar] [CrossRef]
- Zhang, R.; Zhao, Y.; Sun, Y.; Lu, X.; Yang, X. Isolation, characterization, and hepatoprotective effects of the raffinose family oligosaccharides from Rehmannia glutinosa Libosch. J. Agric. Food Chem. 2013, 61, 7786–7793. [Google Scholar] [CrossRef]
- Siddiqui, A.J.; Musharraf, S.G.; Choudhary, M.I. Application of analytical methods in authentication and adulteration of honey. Food Chem. 2017, 217, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Elango, D.; Rajendran, K.; Van der Laan, L.; Sebastiar, S.; Raigne, J.; Thaiparambil, N.A.; El Haddad, N.; Raja, B.; Wang, W.; Ferela, A. Raffinose family oligosaccharides: Friend or foe for human and plant health? Front. Plant Sci. 2022, 13, 829118. [Google Scholar] [CrossRef]
- Martos, I.; Ferreres, F.; Tomás-Barberán, F.A. Identification of flavonoid markers for the botanical origin of Eucalyptus honey. J. Agric. Food Chem. 2000, 48, 1498–1502. [Google Scholar] [CrossRef]
- Truchado, P.; Ferreres, F.; Bortolotti, L.; Sabatini, A.G.; Tomás-Barberán, F.A. Nectar flavonol rhamnosides are floral markers of acacia (Robinia pseudacacia) honey. J. Agric. Food Chem. 2008, 56, 8815–8824. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Q.X. Chemical composition, characterization, and differentiation of honey botanical and geographical origins. Adv. Food Nutr. Res. 2011, 62, 89–137. [Google Scholar] [PubMed]
- Du Fall, L.A.; Solomon, P.S. Role of cereal secondary metabolites involved in mediating the outcome of plant-pathogen interactions. Metabolites 2011, 1, 64–78. [Google Scholar] [CrossRef] [PubMed]
- Luan, F.; Zeng, J.; Yang, Y.; He, X.; Wang, B.; Gao, Y.; Zeng, N. Recent advances in Camellia oleifera Abel: A review of nutritional constituents, biofunctional properties, and potential industrial applications. J. Funct. Foods 2020, 75, 104242. [Google Scholar] [CrossRef]
- Varshney, M.; Kumar, B.; Rana, V.S.; Sethiya, N.K. An overview on therapeutic and medicinal potential of poly-hydroxy flavone viz. Heptamethoxyflavone, Kaempferitrin, Vitexin and Amentoflavone for management of Alzheimer’s and Parkinson’s diseases: A critical analysis on mechanistic insight. Crit. Rev. Food Sci. Nutr. 2021, 1–24. [Google Scholar] [CrossRef]
- Yao, L.; Jiang, Y.; Datta, N.; Singanusong, R.; Liu, X.; Duan, J.; Raymont, K.; Lisle, A.; Xu, Y. HPLC analyses of flavanols and phenolic acids in the fresh young shoots of tea (Camellia sinensis) grown in Australia. Food Chem. 2004, 84, 253–263. [Google Scholar] [CrossRef]
| Parameter | Mean ± SD |
|---|---|
| Fructose, % | 38.27 ± 1.06 |
| Glucose, % | 27.44 ± 0.71 |
| Sucrose, % | 1.56 ± 0.03 |
| Melibiose, % | 0.11 ± 0.002 |
| Manninotriose, % | 1.44 ± 0.03 |
| Raffinose, % | 6.92 ± 0.21 |
| Stachyose, % | 7.85 ± 0.21 |
| Water, % | 17.62 ± 0.16 |
| Acidity, mL/kg | 34.83 ± 0.82 |
| 5-HMF, mg/kg | ND |
| Compound | Standard Curve | LOD | LOQ | Regression (R2) | COH (n = 3) | CON (n = 3) | ||
|---|---|---|---|---|---|---|---|---|
| Content | RSD (%) | Content | RSD (%) | |||||
| Kaempferitrin | y = 10,383.8424 x + 1676.5839 | 0.07 | 0.25 | 0.9989 | 5.98 ± 0.84 | 1.23 | 2.36 ± 0.82 | 1.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Huang, Q.; Zheng, Y.; Zhang, Y.; Liu, B.; Shi, W.; Zeng, Z. Kaempferitrin: A Flavonoid Marker to Distinguish Camellia oleifera Honey. Nutrients 2023, 15, 435. https://doi.org/10.3390/nu15020435
Li Z, Huang Q, Zheng Y, Zhang Y, Liu B, Shi W, Zeng Z. Kaempferitrin: A Flavonoid Marker to Distinguish Camellia oleifera Honey. Nutrients. 2023; 15(2):435. https://doi.org/10.3390/nu15020435
Chicago/Turabian StyleLi, Zhen, Qiang Huang, Yu Zheng, Yong Zhang, Bin Liu, Wenkai Shi, and Zhijiang Zeng. 2023. "Kaempferitrin: A Flavonoid Marker to Distinguish Camellia oleifera Honey" Nutrients 15, no. 2: 435. https://doi.org/10.3390/nu15020435
APA StyleLi, Z., Huang, Q., Zheng, Y., Zhang, Y., Liu, B., Shi, W., & Zeng, Z. (2023). Kaempferitrin: A Flavonoid Marker to Distinguish Camellia oleifera Honey. Nutrients, 15(2), 435. https://doi.org/10.3390/nu15020435







