Association of Polygenic Variants Involved in Immunity and Inflammation with Duodenal Ulcer Risk and Their Interaction with Irregular Eating Habits
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. DU Diagnosis
2.3. Characteristics of the Participants by Interview and Biochemical Assays
2.4. Usual Food Intake and Dietary Patterns Using and Dietary Patterns by Principal Component Analysis (PCA)
2.5. Genotyping Using a Korean Chip
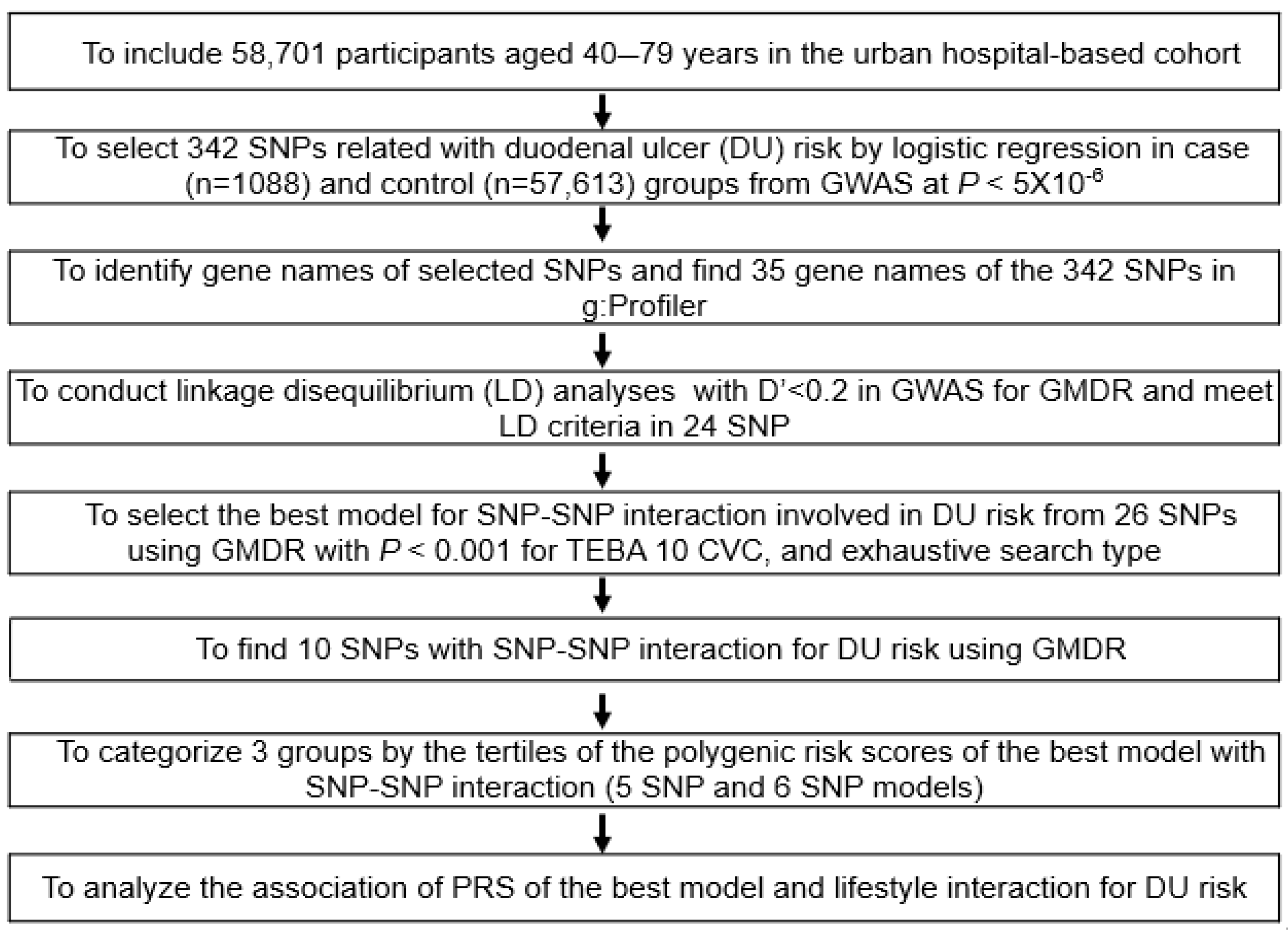
2.6. Selection of the Genetic Variants for DU Risk and SNP-SNP Interaction Model
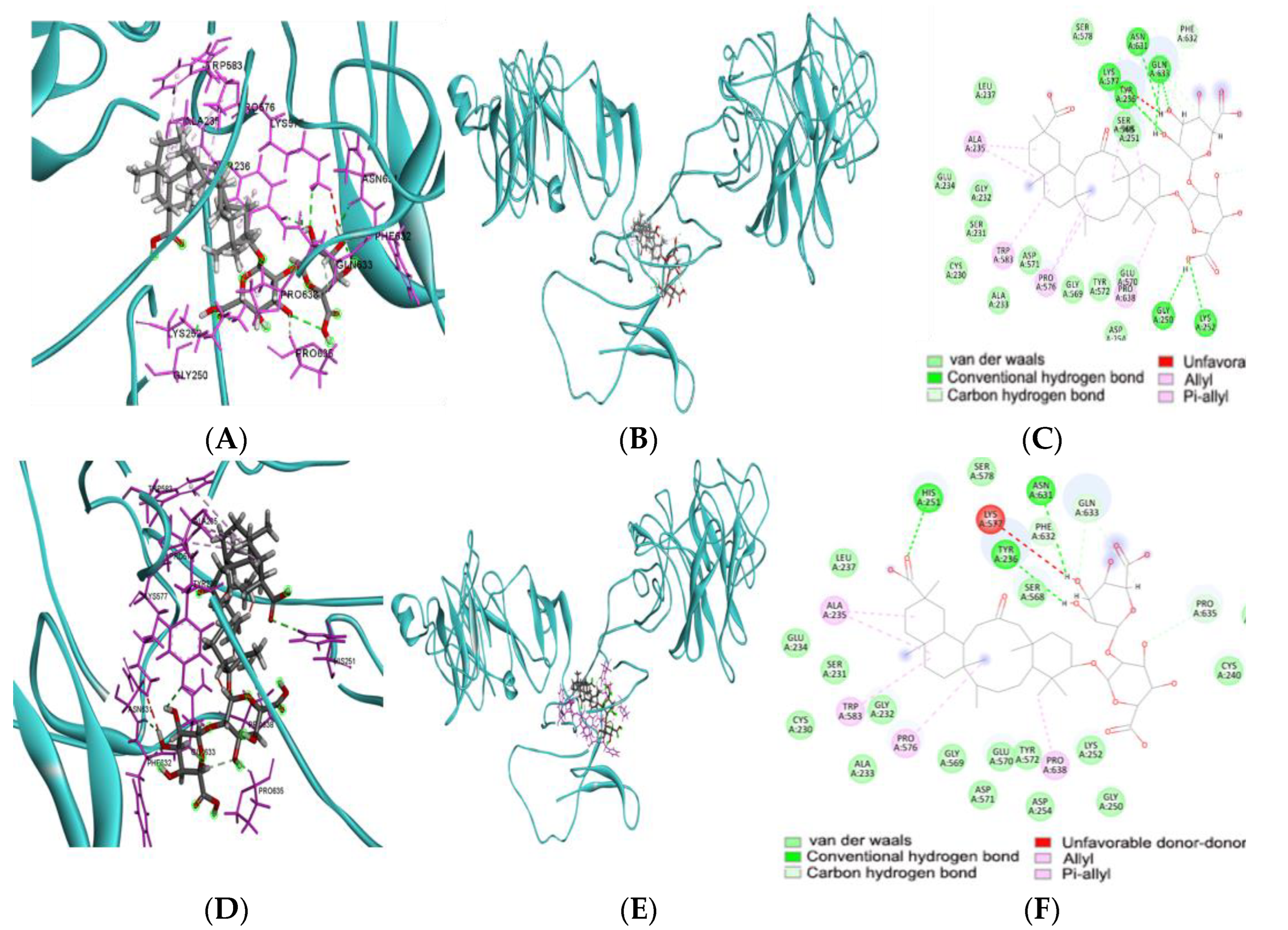
2.7. Molecular Docking of Food Compounds and Targets of Genes Related to DU
2.8. Molecular Dynamics Simulation (MDS)
2.9. Statistical Analysis
3. Results
3.1. General Characteristics According to Their Gender and DU
3.2. Dietary Intake and Lifestyles According to Gender and DU
3.3. Characteristics of Polygenic Variants Involved in DU Risk
3.4. Pathways of DU Risk-Related Genetic Variants
3.5. PRS of Genetic Variants Associated with DU Risk
3.6. Energy Binding Affinity with Food Components and the Foods Containing the Food Components
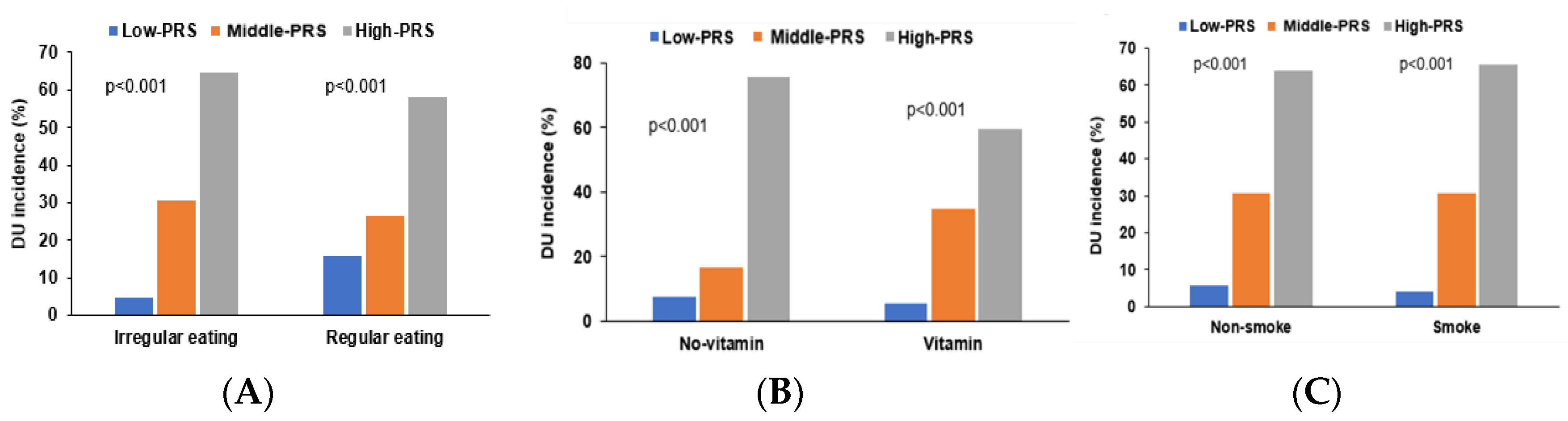
3.7. Interaction of PRS with Lifestyle Factors Influences DU Risk
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xie, X.; Ren, K.; Zhou, Z.; Dang, C.; Zhang, H. The global, regional and national burden of peptic ulcer disease from 1990 to 2019: A population-based study. BMC Gastroenterol. 2022, 22, 58. [Google Scholar] [CrossRef]
- Malik, T.F.; Gnanapandithan, K.; Singh, K. Peptic Ulcer Disease; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Yeo, S.-H.; Yang, C.-H. Peptic Ulcer Disease Associated with Helicobacter pylori Infection. Korean J. Gastroenterol. 2016, 67, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Vona, R.; Pallotta, L.; Cappelletti, M.; Severi, C.; Matarrese, P. The Impact of Oxidative Stress in Human Pathology: Focus on Gastrointestinal Disorders. Antioxidants 2021, 10, 201. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Shu, X.; Wang, J. Helicobacter pylori-Mediated Oxidative Stress and Gastric Diseases: A Review. Front. Microbiol. 2022, 13, 811258. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, L.; Carr, D.F.; Pirmohamed, M. Pharmacogenomics of NSAID-Induced Upper Gastrointestinal Toxicity. Front. Pharm. 2021, 12, 684162. [Google Scholar] [CrossRef] [PubMed]
- Achyut, B.R.; Ghoshal, U.C.; Moorchung, N.; Mittal, B. Association of Toll-like receptor-4 (Asp299Gly and Thr399Ileu) gene polymorphisms with gastritis and precancerous lesions. Hum. Immunol. 2007, 68, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Mărginean, M.O.; Mărginean, C.O.; Meliţ, L.E.; Voidăzan, S.; Moldovan, V.; Bănescu, C. The impact of host’s genetic susceptibility on Helicobacter pylori infection in children. Medicine 2017, 96, e7612. [Google Scholar] [CrossRef]
- Negovan, A.; Iancu, M.; Tripon, F.; Crauciuc, A.; Mocan, S.; Bănescu, C. The CAT-262 C>T, MnSOD Ala16Val, GPX1 Pro198Leu Polymorphisms Related to Oxidative Stress and the Presence of Gastric Lesions. J. Gastrointestin. Liver. Dis. 2018, 27, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Murray, G.K.; Byrne, E.M.; Sidorenko, J.; Visscher, P.M.; Wray, N.R. GWAS of peptic ulcer disease implicates Helicobacter pylori infection, other gastrointestinal disorders and depression. Nat. Commun. 2021, 12, 1146. [Google Scholar] [CrossRef]
- Tanikawa, C.; Urabe, Y.; Matsuo, K.; Kubo, M.; Takahashi, A.; Ito, H.; Tajima, K.; Kamatani, N.; Nakamura, Y.; Matsuda, K. A genome-wide association study identifies two susceptibility loci for duodenal ulcer in the Japanese population. Nat. Genet. 2012, 44, 430–434. [Google Scholar] [CrossRef]
- Tripathi, S.; Ghoshal, U.; Ghoshal, U.C.; Mittal, B.; Krishnani, N.; Chourasia, D.; Agarwal, A.K.; Singh, K. Gastric carcinogenesis: Possible role of polymorphisms of GSTM1, GSTT1, and GSTP1 genes. Scand. J. Gastroenterol. 2008, 43, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.; Lee, C. Association of glycosylated hemoglobin with the gene encoding CDKAL1 in the Korean Association Resource (KARE) study. Hum. Mutat. 2012, 33, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Daily, J.W.; Park, S. Interaction of BDNF rs6265 variants and energy and protein intake in the risk for glucose intolerance and type 2 diabetes in middle-aged adults. Nutrition 2017, 33, 187–194. [Google Scholar] [CrossRef]
- Park, S.; Daily, J.W.; Zhang, X.; Jin, H.S.; Lee, H.J.; Lee, Y.H. Interactions with the MC4R rs17782313 variant, mental stress, and energy intake and the risk of obesity in Genome Epidemiology Study. Nutr. Metab. 2016, 13, 38. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Park, S. An Inverse Relation between Hyperglycemia and Skeletal Muscle Mass Predicted by Using a Machine Learning Approach in Middle-Aged and Older Adults in Large Cohorts. J. Clin. Med. 2021, 10, 2133. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, C.; Wu, X. Development and Validation of an Insulin Resistance Predicting Model Using a Machine-Learning Approach in a Population-Based Cohort in Korea. Diagnostics 2022, 12, 212. [Google Scholar] [CrossRef]
- Park, S.; Ahn, J.; Lee, B.K. Self-rated Subjective Health Status Is Strongly Associated with Sociodemographic Factors, Lifestyle, Nutrient Intakes, and Biochemical Indices, but Not Smoking Status: KNHANES 2007–2012. J. Korean Med. Sci. 2015, 30, 1279–1287. [Google Scholar] [CrossRef]
- Park, S.; Zhang, X.; Lee, N.R.; Jin, H.S. TRPV1 Gene Polymorphisms Are Associated with Type 2 Diabetes by Their Interaction with Fat Consumption in the Korean Genome Epidemiology Study. J. Nutrigenet. Nutr. 2016, 9, 47–61. [Google Scholar] [CrossRef]
- Steel, R.G.; Torrie, J.H.; Dickey, D.A. Principles and Procedures of Statistics: A Biological Approach; McGraw-Hill College: New York, NY, USA, 1997. [Google Scholar]
- Kim, J.O.; Mueller, C.W. Factor Analysis. Statistical Methods and Practical Issues; Sage Publications: Thousand Oaks, CA, USA, 1978. [Google Scholar]
- van Woudenbergh, G.J.; Theofylaktopoulou, D.; Kuijsten, A.; Ferreira, I.; van Greevenbroek, M.M.; van der Kallen, C.J.; Schalkwijk, C.G.; Stehouwer, C.D.; Ocké, M.C.; Nijpels, G.; et al. Adapted dietary inflammatory index and its association with a summary score for low-grade inflammation and markers of glucose metabolism: The Cohort study on Diabetes and Atherosclerosis Maastricht (CODAM) and the Hoorn study. Am. J. Clin. Nutr. 2013, 98, 1533–1542. [Google Scholar] [CrossRef]
- Lee, S.K.; Kim, M.K. Relationship of sodium intake with obesity among Korean children and adolescents: Korea National Health and Nutrition Examination Survey. Br. J. Nutr. 2016, 115, 834–841. [Google Scholar] [CrossRef]
- Kim, Y.; Han, B.-G.; KoGES group. Cohort Profile: The Korean Genome and Epidemiology Study (KoGES) Consortium. Int. J. Epidemiol. 2017, 46, e20. [Google Scholar] [CrossRef]
- Rabbee, N.; Speed, T.P. A genotype calling algorithm for affymetrix SNP arrays. Bioinformatics 2006, 22, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Uma Jyothi, K.; Reddy, B.M. Gene-gene and gene-environment interactions in the etiology of type 2 diabetes mellitus in the population of Hyderabad, India. Meta Gene 2015, 5, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Liu, L.; Zhou, J.; Zhang, T.; Daily, J.W.; Park, S. Bioactive Components of Houttuynia cordata Thunb and Their Potential Mechanisms against COVID-19 Using Network Pharmacology and Molecular Docking Approaches. J. Med. Food 2022, 25, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shi, C.-Y.; Xie, J.; Dai, J.-H.; He, S.-L.; Tian, Y. Identification of potential dipeptidyl peptidase (DPP)-IV inhibitors among Moringa oleifera phytochemicals by virtual screening, molecular docking analysis, ADME/T-based prediction, and in vitro analyses. Molecules 2020, 25, 189. [Google Scholar] [CrossRef] [PubMed]
- SAS/STAT® 14.2 User’s Guide; SAS Inc.: Cary, NC, USA, 2016.
- Kim, H.; Hwang, J.Y.; Kwon, O. Dietary Reference Intakes for Koreans with special consideration to older adults. Nutr. Res. Pract. 2022, 16, S1–S10. [Google Scholar] [CrossRef] [PubMed]
- Yegen, B.C. Lifestyle and Peptic Ulcer Disease. Curr. Pharm. Des. 2018, 24, 2034–2040. [Google Scholar] [CrossRef]
- Karkhah, A.; Ebrahimpour, S.; Rostamtabar, M.; Koppolu, V.; Darvish, S.; Vasigala, V.K.R.; Validi, M.; Nouri, H.R. Helicobacter pylori evasion strategies of the host innate and adaptive immune responses to survive and develop gastrointestinal diseases. Microbiol. Res. 2019, 218, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Khatoon, J.; Prasad, K.N.; Rai, R.P.; Shukla, S.K.; Krishnani, N.; Ghoshal, U.C. Expression levels of A disintegrin and metalloproteases (ADAMs), and Th17-related cytokines and their association with Helicobacter pylori infection in patients with gastroduodenal diseases. Pathog. Dis. 2018, 76, fty078. [Google Scholar] [CrossRef]
- Zhang, B.B.; Liu, X.Z.; Sun, J.; Yin, Y.W.; Sun, Q.Q. Association between TNF α gene polymorphisms and the risk of duodenal ulcer: A meta-analysis. PLoS ONE 2013, 8, e57167. [Google Scholar] [CrossRef]
- Chakravorty, M.; Ghosh, A.; Choudhury, A.; Santra, A.; Hembrum, J.; Roychoudhury, S. Interaction between IL1B gene promoter polymorphisms in determining susceptibility to Helicobacter pylori associated duodenal ulcer. Hum. Mutat. 2006, 27, 411–419. [Google Scholar] [CrossRef]
- Zambon, C.-F.; Basso, D.; Marchet, A.; Fasolo, M.; Stranges, A.; Schiavon, S.; Navaglia, F.; Greco, E.; Fogar, P.; Falda, A.; et al. IL-4 -588C>T polymorphism and IL-4 receptor alpha [Ex5+14A>G.; Ex11+828A>G] haplotype concur in selecting H. pylori cagA subtype infections. Clin. Chim. Acta 2008, 389, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Choi, J.S.; Chun, S.W.; Lee, S.; Han, K.J.; Kim, H.M. The IL-1B Genetic Polymorphism Is Associated with Aspirin-Induced PepticUlcers in a Korean Ethnic Group. Gut Liver 2016, 10, 362–368. [Google Scholar] [CrossRef]
- Lee, D.H.; Hahm, K.-B. Inflammatory cytokine gene polymorphisms and gastric cancer. J. Gastrogenterol. Hepatol. 2008, 23, 1470–1472. [Google Scholar] [CrossRef]
- Jia, Z.F.; Zhang, S.L.; Cao, X.Y.; Zhou, B.S.; Jiang, J. Interaction between Helicobacter pylori and host genetic variants in gastric carcinogenesis. Future Oncol. 2016, 12, 2127–2134. [Google Scholar] [CrossRef]
- Gareb, B.; Otten, A.T.; Frijlink, H.W.; Dijkstra, G.; Kosterink, J.G.W. Review: Local Tumor Necrosis Factor-α Inhibition in Inflammatory Bowel Disease. Pharmaceutics 2020, 12, 539. [Google Scholar] [CrossRef]
- Street, V.A.; Bennett, C.L.; Goldy, J.D.; Shirk, A.J.; Kleopa, K.A.; Tempel, B.L.; Lipe, H.P.; Scherer, S.S.; Bird, T.D.; Chance, P.F. Mutation of a putative protein degradation gene LITAF/SIMPLE in Charcot-Marie-Tooth disease 1C. Neurology 2003, 60, 22–26. [Google Scholar] [CrossRef]
- García-González, M.A.; Bujanda, L.; Quintero, E.; Santolaria, S.; Benito, R.; Strunk, M.; Sopeña, F.; Thomson, C.; Pérez-Aisa, A.; Nicolás-Pérez, D.; et al. Association of PSCA rs2294008 gene variants with poor prognosis and increased susceptibility to gastric cancer and decreased risk of duodenal ulcer disease. Int. J. Cancer 2015, 137, 1362–1373. [Google Scholar] [CrossRef]
- Cui, H.; Tang, M.; Zhang, M.; Liu, S.; Chen, S.; Zeng, Z.; Shen, Z.; Song, B.; Lu, J.; Jia, H.; et al. Variants in the PSCA gene associated with risk of cancer and nonneoplastic diseases: Systematic research synopsis, meta-analysis and epidemiological evidence. Carcinogenesis 2019, 40, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Wen, S.; Cao, L.; Zhou, Y.; Feng, Z. Effect of eradication of Helicobacter pylori on expression levels of FHIT, IL-8, and P73 in gastric mucosa of first-degree relatives of gastric cancer patients. PLoS ONE 2015, 10, e0124576. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, D.V.; Vindrieux, D.; Goehrig, D.; Jaber, S.; Collin, G.; Griveau, A.; Wiel, C.; Bendridi, N.; Djebali, S.; Farfariello, V.; et al. Calcium channel ITPR2 and mitochondria–ER contacts promote cellular senescence and aging. Nat. Commun. 2021, 12, 720. [Google Scholar] [CrossRef] [PubMed]
- Engevik, K.A.; Karns, R.A.; Oshima, Y.; Montrose, M.H. Multiple calcium sources are required for intracellular calcium mobilization during gastric organoid epithelial repair. Physiol. Rep. 2020, 8, e14384. [Google Scholar] [CrossRef] [PubMed]
- Cournia, Z.; Allen, B.; Sherman, W. Relative Binding Free Energy Calculations in Drug Discovery: Recent Advances and Practical Considerations. J. Chem. Inform. Model. 2017, 57, 2911–2937. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.H. CD6 as a Cell Surface Receptor and As a Target for Regulating Immune Responses. Curr. Drug Targets 2016, 17, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Alsinnari, Y.M.; Alqarni, M.S.; Attar, M.; Bukhari, Z.M.; Almutairi, M.; Baabbad, F.M.; Hasosah, M. Risk Factors for Recurrence of Peptic Ulcer Disease: A Retrospective Study in Tertiary Care Referral Center. Cureus 2022, 14, e22001. [Google Scholar] [CrossRef] [PubMed]



| Men | Women | Adjusted ORs (95% CI) | |||
|---|---|---|---|---|---|
| Non-Ulcer (n = 19,725) | DU (n = 540) | Non-Ulcer (n = 37,810) | DU (n = 548) | ||
| Age (years) 3 | 57.1 ± 0.09 b 1 | 58.3 ± 0.42 a | 52.3 ± 0.06 c | 53.4 ± 0.45 c *** +++ | 1.40 (1.22–1.61) 2 |
| Gender (%) | 36.1 | 49.1 ⁑⁑⁑ | 63.9 | 50.9 ⁑⁑⁑ | 0.686 (0.570–0.827) |
| BMI (kg/m2) 4 | 24.5 ± 0.03 a | 23.8 ± 0.16 b | 23.5 ± 0.02 bc | 23.4 ± 0.17 c *** +++ | 0.777 (0.670–0.900) |
| Waist circumferences (cm) 5 | 85.3 ± 0.07 a | 84.1 ± 0.35 b | 78.3 ± 0.05 c | 77.8 ± 0.35 c *** ++ | 0.781 (0.657–0.928) |
| Serum glucose (mg/dL) 6 | 97.7 ± 0.19 a | 94.9 ± 0.91 b | 93.8 ± 0.12 b | 92.9 ± 0.90 b *** ++ | 0.796 (0.628–1.009) |
| Blood HbA1c (%) 7 | 5.68 ± 0.01 b | 5.56 ± 0.05 b | 5.72 ± 0.01 a | 5.78 ± 0.05 a *** # | 0.720 (0.506–1.025) |
| WBC (109/L) 8 | 5.76 ± 0.02 b | 5.84 ± 0.09 a | 5.67 ± 0.01 c | 5.47 ± 0.09 d *** | 0.753 (0.657–0.863) |
| Serum hs-CRP (mg/dL) 9 | 0.14 ± 0.004 | 0.13 ± 0.02 | 0.14 ± 0.002 | 0.13 ± 0.02 | 0.794 (0.436–1.448) |
| MetS (n, Yes%) | 3503 (17.8) | 88 (16.3) | 4625 (12.2) | 71 (13.0) | 0.900 (0.748–1.083) |
| Bronchitis (n, Yes%) | 135 (0.61) | 23 (1.85) ⁑⁑ | 257 (0.62) | 15 (1.83) ⁑⁑ | 2.42 (1.38–4.25) |
| Asthma (n, Yes%) | 261 (1.38) | 16 (3.14) ⁑⁑ | 630 (1.77) | 18 (3.53) ⁑⁑ | 2.17 (1.50–3.13) |
| Arthritis (n, %) | 727 (3.85) | 39 (7.65) ⁑⁑⁑ | 3886 (10.9) | 110 (21.5) ⁑⁑⁑ | 2.19 (1.80–2.67) |
| Allergy (n, %) | 1011 (5.53) | 53 (10.4) ⁑⁑⁑ | 2716 (7.64) | 73 (14.3) ⁑⁑⁑ | 2.06 (1.68–2.53) |
| Gastritis (n, %) | 1438 (7.61) | 102 (20.0) ⁑⁑⁑ | 3720 (10.5) | 152 (29.8) ⁑⁑⁑ | 3.34 (2.86–3.91) |
| Periodontitis (n, Yes%) | 1394 (7.38) | 74 (14.5) ⁑⁑⁑ | 2221 (6.25) | 68 (13.3) ⁑⁑⁑ | 2.18 (1.79–2.67) |
| Osteoporosis (n, Yes%) | 123 (0.62) | 9 (1.67) ⁑⁑ | 2613 (7.35) | 70 (13.7) ⁑⁑⁑ | 1.93 (1.49–2.50) |
| Men | Women | Adjusted ORs (95% CI) | |||
|---|---|---|---|---|---|
| No-Ulcer (n = 19,725) | Ulcer (n = 540) | No-Ulcer (n = 37,810) | Ulcer (n = 548) | ||
| Energy intake (kcal/day) 3 | 90.0 ± 0.38 b 1 | 90.1 ± 1.82 b | 101 ± 0.27 a | 102 ± 1.93 a *** | 1.011 (0.824–1.239) 2 |
| KBD (N, Yes%) 4 | 7852 (40.1) | 209 (41.0) | 10,686 (30.1) | 143 (27.9) | 0.989 (0.850–1.150) |
| PBD (N, Yes%) 4 | 3833 (20.3) | 103 (20.2) | 14,151 (39.8) | 206 (40.2) | 1.022 (0.874–1.196) |
| WSD (N, Yes%) 4 | 9968 (52.7) | 230 (45.1) ⁑⁑ | 12,619 (35.5) | 155 (30.3) ⁑ | 0.809 (0.695–0.941) |
| RMD (N, Yes%) 4 | 6019 (31.8) | 162 (31.8) | 12,131 (34.1) | 159 (31.1) | 0.918 (0.791–1.064) |
| Irregular meals | 67 (1.13) | 3 (1.58) | 232 (2.22) | 9 (4.57) ⁑ | 1.965 (1.029–3.751) |
| Less cooked meats (N, Yes%) | 11,792 (62.5) | 336 (66.1) ⁑ | 18,788 (53.0) | 302 (59.1) ⁑⁑ | 1.243 (1.082–1.428) |
| Burnt meats (N, Yes%) | 3493 (19.3) | 101 (20.7) | 4371 (12.6) | 72 (14.4) | 1.197 (1.001–1.431) |
| Fried foods (N, Yes%) 5 | 11,452 (60.6) | 294 (57.7) | 23,312 (65.6) | 345 (67.4) | 0.940 (0.817–1.081) |
| Coffee (g/day) 6 | 3.65 ± 0.03 a | 3.28 ± 0.14 b | 3.69 ± 0.02 a | 3.03 ± 0.13 b +++ | 0.648 (0.567–0.740) |
| Tea (g/day) 7 45 | 43.9 ± 0.81 | 40.5 ± 3.89 | 42.9 ± 0.53 | 45.9 ± 3.84 | 1.115 (0.959–1.297) |
| Alcohol (g/day) 8 | 30.6 ± 0.64 a | 30.2 ± 3.05 a | 9.13 ± 0.45 b | 9.09 ± 3.23 b *** | 1.019 (0.880–1.179) |
| Multivitamin (N, Yes%) | 14,647 (74.3) | 378 (70.0) ⁑⁑ | 29,158 (77.1) | 396 (72.3) ⁑⁑ | 0.779 (0.673–0.903) |
| Physical activity (N, Yes%) | 11,611 (59.0) | 330 (61.5) | 19,725 (52.3) | 287 (52.7) | 1.451 (0.201–10.46) |
| Former smoker (N, %) | 8515 (43.3) | 272 (50.5) ⁑⁑⁑ | 449 (1.19) | 11 (2.01) | 1.516 (1.220–1.883) |
| Current smoker (N, %) | 5501 (28.0) | 157 (29.1) | 737 (1.96) | 12 (2.19) | 1.475 (1.162–1.872) |
| CHR | SNP | BP | A1 | A2 | OR | SE | p | MAF | HWE_P | Gene Names | Functional SEQUENCES |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | rs576376935 | 218,990,311 | G | T | 1.945 | 0.159 | 2.88 × 10−6 | 0.0113 | 0.3496 | CXCR2 | Intron |
| 3 | rs77063016 | 60,431,863 | C | G | 1.314 | 0.06484 | 2.52 × 10−6 | 0.1183 | 0.8432 | FHIT | Intron |
| 5 | rs10055925 | 40,688,059 | A | G | 0.7662 | 0.04673 | 1.21 × 10−8 | 0.4778 | 0.0509 | TTC33 | Intron |
| 8 | rs2978977 | 143,755,720 | A | C | 0.6365 | 0.0509 | 6.86 × 10−19 | 0.3857 | 0.5421 | PSCA | Intron |
| 10 | rs6584283 | 101,290,301 | T | C | 1.204 | 0.04625 | 4.13 × 10−6 | 0.4633 | 0.7842 | LINC01475 | Intron |
| 11 | rs11230563 (R225W) | 60,776,209 | T | C | 0.808 | 0.06226 | 4.18 × 10−6 | 0.1932 | 0.7708 | CD6 | Missense |
| 12 | rs7309887 | 26,583,100 | C | A | 0.8127 | 0.04997 | 3.32 × 10−6 | 0.3536 | 0.7112 | ITPR2 | NMD transcript |
| 13 | rs78141015 | 43,664,299 | T | C | 1.702 | 0.1124 | 2.21 × 10−6 | 0.0276 | 0.7582 | DNAJC15 | Intron |
| 16 | rs111690253 | 11,688,746 | T | A | 1.914 | 0.1251 | 2.12 × 10−7 | 0.0192 | 0.1524 | LITAF | Intron |
| 19 | rs796980537 | 49,203,590 | T | A | 0.7424 | 0.0753 | 6.03 × 10−6 | 0.1359 | 0.1003 | FUT2 | Intron |
| Pathways | No. of Genes | Beta | SD | p Value Bonferroni | Participating Genes |
|---|---|---|---|---|---|
| GO BP: GO Actin modification | 5 | 1.6621 | 0.027085 | 6.6387 × 10−5 | CXCR2, FHIT, CD6, ITPR2, DNAJC15 |
| GO MF: GO LRR domain binding | 17 | 0.72595 | 0.021805 | 9.8491 × 10−5 | CD6, FUT2, FHIT |
| Curated gene sets: Shaffer IRF4 targets in myeloma vs. mature B lymphocyte | 96 | 0.30049 | 0.021404 | 0.00014762 | CXCR2, CD6, ITPR2, FUT2 |
| Curated gene sets: Reactome runx3 regulates immune response and cell migration | 6 | 1.2508 | 0.022326 | 0.00019992 | CXCR2, FHIT, CD6, ITPR2, DNAJC15 |
| Active Ingredients | Effective Food | ΔG of wild CD6 | ΔG of Mutant CD6 |
|---|---|---|---|
| Azaspiracid 2 | Blue mussel | −13.2 | −13.2 |
| Glycyrrhizin | Liquorice | −11.7 | - |
| Physalin B | Winter cherry | −12.4 | - |
| Janthitrem F | Penicillium Janthinellum | −12 | - |
| Casuarinin | Siberian filbert | −11.5 | - |
| Plastoquinone 8 | Sweet corn | - | −12.3 |
| Solamargine | Solanaceae family | - | −12.2 |
| Saponin D | Hovenia dulcis | - | −11.2 |
| Matesaponin 2 | Ilex paraguariensis | - | −11.9 |
| Low-PRS (n = 5912) | Middle-PRS (n = 23,471) | High-PRS (n = 29,240) | Interaction | |
|---|---|---|---|---|
| Irregular meal | 1 | 2.237 (1.196–4.185) | 3.674 (1.996–6.762) | 0.0047 |
| Regular meal | 1 | 0.062 (0.003–1.012) | 0.742 (0.115–4.773) | |
| Non-smoking + former | 1 | 1.718 (0.934–3.161) | 3.041 (1.689–5.475) | 0.0015 |
| Smoking | 1 | 4.285 (0.566–32.43) | 5.301 (0.713–39.38) | |
| No multivitamin | 1 | 2.215 (1.146–4.278) | 3.057 (1.606–5.821) | 0.0055 |
| Multivitamin | 1 | 1.016 (0.287–3.600) | 3.789 (1.174–12.23) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Liu, M.; Huang, S. Association of Polygenic Variants Involved in Immunity and Inflammation with Duodenal Ulcer Risk and Their Interaction with Irregular Eating Habits. Nutrients 2023, 15, 296. https://doi.org/10.3390/nu15020296
Park S, Liu M, Huang S. Association of Polygenic Variants Involved in Immunity and Inflammation with Duodenal Ulcer Risk and Their Interaction with Irregular Eating Habits. Nutrients. 2023; 15(2):296. https://doi.org/10.3390/nu15020296
Chicago/Turabian StylePark, Sunmin, Meiling Liu, and Shaokai Huang. 2023. "Association of Polygenic Variants Involved in Immunity and Inflammation with Duodenal Ulcer Risk and Their Interaction with Irregular Eating Habits" Nutrients 15, no. 2: 296. https://doi.org/10.3390/nu15020296
APA StylePark, S., Liu, M., & Huang, S. (2023). Association of Polygenic Variants Involved in Immunity and Inflammation with Duodenal Ulcer Risk and Their Interaction with Irregular Eating Habits. Nutrients, 15(2), 296. https://doi.org/10.3390/nu15020296







