Changes in Eating Behaviors and Their Associations with Weight Loss in Japanese Patients Who Underwent Laparoscopic Sleeve Gastrectomy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Assessment of Eating Behaviors
2.3. Definitions
2.4. Statistical Analysis
3. Results
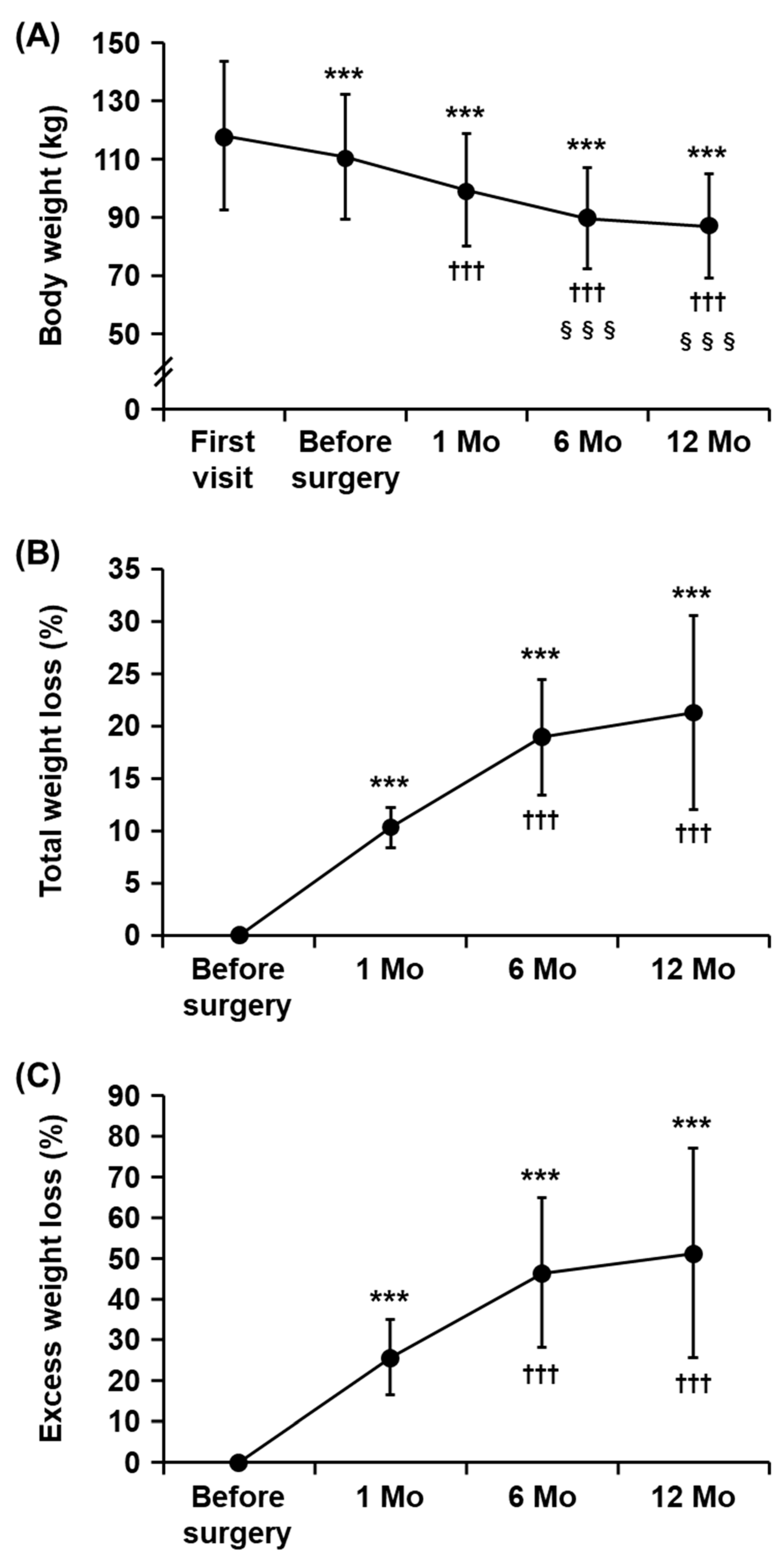
3.1. Characteristics of the Study Subjects and Changes in Body Weight after Metabolic and Bariatric Surgery
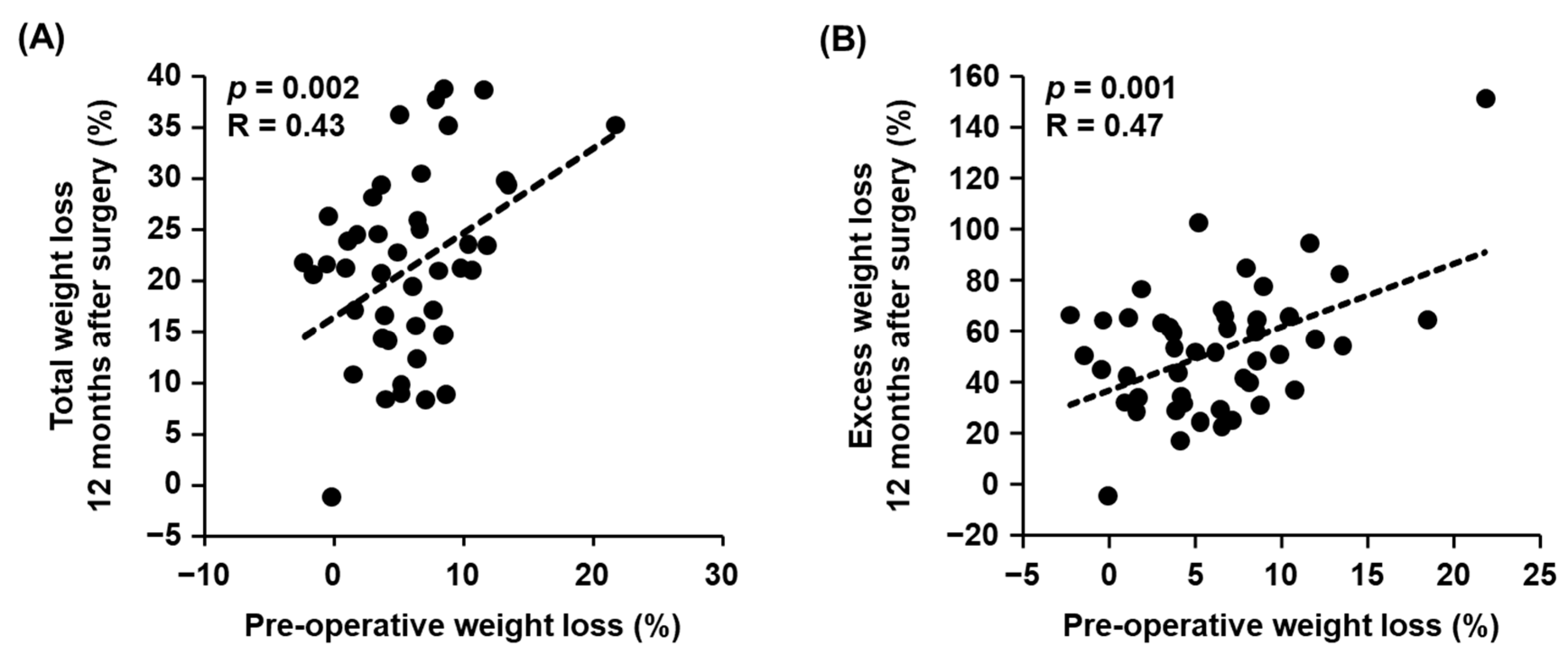
3.2. Correlations between Pre-Operative Clinical Parameters and Weight Loss after Metabolic and Bariatric Surgery
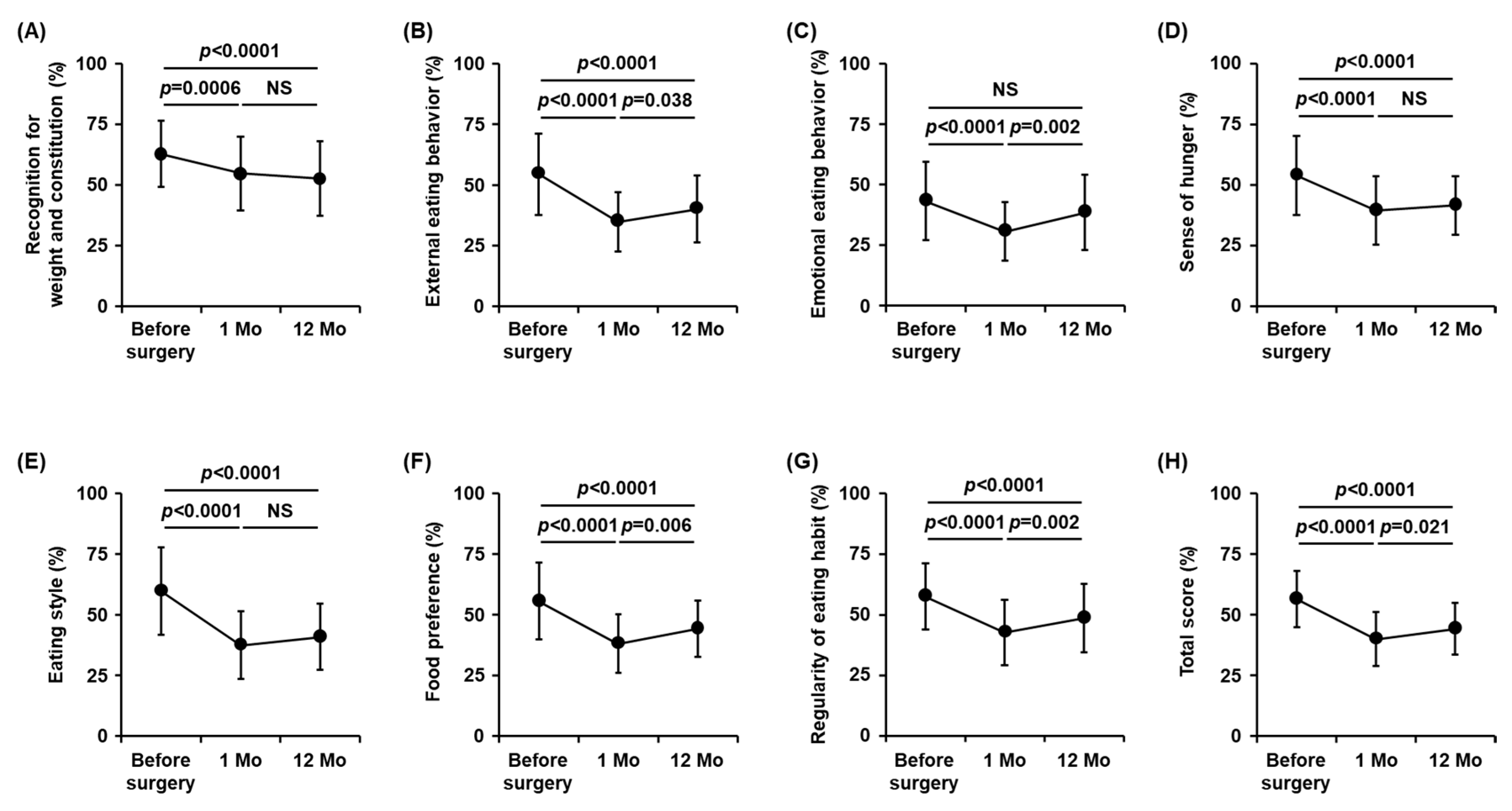
3.3. Changes in Eating Behavior Scores after Metabolic and Bariatric Surgery
3.4. Correlations between Changes in Eating Behavior Scores and Weight Loss after Metabolic and Bariatric Surgery
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miyazawa, I.; Kadota, A.; Miura, K.; Okamoto, M.; Nakamura, T.; Ikai, T.; Maegawa, H.; Ohnishi, A. Twelve-Year Trends of Increasing Overweight and Obesity in Patients with Diabetes: The Shiga Diabetes Clinical Survey. Endocr. J. 2018, 65, 527–536. [Google Scholar] [CrossRef]
- Jaacks, L.M.; Vandevijvere, S.; Pan, A.; McGowan, C.J.; Wallace, C.; Imamura, F.; Mozaffarian, D.; Swinburn, B.; Ezzati, M. The Obesity Transition: Stages of the Global Epidemic. Lancet Diabetes Endocrinol. 2019, 7, 231–240. [Google Scholar] [CrossRef]
- Rubino, F.; Nathan, D.M.; Eckel, R.H.; Schauer, P.R.; Alberti, K.G.M.M.; Zimmet, P.Z.; Del Prato, S.; Ji, L.; Sadikot, S.M.; Herman, W.H.; et al. Metabolic Surgery in the Treatment Algorithm for Type 2 Diabetes: A Joint Statement by International Diabetes Organizations. Diabetes Care 2016, 39, 861–877. [Google Scholar] [CrossRef] [PubMed]
- Sjöström, L.; Narbro, K.; Sjöström, C.D.; Karason, K.; Larsson, B.; Wedel, H.; Lystig, T.; Sullivan, M.; Bouchard, C.; Carlsson, B.; et al. Effects of Bariatric Surgery on Mortality in Swedish Obese Subjects. N. Engl. J. Med. 2007, 357, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Haruta, H.; Kasama, K.; Ohta, M.; Sasaki, A.; Yamamoto, H.; Miyazaki, Y.; Oshiro, T.; Naitoh, T.; Hosoya, Y.; Togawa, T.; et al. Long-Term Outcomes of Bariatric and Metabolic Surgery in Japan: Results of a Multi-Institutional Survey. Obes. Surg. 2017, 27, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, A.; Yokote, K.; Naitoh, T.; Fujikura, J.; Hayashi, K.; Hirota, Y.; Inagaki, N.; Ishigaki, Y.; Kasama, K.; Kikkawa, E.; et al. Metabolic Surgery in Treatment of Obese Japanese Patients with Type 2 Diabetes: A Joint Consensus Statement from the Japanese Society for Treatment of Obesity, the Japan Diabetes Society, and the Japan Society for the Study of Obesity. Diabetol. Int. 2022, 13, 1–30. [Google Scholar] [CrossRef]
- Heymsfield, S.B.; Wadden, T.A. Mechanisms, Pathophysiology, and Management of Obesity. N. Engl. J. Med. 2017, 376, 254–266. [Google Scholar] [CrossRef]
- Simon, G.E.; Von Korff, M.; Saunders, K.; Miglioretti, D.L.; Crane, P.K.; van Belle, G.; Kessler, R.C. Association Between Obesity and Psychiatric Disorders in the US Adult Population. Arch. Gen. Psychiatry 2006, 63, 824. [Google Scholar] [CrossRef]
- Sarwer, D.B.; Allison, K.C.; Wadden, T.A.; Ashare, R.; Spitzer, J.C.; McCuen-Wurst, C.; LaGrotte, C.; Williams, N.N.; Edwards, M.; Tewksbury, C.; et al. Psychopathology, Disordered Eating, and Impulsivity as Predictors of Outcomes of Bariatric Surgery. Surg. Obes. Relat. Dis. 2019, 15, 650–655. [Google Scholar] [CrossRef]
- Fujishima, Y.; Maeda, N.; Inoue, K.; Kashine, S.; Nishizawa, H.; Hirata, A.; Kozawa, J.; Yasuda, T.; Okita, K.; Imagawa, A.; et al. Efficacy of Liraglutide, a Glucagon-like Peptide-1 (GLP-1) Analogue, on Body Weight, Eating Behavior, and Glycemic Control, in Japanese Obese Type 2 Diabetes. Cardiovasc. Diabetol. 2012, 11, 107. [Google Scholar] [CrossRef]
- Fukuda, S.; Hirata, A.; Nishizawa, H.; Nagao, H.; Kashine, S.; Kimura, T.; Inoue, K.; Fujishima, Y.; Yamaoka, M.; Kozawa, J.; et al. Systemic Arteriosclerosis and Eating Behavior in Japanese Type 2 Diabetic Patients with Visceral Fat Accumulation. Cardiovasc. Diabetol. 2015, 14, 8. [Google Scholar] [CrossRef]
- Japan Society for the Study of Obesity. Guideline for the Management of Obesity; Japan Society for the Study of Obesity: Osaka, Japan, 2016. [Google Scholar]
- Inoue, K.; Maeda, N.; Kashine, S.; Fujishima, Y.; Kozawa, J.; Hiuge-Shimizu, A.; Okita, K.; Imagawa, A.; Funahashi, T.; Shimomura, I. Short-Term Effects of Liraglutide on Visceral Fat Adiposity, Appetite, and Food Preference: A Pilot Study of Obese Japanese Patients with Type 2 Diabetes. Cardiovasc. Diabetol. 2011, 10, 109. [Google Scholar] [CrossRef]
- Masaki, T.; Ozeki, Y.; Yoshida, Y.; Okamoto, M.; Miyamoto, S.; Gotoh, K.; Shibata, H. Glucagon-Like Peptide-1 Receptor Agonist Semaglutide Improves Eating Behavior and Glycemic Control in Japanese Obese Type 2 Diabetic Patients. Metabolites 2022, 12, 147. [Google Scholar] [CrossRef] [PubMed]
- Livhits, M.; Mercado, C.; Yermilov, I.; Parikh, J.A.; Dutson, E.; Mehran, A.; Ko, C.Y.; Gibbons, M.M. Does Weight Loss Immediately before Bariatric Surgery Improve Outcomes: A Systematic Review. Surg. Obes. Relat. Dis. 2009, 5, 713–721. [Google Scholar] [CrossRef]
- Kourounis, G.; Kong, C.Y.; Logue, J.; Gibson, S. Weight Loss in Adults Following Bariatric Surgery, a Systematic Review of Preoperative Behavioural Predictors. Clin. Obes. 2020, 10, e12392. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Seki, Y.; Haruta, H.; Kikkawa, E.; Kasama, K. Preoperative Weight Loss and Operative Outcome After Laparoscopic Sleeve Gastrectomy. Obes. Surg. 2017, 27, 2515–2521. [Google Scholar] [CrossRef]
- Pekkarinen, T.; Mustonen, H.; Sane, T.; Jaser, N.; Juuti, A.; Leivonen, M. Long-Term Effect of Gastric Bypass and Sleeve Gastrectomy on Severe Obesity: Do Preoperative Weight Loss and Binge Eating Behavior Predict the Outcome of Bariatric Surgery? Obes. Surg. 2016, 26, 2161–2167. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.J.; Lee, C.M.; Rigas, G.; Tam, C.S. Predictors of Weight Loss 2 Years after Laparoscopic Sleeve Gastrectomy: Weight Loss 2 Years after LSG. Asian J. Endosc. Surg. 2015, 8, 328–332. [Google Scholar] [CrossRef]
- Brown, W.A.; Moszkowicz, J.; Brennan, L.; Burton, P.R.; Anderson, M.; O’Brien, P.E. Pre-Operative Weight Loss Does Not Predict Weight Loss Following Laparoscopic Adjustable Gastric Banding. Obes. Surg. 2013, 23, 1611–1615. [Google Scholar] [CrossRef]
- Conaty, E.A.; Bonamici, N.J.; Gitelis, M.E.; Johnson, B.J.; DeAsis, F.; Carbray, J.M.; Lapin, B.; Joehl, R.; Denham, W.; Linn, J.G.; et al. Efficacy of a Required Preoperative Weight Loss Program for Patients Undergoing Bariatric Surgery. J. Gastrointest. Surg. 2016, 20, 667–673. [Google Scholar] [CrossRef]
- Alvarado, R.; Alami, R.S.; Hsu, G.; Safadi, B.Y.; Sanchez, B.R.; Morton, J.M.; Curet, M.J. The Impact of Preoperative Weight Loss in Patients Undergoing Laparoscopic Roux-En-Y Gastric Bypass. Obes. Surg. 2005, 15, 1282–1286. [Google Scholar] [CrossRef]
- Ruiz-Tovar, J.; Boix, E.; Bonete, J.M.; Martínez, R.; Zubiaga, L.; Díez, M.; Calpena, R. Effect of Preoperative Eating Patterns and Preoperative Weight Loss on the Short- and Mid-Term Weight Loss Results of Sleeve Gastrectomy. Cir. Esp. Engl. Ed. 2015, 93, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Campos, G.M. Factors Associated with Weight Loss After Gastric Bypass. Arch. Surg. 2008, 143, 877. [Google Scholar] [CrossRef]
- Faria, G.; Preto, J.; Almeida, A.B.; Guimarães, J.T.; Calhau, C.; Taveira-Gomes, A. Fasting Glycemia: A Good Predictor of Weight Loss after RYGB. Surg. Obes. Relat. Dis. 2014, 10, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Pagoto, S.; Olendzki, B.; Hafner, A.; Perugini, R.; Mason, R.; Kelly, J. Predictors of Weight Status Following Laparoscopic Gastric Bypass. Obes. Surg. 2006, 16, 1227–1231. [Google Scholar] [CrossRef] [PubMed]
- Romano, L.; Necozione, S.; Cianca, G.; Schietroma, M.; Carlei, F.; Giuliani, A. Weight Loss after Sleeve Gastrectomy in Patients with Diabetes: Preliminary Study in One Year of Activity. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 4317–4324. [Google Scholar] [CrossRef] [PubMed]
- Goodarzi, M.O. Genetics of Obesity: What Genetic Association Studies Have Taught Us about the Biology of Obesity and Its Complications. Lancet Diabetes Endocrinol. 2018, 6, 223–236. [Google Scholar] [CrossRef]
- Ciudin, A.; Fidilio, E.; Gutiérrez-Carrasquilla, L.; Caixàs, A.; Vilarrasa, N.; Pellitero, S.; Simó-Servat, A.; Vilallonga, R.; Ruiz, A.; de la Fuente, M.; et al. A Clinical-Genetic Score for Predicting Weight Loss after Bariatric Surgery: The OBEGEN Study. J. Pers. Med. 2021, 11, 1040. [Google Scholar] [CrossRef]
- Bandstein, M.; Voisin, S.; Nilsson, E.K.; Schultes, B.; Ernst, B.; Thurnheer, M.; Benedict, C.; Mwinyi, J.; Schiöth, H.B. A Genetic Risk Score Is Associated with Weight Loss Following Roux-En Y Gastric Bypass Surgery. Obes. Surg. 2016, 26, 2183–2189. [Google Scholar] [CrossRef]
- Still, C.D.; Wood, G.C.; Chu, X.; Erdman, R.; Manney, C.H.; Benotti, P.N.; Petrick, A.T.; Strodel, W.E.; Mirshahi, U.L.; Mirshahi, T.; et al. High Allelic Burden of Four Obesity SNPs Is Associated with Poorer Weight Loss Outcomes Following Gastric Bypass Surgery. Obesity 2011, 19, 1676–1683. [Google Scholar] [CrossRef]
- Abu-Hamdah, R.; Rabiee, A.; Meneilly, G.S.; Shannon, R.P.; Andersen, D.K.; Elahi, D. The Extrapancreatic Effects of Glucagon-Like Peptide-1 and Related Peptides. J. Clin. Endocrinol. Metab. 2009, 94, 1843–1852. [Google Scholar] [CrossRef] [PubMed]
- Zakeri, R.; Batterham, R.L. Potential Mechanisms Underlying the Effect of Bariatric Surgery on Eating Behaviour. Curr. Opin. Endocrinol. Diabetes Obes. 2018, 25, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Meek, C.L.; Lewis, H.B.; Reimann, F.; Gribble, F.M.; Park, A.J. The Effect of Bariatric Surgery on Gastrointestinal and Pancreatic Peptide Hormones. Peptides 2016, 77, 28–37. [Google Scholar] [CrossRef]
- Santo, M.A.; Riccioppo, D.; Pajecki, D.; Kawamoto, F.; de Cleva, R.; Antonangelo, L.; Marçal, L.; Cecconello, I. Weight Regain After Gastric Bypass: Influence of Gut Hormones. Obes. Surg. 2016, 26, 919–925. [Google Scholar] [CrossRef]
- Duarte-Guerra, L.S.; Coêlho, B.M.; Santo, M.A.; Lotufo-Neto, F.; Wang, Y.-P. Morbidity Persistence and Comorbidity of Mood, Anxiety, and Eating Disorders among Preoperative Bariatric Patients. Psychiatry Res. 2017, 257, 1–6. [Google Scholar] [CrossRef]
- Sarwer, D.B.; Heinberg, L.J. A Review of the Psychosocial Aspects of Clinically Severe Obesity and Bariatric Surgery. Am. Psychol. 2020, 75, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Dawes, A.J.; Maggard-Gibbons, M.; Maher, A.R.; Booth, M.J.; Miake-Lye, I.; Beroes, J.M.; Shekelle, P.G. Mental Health Conditions Among Patients Seeking and Undergoing Bariatric Surgery: A Meta-Analysis. JAMA 2016, 315, 150. [Google Scholar] [CrossRef]
- Livhits, M.; Mercado, C.; Yermilov, I.; Parikh, J.A.; Dutson, E.; Mehran, A.; Ko, C.Y.; Gibbons, M.M. Preoperative Predictors of Weight Loss Following Bariatric Surgery: Systematic Review. Obes. Surg. 2012, 22, 70–89. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, D.; Shikora, S.A.; Aarts, E.; Aminian, A.; Angrisani, L.; Cohen, R.V.; de Luca, M.; Faria, S.L.; Goodpaster, K.P.S.; Haddad, A.; et al. 2022 American Society of Metabolic and Bariatric Surgery (ASMBS) and International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO) Indications for Metabolic and Bariatric Surgery. Obes. Surg. 2022, 1–12. [Google Scholar] [CrossRef]
- Chesler, B.E. Emotional Eating: A Virtually Untreated Risk Factor for Outcome Following Bariatric Surgery. Sci. World J. 2012, 2012, 1–6. [Google Scholar] [CrossRef]
- Subramaniam, K.; Low, W.-Y.; Lau, P.-C.; Chin, K.-F.; Chinna, K.; Kosai, N.; Taher, M.; Rajan, R. Eating Behaviour Predicts Weight Loss Six Months after Bariatric Surgery: A Longitudinal Study. Nutrients 2018, 10, 1616. [Google Scholar] [CrossRef] [PubMed]
- Forman, E.M.; Butryn, M.L.; Juarascio, A.S.; Bradley, L.E.; Lowe, M.R.; Herbert, J.D.; Shaw, J.A. The Mind Your Health Project: A Randomized Controlled Trial of an Innovative Behavioral Treatment for Obesity: Innovative Behavioral Treatment for Obesity. Obesity 2013, 21, 1119–1126. [Google Scholar] [CrossRef] [PubMed]

| Clinical Parameters | |
|---|---|
| n (males/females) | 49 (25/24) |
| Age (years) | 44.7 ± 8.7 |
| Obesity-related complications | |
| Type 2 diabetes | 27 (55.1%) |
| Hypertension | 28 (57.1%) |
| Dyslipidemia | 26 (53.1%) |
| (At the first visit) | |
| BW (kg) | 118.0 ± 25.5 |
| BMI (kg/m2) | 42.0 ± 8.3 |
| (Before surgery) | |
| BW (kg) | 110.7 ± 21.5 |
| BMI (kg/m2) | 39.8 ± 7.8 |
| Excess weight (kg) | 49.1 ± 19.8 |
| Eating behaviors | |
| Recognition for weight and constitution (%) | 62.7 ± 13.6 |
| External eating behavior (%) | 54.5 ± 16.8 |
| Emotional eating behavior (%) | 43.2 ± 16.1 |
| Sense of hunger (%) | 54.0 ± 16.4 |
| Eating style (%) | 59.7 ± 18.0 |
| Food preference (%) | 55.5 ± 15.9 |
| Regularity of eating habits (%) | 57.6 ± 13.7 |
| Total score (%) | 56.5 ± 11.7 |
| Days from the first visit to surgery | 147 (104–242) |
| Sex, Age, and Pre-Operative BMI-Adjusted | ||||
|---|---|---|---|---|
| Unadjusted | ||||
| Clinical Parameters before Surgery | R | p Value | Std β | p Value |
| Sex | - | 0.560 | - | - |
| Age | −0.19 | 0.199 | - | - |
| BMI | 0.18 | 0.208 | - | - |
| Pre-operative weight loss (%) | 0.43 | 0.002 | 0.44 | 0.002 |
| Obesity-related complications | ||||
| Type 2 diabetes | - | 0.006 | 0.35 | 0.020 |
| Hypertension | - | 0.420 | 0.05 | 0.764 |
| Dyslipidemia | - | 0.923 | −0.03 | 0.860 |
| Eating behaviors | ||||
| Recognition for weight and constitution | −0.18 | 0.222 | −0.32 | 0.055 |
| External eating behavior | 0.05 | 0.753 | 0.03 | 0.858 |
| Emotional eating behavior | −0.08 | 0.566 | −0.14 | 0.347 |
| Sense of hunger | 0.10 | 0.519 | 0.05 | 0.768 |
| Eating style | 0.08 | 0.589 | 0.03 | 0.854 |
| Food preference | −0.13 | 0.369 | −0.23 | 0.131 |
| Regularity of eating habits | 0.04 | 0.773 | 0.005 | 0.974 |
| Total score | 0.01 | 0.968 | −0.05 | 0.768 |
| Sex, Age, and Pre-Operative BMI-Adjusted | ||||
|---|---|---|---|---|
| Unadjusted | ||||
| Clinical Parameters before Surgery | R | p Value | Std β | p Value |
| Sex | - | 0.667 | - | - |
| Age | −0.14 | 0.353 | - | - |
| BMI | −0.28 | 0.054 | - | - |
| Pre-operative weight loss (%) | 0.47 | 0.001 | 0.53 | 0.0007 |
| Obesity-related complications | ||||
| Type 2 diabetes | - | 0.210 | 0.28 | 0.062 |
| Hypertension | - | 0.342 | 0.13 | 0.383 |
| Dyslipidemia | - | 0.742 | −0.03 | 0.847 |
| Eating behaviors | ||||
| Recognition for weight and constitution | −0.24 | 0.102 | −0.38 | 0.017 |
| External eating behavior | −0.12 | 0.426 | −0.03 | 0.814 |
| Emotional eating behavior | −0.23 | 0.119 | −0.19 | 0.197 |
| Sense of hunger | −0.09 | 0.534 | −0.01 | 0.924 |
| Eating style | 0.03 | 0.854 | 0.04 | 0.794 |
| Food preference | −0.22 | 0.127 | −0.23 | 0.116 |
| Regularity of eating habits | −0.05 | 0.713 | −0.09 | 0.531 |
| Total score | −0.19 | 0.190 | −0.13 | 0.390 |
| Sex, Age, and Pre-Operative BMI-Adjusted | ||||
|---|---|---|---|---|
| Changes in Eating Behaviors (From 1 to 12 Months after Surgery) | Unadjusted | |||
| R | p Value | Std β | p Value | |
| Δ Recognition for weight and constitution | −0.15 | 0.326 | −0.13 | 0.419 |
| Δ External eating behavior | −0.14 | 0.350 | −0.16 | 0.308 |
| Δ Emotional eating behavior | −0.38 | 0.009 | −0.37 | 0.013 |
| Δ Sense of hunger | −0.26 | 0.076 | −0.29 | 0.060 |
| Δ Eating style | −0.12 | 0.413 | −0.17 | 0.281 |
| Δ Food preference | −0.11 | 0.480 | −0.06 | 0.710 |
| Δ Regularity of eating habits | −0.17 | 0.263 | −0.20 | 0.182 |
| Δ Total score | −0.22 | 0.137 | −0.24 | 0.127 |
| Sex, Age, and Pre-Operative BMI-Adjusted | ||||
|---|---|---|---|---|
| Changes in Eating Behaviors (From 1 to 12 Months after Surgery) | Unadjusted | |||
| R | p Value | Std β | p Value | |
| Δ Recognition for weight and constitution | −0.07 | 0.620 | −0.12 | 0.437 |
| Δ External eating behavior | −0.09 | 0.541 | −0.12 | 0.432 |
| Δ Emotional eating behavior | −0.27 | 0.062 | −0.36 | 0.014 |
| ΔSense of hunger | −0.29 | 0.048 | −0.30 | 0.045 |
| Δ Eating style | −0.11 | 0.458 | −0.17 | 0.262 |
| Δ Food preference | 0.045 | 0.761 | −0.08 | 0.616 |
| Δ Regularity of eating habits | −0.19 | 0.192 | −0.23 | 0.117 |
| Δ Total score | −0.16 | 0.282 | −0.24 | 0.115 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kimura, Y.; Fujishima, Y.; Nishizawa, H.; Saito, T.; Miyazaki, Y.; Shirahase, K.; Tokuzawa, C.; Nagai, N.; Fukuda, S.; Maeda, K.; et al. Changes in Eating Behaviors and Their Associations with Weight Loss in Japanese Patients Who Underwent Laparoscopic Sleeve Gastrectomy. Nutrients 2023, 15, 353. https://doi.org/10.3390/nu15020353
Kimura Y, Fujishima Y, Nishizawa H, Saito T, Miyazaki Y, Shirahase K, Tokuzawa C, Nagai N, Fukuda S, Maeda K, et al. Changes in Eating Behaviors and Their Associations with Weight Loss in Japanese Patients Who Underwent Laparoscopic Sleeve Gastrectomy. Nutrients. 2023; 15(2):353. https://doi.org/10.3390/nu15020353
Chicago/Turabian StyleKimura, Yu, Yuya Fujishima, Hitoshi Nishizawa, Takuro Saito, Yasuhiro Miyazaki, Keiko Shirahase, Chie Tokuzawa, Naoko Nagai, Shiro Fukuda, Kazuhisa Maeda, and et al. 2023. "Changes in Eating Behaviors and Their Associations with Weight Loss in Japanese Patients Who Underwent Laparoscopic Sleeve Gastrectomy" Nutrients 15, no. 2: 353. https://doi.org/10.3390/nu15020353
APA StyleKimura, Y., Fujishima, Y., Nishizawa, H., Saito, T., Miyazaki, Y., Shirahase, K., Tokuzawa, C., Nagai, N., Fukuda, S., Maeda, K., Maeda, N., Doki, Y., & Shimomura, I. (2023). Changes in Eating Behaviors and Their Associations with Weight Loss in Japanese Patients Who Underwent Laparoscopic Sleeve Gastrectomy. Nutrients, 15(2), 353. https://doi.org/10.3390/nu15020353






