D-Limonene Promotes Anti-Obesity in 3T3-L1 Adipocytes and High-Calorie Diet-Induced Obese Rats by Activating the AMPK Signaling Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Culture and Adipocyte Differentiation
2.3. Cell Viability Assay
2.4. Intracellular Lipid Assay
2.4.1. Oil Red O Staining Assay
2.4.2. Intracellular Triglyceride Assay
2.5. Animals and Diet
2.6. Animal Growth Characteristics Assay
2.7. Biochemical Parameters Assay
2.8. Histological Assay
2.9. mRNA Expression Assay
2.10. Protein Expression Assay
2.11. Statistical Assay
3. Results
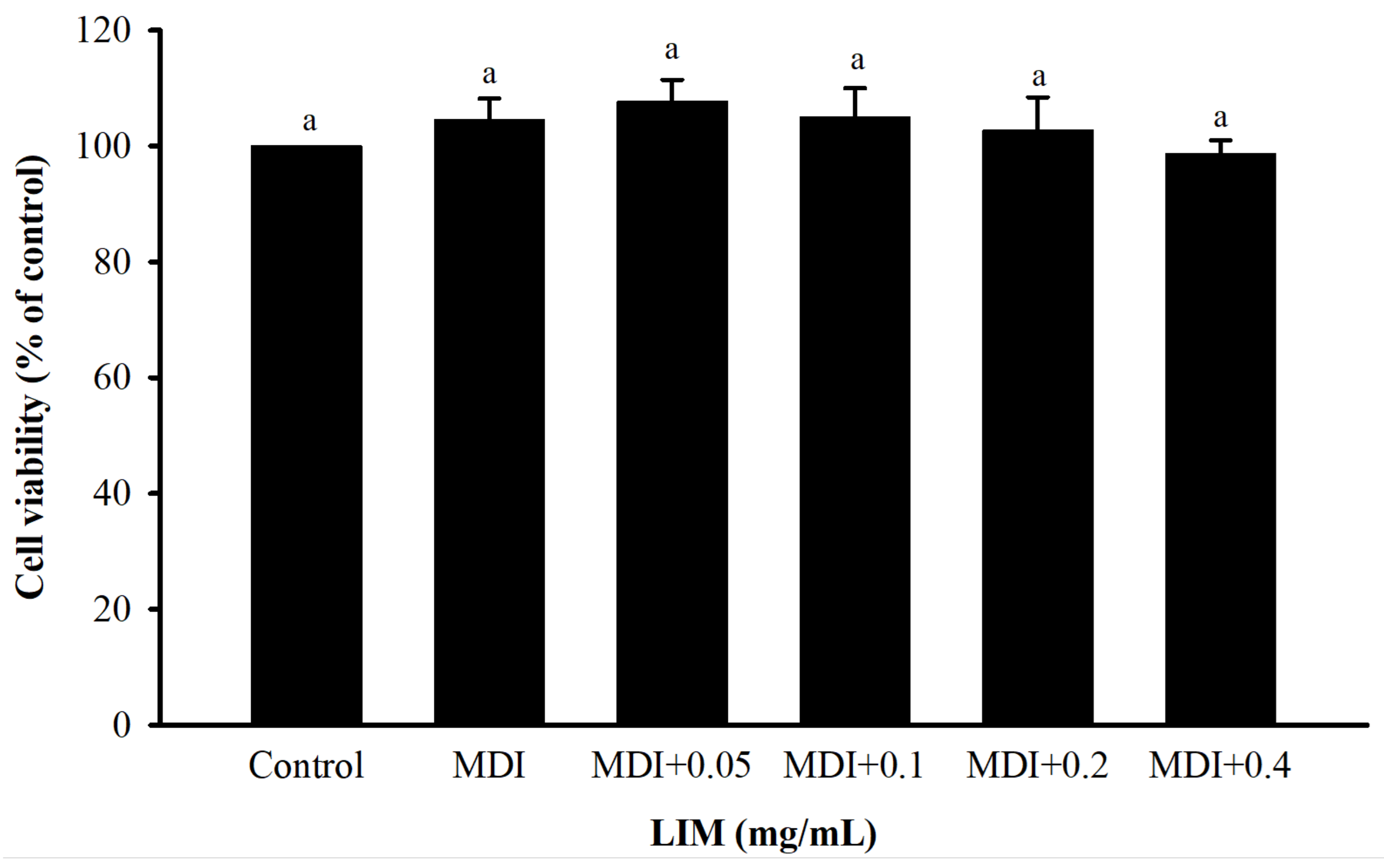
3.1. Effect of LIM on Cell Viability and Proliferation Ability of 3T3-L1 Adipocytes
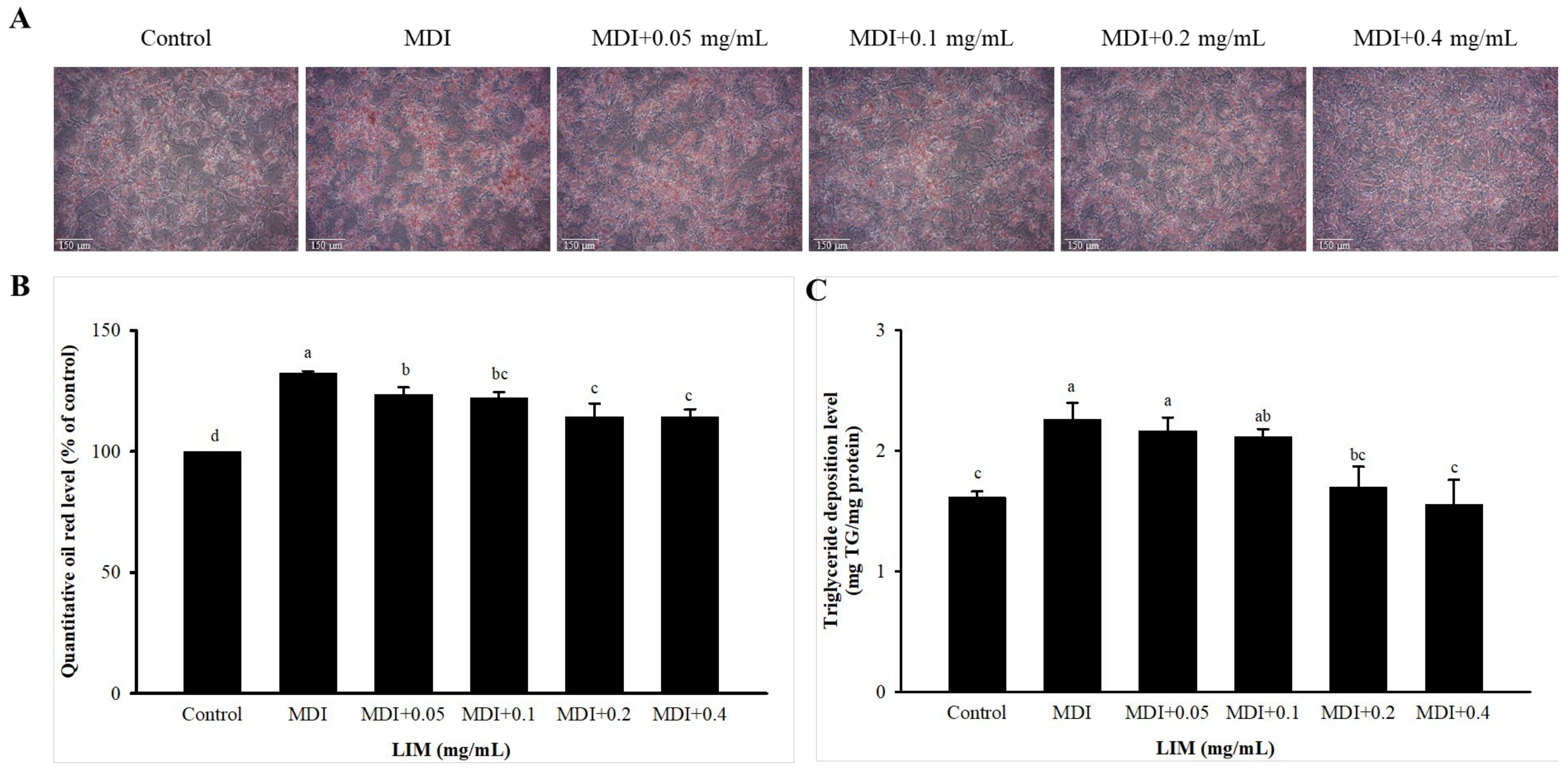
3.2. Effect of LIM on Lipid Accumulation in 3T3-L1 Adipocytes
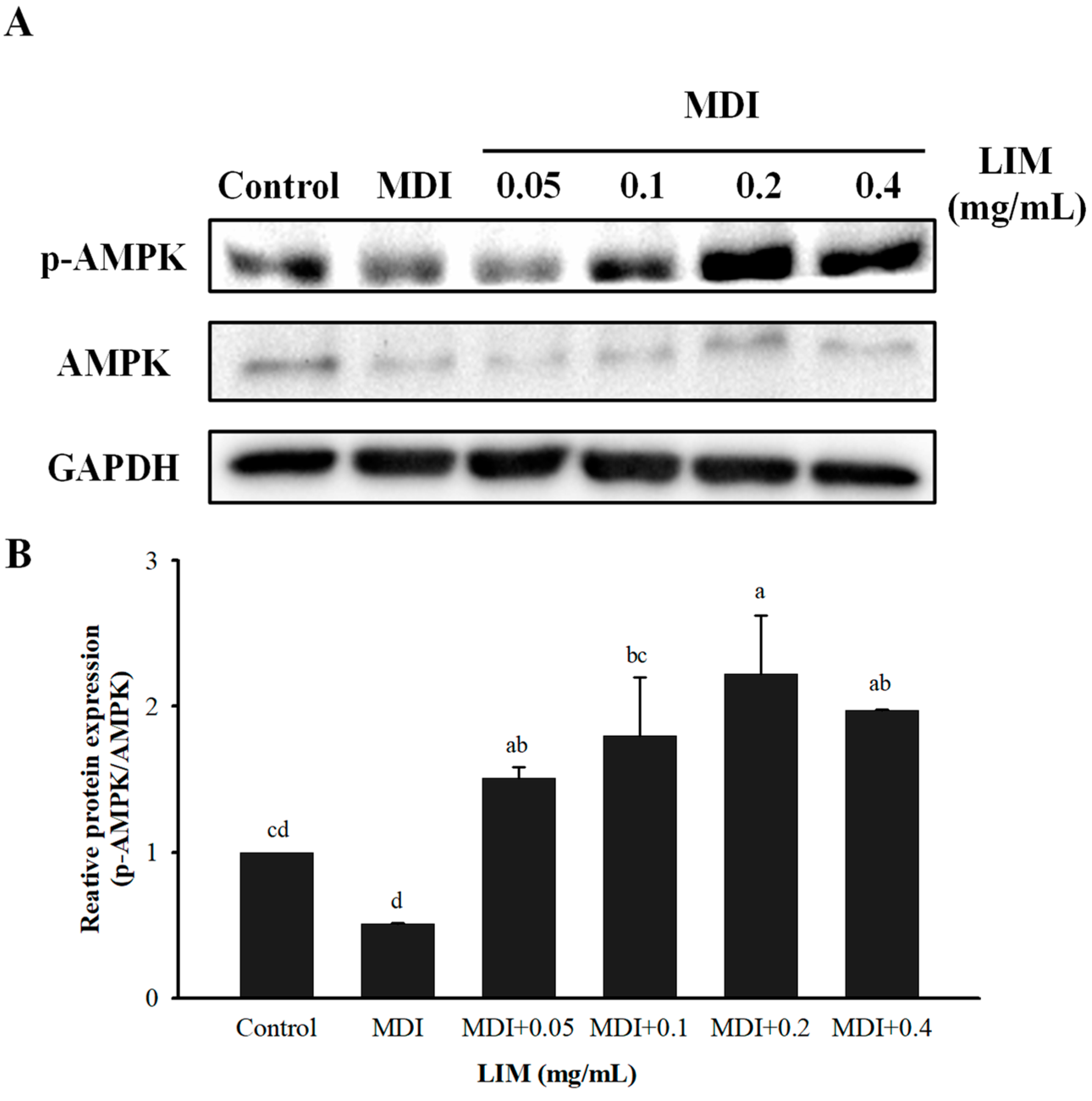
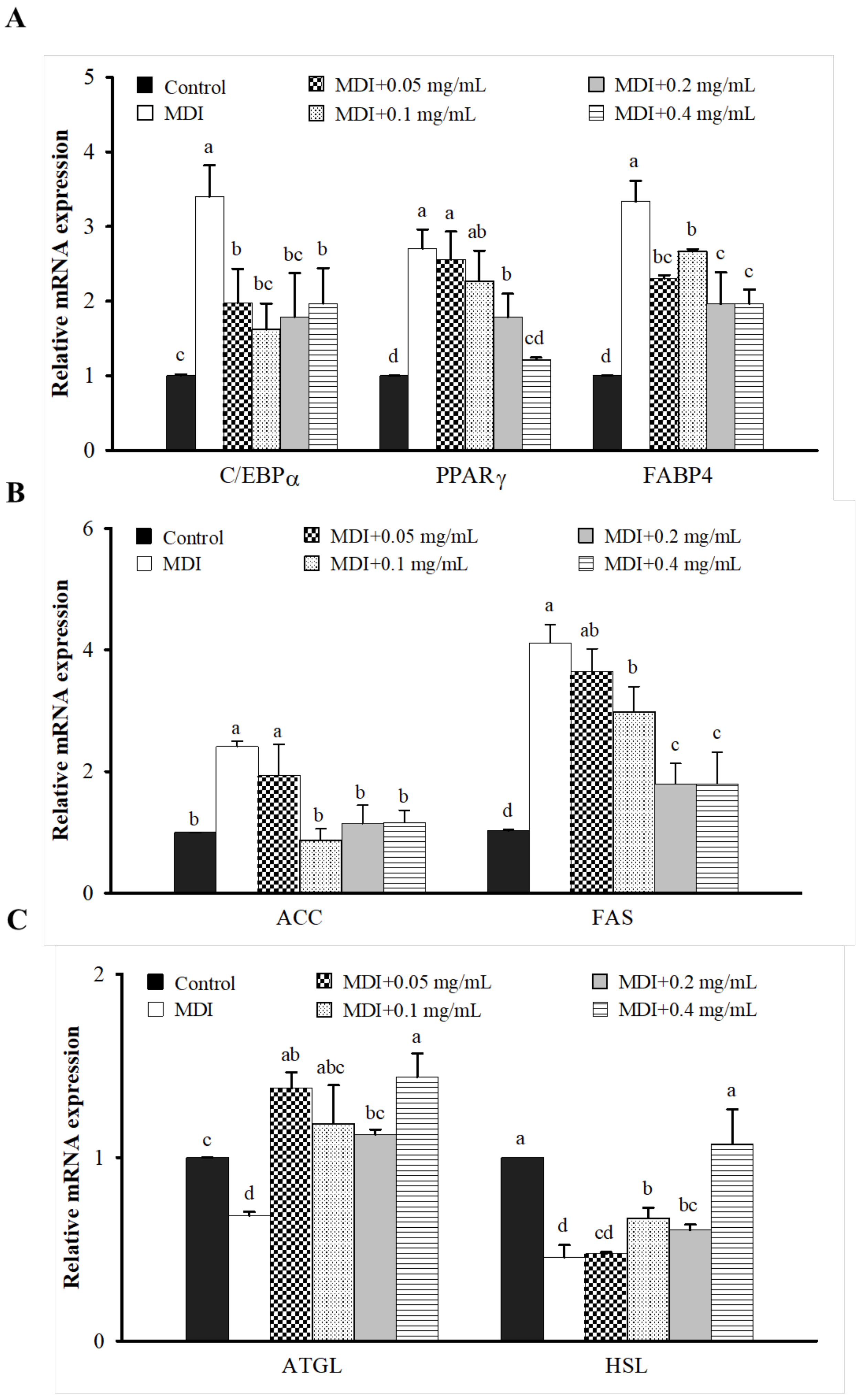
3.3. Effect of LIM on Protein and mRNA Expression Related to AMPK Signaling Pathway in 3T3-L1 Adipocytes
3.4. Effect of LIM on the Growth Characteristics in High-Calorie Diet-Induced Obese Rats
3.5. Effect of LIM on Biochemical Parameters in High-Calorie Diet-Induced Obese Rats
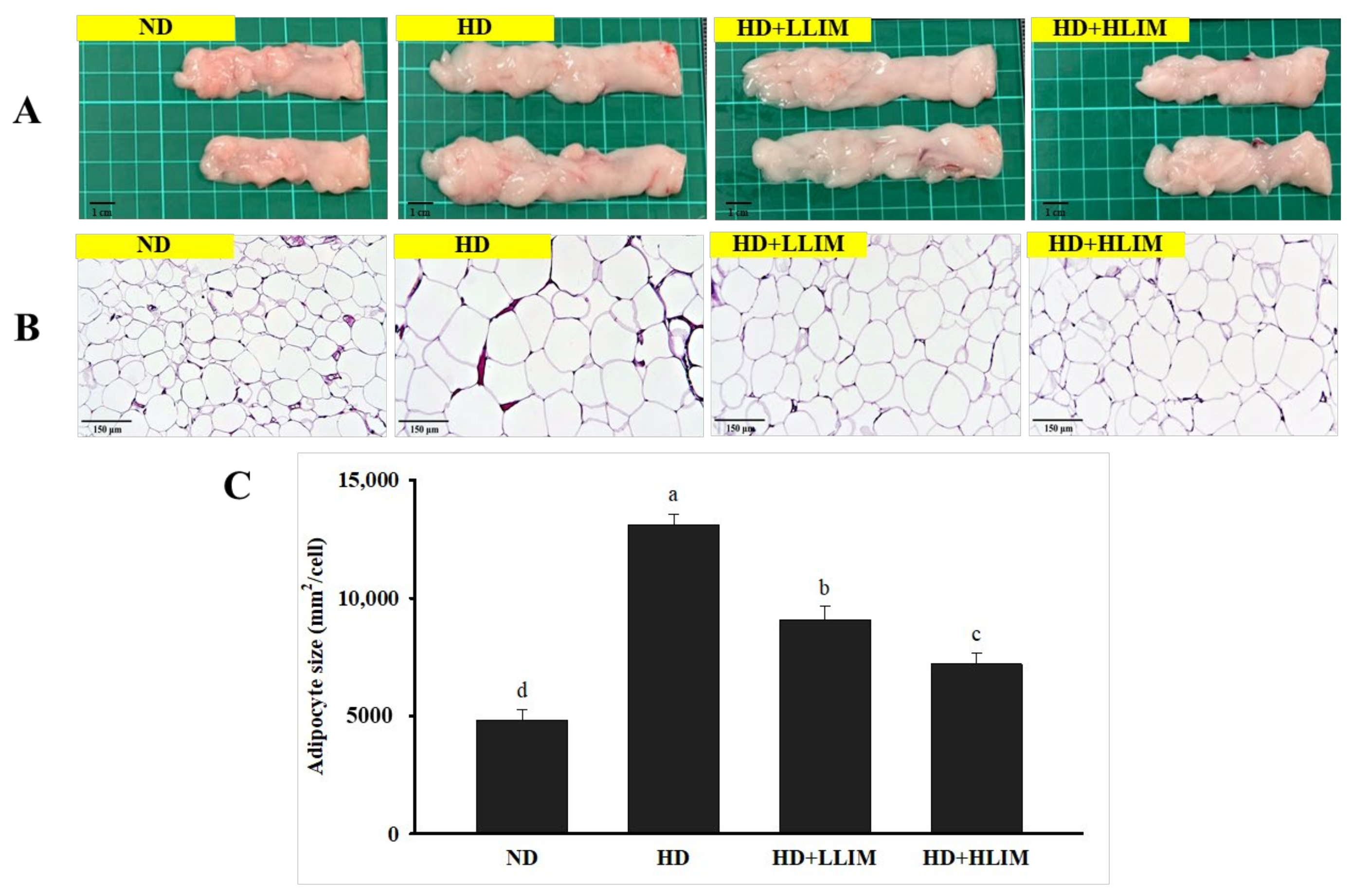
3.6. Effect of LIM on Epididymal Adipose Tissue Sections in High-Calorie Diet-Induced Obese Rats
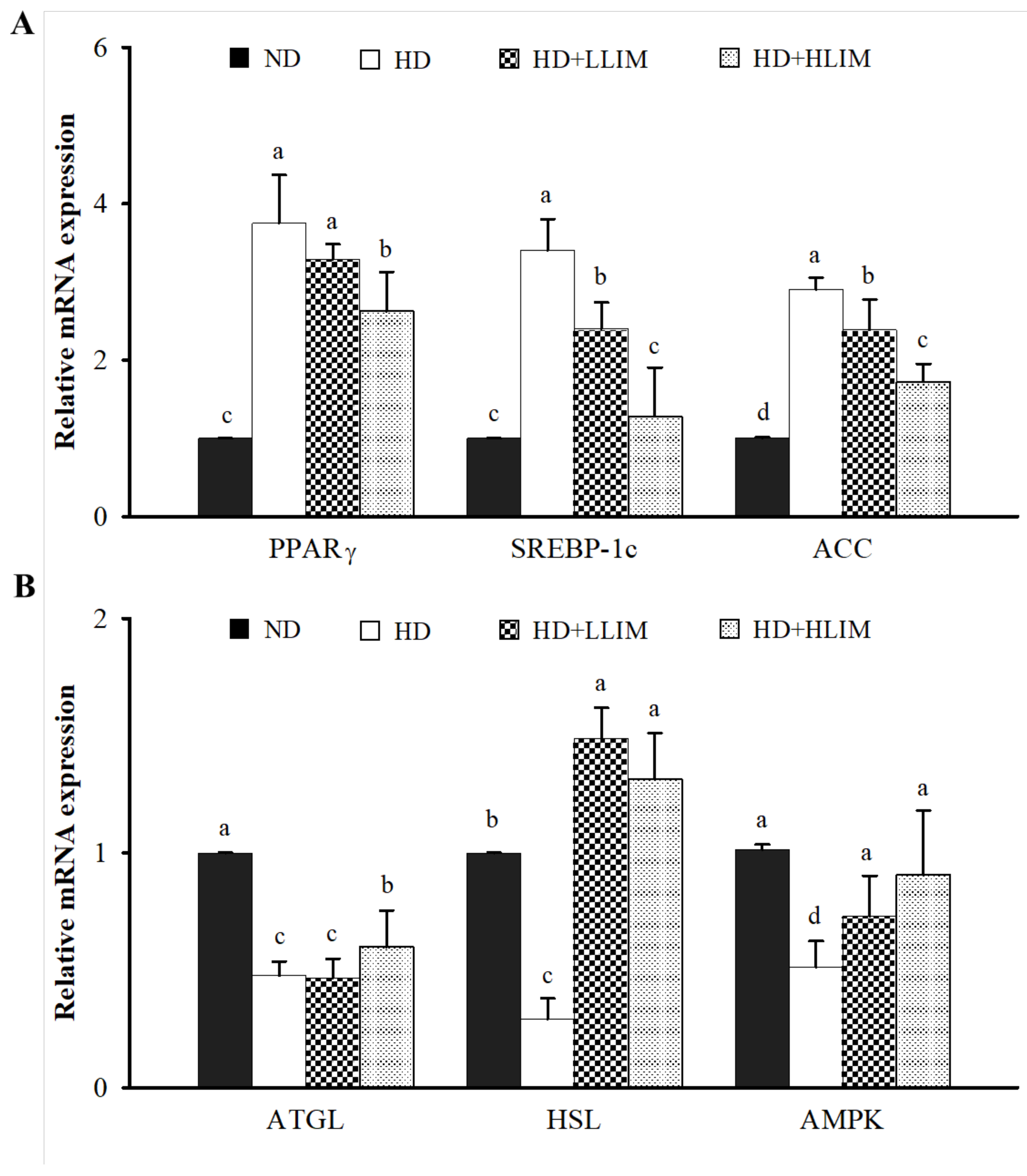
3.7. Effect of LIM on mRNA Expression of Genes Related to AMPK Signaling Pathway in High-Calorie Diet-Induced Obese Rats
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Romieu, I.; Dossus, L.; Barquera, S.; Blottière, H.M.; Franks, P.W.; Gunter, M.; Hwalla, N.; Hursting, S.D.; Leitzmann, M.; Margetts, B. Energy balance and obesity: What are the main drivers? Cancer Causes Control 2017, 28, 247–258. [Google Scholar] [CrossRef] [PubMed]
- World Obesity Federation (WOF). World Obesity Atlas 2022. 2022. Available online: https://www.worldobesity.org/resources/resource-library/world-obesity-atlas-2022 (accessed on 10 December 2022).
- Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity: Implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer. Obes. Res. Clin. Pract. 2013, 7, e330–e341. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Seo, M.G.; Cominguez, D.C.; Han, I.; An, H.J. Atractylodes Chinensis water extract ameliorates obesity via promotion of the SIRT1/AMPK expression in high-fat diet-induced obese mice. Nutrients 2021, 13, 2992. [Google Scholar] [CrossRef] [PubMed]
- Fabbrini, E.; Sullivan, S.; Klein, S. Obesity and nonalcoholic fatty liver disease: Biochemical, metabolic, and clinical implications. Hepatology 2010, 51, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Klop, B.; Elte, J.W.; Cabezas, M.C. Dyslipidemia in obesity: Mechanisms and potential targets. Nutrients 2013, 5, 1218–1240. [Google Scholar] [CrossRef]
- Bijland, S.; Mancini, S.J.; Salt, I.P. Role of AMP-activated protein kinase in adipose tissue metabolism and inflammation. Clin. Sci. 2013, 124, 491–507. [Google Scholar] [CrossRef]
- Jeon, S.M. Regulation and function of AMPK in physiology and diseases. Exp. Mol. Med. 2016, 48, e245. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, L.; Huang, Z.; Lin, L.; Zheng, G. Synergistic effects of caffeine and catechins on lipid metabolism in chronically fed mice via the AMP-activated protein kinase signaling pathway. Eur. J. Nutr. 2017, 56, 2309–2318. [Google Scholar] [CrossRef]
- Lai, C.S.; Liao, S.N.; Tsai, M.L.; Kalyanam, N.; Majeed, M.; Majeed, A.; Ho, C.T.; Pan, M.H. Calebin-A inhibits adipogenesis and hepatic steatosis in high-fat diet-induced obesity via activation of AMPK signaling. Mol. Nutr. Food Res. 2015, 59, 1883–1895. [Google Scholar] [CrossRef]
- Sri Devi, S.; Ashokkumar, N. Citral, a monoterpene inhibits adipogenesis through modulation of adipogenic transcription factors in 3T3-L1 cells. Indian J. Clin. Biochem. 2018, 33, 414–421. [Google Scholar] [CrossRef]
- Mohamed, G.A.; Ibrahim, S.R.M.; Elkhayat, E.S.; El Dine, R.S. Natural anti-obesity agents. Bull. Fac. Pharm. Cairo Univ. 2014, 52, 269–284. [Google Scholar] [CrossRef]
- Negro, V.; Mancini, G.; Ruggeri, B.; Fino, D. Citrus waste as feedstock for bio-based products recovery: Review on limonene case study and energy valorization. Bioresour. Technol. 2016, 214, 806–815. [Google Scholar] [CrossRef]
- Wu, C.C.; Huang, Y.W.; Hou, C.Y.; Chen, Y.T.; Dong, C.D.; Chen, C.W.; Singhania, R.R.; Leang, J.Y.; Hsieh, S.L. The anti-obesity effects of lemon fermented products in 3T3-L1 preadipocytes and in a rat model with high-calorie diet-induced obesity. Nutrients 2021, 13, 2809. [Google Scholar] [CrossRef]
- Sun, J. D-Limonene: Safety and clinical applications. Altern. Med. Rev. 2007, 12, 259–264. [Google Scholar]
- Roberto, D.; Micucci, P.; Sebastian, T.; Graciela, F.; Anesini, C. Antioxidant activity of limonene on normal murine lymphocytes: Relation to H2O2 modulation and cell proliferation. Basic. Clin. Pharmacol. Toxicol. 2010, 106, 38–44. [Google Scholar] [CrossRef]
- Yu, L.; Yan, J.; Sun, Z. D-limonene exhibits anti-inflammatory and antioxidant properties in an ulcerative colitis rat model via regulation of iNOS, COX-2, PGE2 and ERK signaling pathways. Mol. Med. Rep. 2017, 15, 2339–2346. [Google Scholar] [CrossRef]
- Santiago, J.V.A.; Jayachitra, J.; Shenbagam, M.; Nalini, N. D-limonene attenuates blood pressure and improves the lipid and antioxidant status in high-fat diet and L-NAME treated rats. J. Pharm. Sci. Res. 2010, 2, 752–758. [Google Scholar]
- Jing, L.; Zhang, Y.; Fan, S.; Gu, M.; Guan, Y.; Lu, X.; Huang, C.; Zhou, Z. Preventive and ameliorating effects of citrus d-limonene on dyslipidemia and hyperglycemia in mice with high-fat diet-induced obesity. Eur. J. Pharmacol. 2013, 715, 46–55. [Google Scholar] [CrossRef]
- Valerii, M.C.; Turroni, S.; Ferreri, C.; Zaro, M.; Sansone, A.; Dalpiaz, A.; Botti, G.; Ferraro, L.; Spigarelli, R.; Bellocchio, I. Effect of a fiber d-limonene-enriched food supplement on intestinal microbiota and metabolic parameters of mice on a high-fat diet. Pharmaceutics 2021, 13, 1753. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Perrin, D.; Soulage, C.; Pequignot, J.M.; Géloën, A. Resistance to obesity in Lou/C rats prevents aging-associated metabolic alterations. Diabetologia 2003, 46, 1489–1496. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Do Nascimento, C.M.; Cassol, T.; Da Silva, F.S.; Bonfleur, M.L.; Nassar, C.A.; Nassar, P.O. Radiographic evaluation of the effect of obesity on alveolar bone in rats with ligature-induced periodontal disease. Diabet. Metab. Synd. Obes. 2013, 6, 365–370. [Google Scholar] [CrossRef]
- Enders, J.F.; Peebles, T.C. Propagation in tissue cultures of cytopathogenic agents from patients with measles. Proc. Soc. Exp. Biol. Med. 1954, 86, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.; Al-Numair, K.S.; Balakrishnan, A. Docking studies on the interaction of flavonoids with fat mass and obesity-associated protein. Pak. J. Pharm. Sci. 2015, 28, 1647–1653. [Google Scholar] [PubMed]
- Church, C.; Lee, S.; Bagg, E.A.; McTaggart, J.S.; Deacon, R.; Gerken, T.; Lee, A.; Moir, L.; Mecinović, J.; Quwailid, M.M. A mouse model for the metabolic effects of the human fat mass and obesity-associated FTO gene. PLoS Genet. 2009, 5, e1000599. [Google Scholar] [CrossRef]
- Ahmed, M.; Hameed, S.; Ali, M.; Hizbullah, S.; Zaheer, A. In silico molecular docking analysis of limonene with the fat mass and obesity-associated protein by using autodock vina. Sci. J. Inform. 2021, 8, 154–160. [Google Scholar] [CrossRef]
- Fryer, L.G.D.; Carling, D. AMP-activated protein kinase and the metabolic syndrome. Biochem. Soc. Trans. 2005, 33, 362–366. [Google Scholar] [CrossRef]
- Ahmad, B.; Serpell, C.J.; Fong, I.L.; Wong, E.H. Molecular mechanisms of adipogenesis: The anti-adipogenic role of AMP-activated protein kinase. Front. Mol. Biosci. 2020, 7, 76. [Google Scholar] [CrossRef]
- Kraus, N.A.; Ehebauer, F.; Zapp, B.; Rudolphi, B.; Kraus, B.J.; Kraus, D. Quantitative assessment of adipocyte differentiation in cell culture. Adipocyte 2016, 5, 351–358. [Google Scholar] [CrossRef]
- Sørensen, T.I.A. From fat cells through an obesity theory. Eur. J. Clin. Nutr. 2018, 72, 1329–1335. [Google Scholar] [CrossRef]
- Tan, X.C.; Chua, K.H.; Ram, M.R.; Kuppusamy, U.R. Monoterpenes: Novel insights into their biological effects and roles on glucose uptake and lipid metabolism in 3T3-L1 adipocytes. Food Chem. 2016, 196, 242–250. [Google Scholar] [CrossRef]
- Ngamdokmai, N.; Paracha, T.U.; Waranuch, N.; Chootip, K.; Wisuitiprot, W.; Suphrom, N.; Insumrong, K.; Ingkaninan, K. Effects of essential oils and some constituents from ingredients of anti-cellulite herbal compress on 3T3-L1 adipocytes and rat aortae. Pharmaceuticals 2021, 14, 253. [Google Scholar] [CrossRef]
- Soundharrajan, I.; Kim, D.H.; Srisesharam, S.; Kuppusamy, P.; Choi, K.C. R-Limonene enhances differentiation and 2-deoxy-d-glucose uptake in 3T3-L1 preadipocytes by activating the akt signaling pathway. Evid.-Based Complement. Altern. Med. 2018, 2018, 4573254. [Google Scholar] [CrossRef]
- Khan, A.; Siddiqui, H.; Mahmood, T.; Ahsan, F. A comparative evaluation study of Citrus limetta and metformin against hyperlipidemia in diabetic and non-diabetic rats. Res. J. Pharm. Technol. 2019, 12, 1244–1250. [Google Scholar] [CrossRef]
- Vigneshwar, R.; Arivuchelvan, A.; Mekala, P.; Imayarasi, K. Sex-specific reference intervals for Wistar albino rats: Hematology and clinical biochemistry. Indian J. Anim. Health 2021, 60, 58–65. [Google Scholar] [CrossRef]
- Sun, D.; Gao, W.; Hu, H.; Zhou, S. Why 90% of clinical drug development fails and how to improve it? Acta Pharm. Sin. B 2022, 12, 3049–3062. [Google Scholar] [CrossRef]
- Huang, C.H.; Hsiao, Y.H.; Lin, Y.H.; Tsai, G.J. Effects of fermented citrus peel on ameliorating obesity in rats fed with high-fat diet. Molecules 2022, 27, 8966. [Google Scholar] [CrossRef]
| ND | HD | HD + LLIM | HD + HLIM | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Body weight (g) | 493.50 | ± | 9.32 b | 564.25 | ± | 47.14 a | 503.13 | ± | 42.60 b | 477.23 | ± | 41.07 b |
| Weight gain (%) | 94.15 | ± | 4.39 c | 123.38 | ± | 16.38 a | 111.01 | ± | 3.63 b | 100.37 | ± | 6.52 bc |
| Lee’s index (g/cm) | 0.30 | ± | 0.01 c | 0.35 | ± | 0.01 a | 0.32 | ± | 0.00 b | 0.30 | ± | 0.00 c |
| Food intake (g/rat/day) | 25.89 | ± | 1.07 a | 18.23 | ± | 0.96 b | 19.02 | ± | 1.11 b | 19.11 | ± | 0.87 b |
| Feed conversion ratio (%) | 8.77 | ± | 0.37 d | 16.12 | ± | 1.21 a | 13.41 | ± | 0.36 b | 12.04 | ± | 0.57 c |
| Liver (%) | 3.46 | ± | 0.30 a | 3.22 | ± | 0.27 a | 3.50 | ± | 0.41 a | 3.63 | ± | 0.44 a |
| Kidney (%) | 0.81 | ± | 0.06 a | 0.65 | ± | 0.04 b | 0.69 | ± | 0.11 b | 0.82 | ± | 0.05 a |
| Spleen (%) | 0.19 | ± | 0.01 a | 0.17 | ± | 0.02 a | 0.17 | ± | 0.01 a | 0.16 | ± | 0.02 a |
| Epididymal adipose tissue (%) | 1.55 | ± | 0.18 b | 2.83 | ± | 0.94 a | 2.22 | ± | 0.46 ab | 1.83 | ± | 0.35 b |
| Mesenteric adipose tissue (%) | 1.02 | ± | 0.04 c | 1.90 | ± | 0.15 a | 1.43 | ± | 0.20 b | 1.17 | ± | 0.16 c |
| Retroperitoneal adipose tissue (%) | 1.23 | ± | 0.05 c | 3.16 | ± | 0.64 a | 2.04 | ± | 0.43 b | 2.05 | ± | 0.17 b |
| Total fat tissue (%) | 3.81 | ± | 0.59 c | 8.25 | ± | 1.11 a | 5.78 | ± | 1.02 b | 5.24 | ± | 0.58 b |
| ND | HD | HD + LLIM | HD + HLIM | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Triglyceride (mg/dL) | 75.11 | ± | 7.26 a | 84.05 | ± | 4.54 a | 74.10 | ± | 9.51 a | 45.79 | ± | 1.81 b |
| Total cholesterol (mg/dL) | 78.84 | ± | 2.71 a | 72.36 | ± | 2.16 b | 77.95 | ± | 2.78 a | 73.29 | ± | 2.84 b |
| Low-density lipoprotein cholesterol (mg/dL) | 9.50 | ± | 0.58 c | 12.67 | ± | 0.58 a | 11.00 | ± | 0.00 b | 11.67 | ± | 0.58 b |
| High-density lipoprotein cholesterol (mg/dL) | 48.00 | ± | 5.48 ab | 41.67 | ± | 2.31 b | 43.67 | ± | 2.08 ab | 51.00 | ± | 6.00 a |
| Free fatty acid (mmol/L) | 1.44 | ± | 0.12 a | 1.32 | ± | 0.23 a | 1.43 | ± | 0.06 a | 1.38 | ± | 0.13 a |
| Lipase (mmol/L) | 7.20 | ± | 0.45 a | 6.40 | ± | 0.55 b | 6.57 | ± | 0.53 ab | 7.20 | ± | 0.45 a |
| Ketone body (mmol/L) | 1.24 | ± | 0.21 a | 1.52 | ± | 0.13 a | 1.34 | ± | 0.15 a | 1.42 | ± | 0.16 a |
| Aspartate aminotransferase (U/L) | 136.20 | ± | 28.60 a | 134.60 | ± | 7.64 a | 131.29 | ± | 12.40 a | 135.35 | ± | 27.32 a |
| Alanine aminotransferase (U/L) | 50.20 | ± | 5.26 a | 49.40 | ± | 3.83 a | 45.79 | ± | 7.38 a | 43.67 | ± | 5.13 a |
| Creatinine (mg/dL) | 0.71 | ± | 0.03 a | 0.75 | ± | 0.08 a | 0.67 | ± | 0.06 ab | 0.62 | ± | 0.06 b |
| Uric acid (mg/dL) | 2.18 | ± | 0.33 b | 3.10 | ± | 0.56 a | 3.92 | ± | 0.59 a | 3.13 | ± | 0.57 a |
| Sodium (mmol/L) | 150.82 | ± | 2.50 a | 150.33 | ± | 2.52 a | 150.69 | ± | 2.20 a | 149.65 | ± | 2.56 a |
| Potassium (mmol/L) | 7.22 | ± | 1.61 a | 7.16 | ± | 0.67 a | 7.99 | ± | 0.79 a | 7.78 | ± | 0.80 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, J.-T.; Huang, Y.-W.; Hou, C.-Y.; Wang, J.-J.; Wu, C.-C.; Hsieh, S.-L. D-Limonene Promotes Anti-Obesity in 3T3-L1 Adipocytes and High-Calorie Diet-Induced Obese Rats by Activating the AMPK Signaling Pathway. Nutrients 2023, 15, 267. https://doi.org/10.3390/nu15020267
Liao J-T, Huang Y-W, Hou C-Y, Wang J-J, Wu C-C, Hsieh S-L. D-Limonene Promotes Anti-Obesity in 3T3-L1 Adipocytes and High-Calorie Diet-Induced Obese Rats by Activating the AMPK Signaling Pathway. Nutrients. 2023; 15(2):267. https://doi.org/10.3390/nu15020267
Chicago/Turabian StyleLiao, Jin-Ting, Yu-Wen Huang, Chih-Yao Hou, Jyh-Jye Wang, Chih-Chung Wu, and Shu-Ling Hsieh. 2023. "D-Limonene Promotes Anti-Obesity in 3T3-L1 Adipocytes and High-Calorie Diet-Induced Obese Rats by Activating the AMPK Signaling Pathway" Nutrients 15, no. 2: 267. https://doi.org/10.3390/nu15020267
APA StyleLiao, J.-T., Huang, Y.-W., Hou, C.-Y., Wang, J.-J., Wu, C.-C., & Hsieh, S.-L. (2023). D-Limonene Promotes Anti-Obesity in 3T3-L1 Adipocytes and High-Calorie Diet-Induced Obese Rats by Activating the AMPK Signaling Pathway. Nutrients, 15(2), 267. https://doi.org/10.3390/nu15020267









