Plant Protein but Not Animal Protein Consumption Is Associated with Frailty through Plasma Metabolites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Dietary Assessment
2.3. Plasma Metabolomic Assessment
2.4. Construction of Frailty Index (FI)
2.5. Assessment of Covariates
2.6. Statistical Analysis
3. Results
3.1. Cross-Sectional Associations of Protein Intake with Frailty Index
3.2. Plasma Metabolomic Profile of Vegetable Protein Intake
3.3. Plasma Metabolites Mediate the Association between Plant Protein Intake and Frailty Index
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schumacher, B.T.; Di, C.; Bellettiere, J.; Lamonte, M.J.; Simonsick, E.M.; Parada, H., Jr.; Hooker, S.P.; Lacroix, A.Z. Validation, Recalibration, and Predictive Accuracy of Published VO 2max Prediction Equations for Adults Ages 50–96 Yr. Med. Sci. Sports Exerc. 2023, 55, 322–332. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Tanaka, T.; Talegawkar, S.A.; Jin, Y.; Bandinelli, S.; Ferrucci, L. Association of Adherence to the Mediterranean-Style Diet with Lower Frailty Index in Older Adults. Nutrients 2021, 13, 1129. [Google Scholar] [CrossRef]
- Kojima, G.; Avgerinou, C.; Iliffe, S.; Walters, K. Adherence to Mediterranean Diet Reduces Incident Frailty Risk: Systematic Review and Meta-Analysis. J. Am. Geriatr. Soc. 2018, 66, 783–788. [Google Scholar] [CrossRef]
- Tanaka, T.; Kafyra, M.; Jin, Y.; Chia, C.W.; Dedoussis, G.V.; Talegawkar, S.A.; Ferrucci, L. Quality Specific Associations of Carbohydrate Consumption and Frailty Index. Nutrients 2022, 14, 5072. [Google Scholar] [CrossRef]
- Chuy, V.; Gentreau, M.; Artero, S.; Berticat, C.; Rigalleau, V.; Peres, K.; Helmer, C.; Samieri, C.; Feart, C. Simple Carbohydrate Intake and Higher Risk for Physical Frailty Over 15 Years in Community-Dwelling Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 10–18. [Google Scholar] [CrossRef]
- Phillips, S.M.; Chevalier, S.; Leidy, H.J. Protein “requirements” beyond the RDA: Implications for optimizing health. Appl. Physiol. Nutr. Metab. 2016, 41, 565–572. [Google Scholar] [CrossRef]
- Sandoval-Insausti, H.; Perez-Tasigchana, R.F.; Lopez-Garcia, E.; Garcia-Esquinas, E.; Rodriguez-Artalejo, F.; Guallar-Castillon, P. Macronutrients Intake and Incident Frailty in Older Adults: A Prospective Cohort Study. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 1329–1334. [Google Scholar] [CrossRef]
- Struijk, E.A.; Fung, T.T.; Rodriguez-Artalejo, F.; Bischoff-Ferrari, H.A.; Hu, F.B.; Willett, W.C.; Lopez-Garcia, E. Protein intake and risk of frailty among older women in the Nurses’ Health Study. J. Cachexia Sarcopenia Muscle 2022, 13, 1752–1761. [Google Scholar] [CrossRef]
- Struijk, E.A.; Fung, T.T.; Sotos-Prieto, M.; Rodriguez-Artalejo, F.; Willett, W.C.; Hu, F.B.; Lopez-Garcia, E. Red meat consumption and risk of frailty in older women. J. Cachexia Sarcopenia Muscle 2022, 13, 210–219. [Google Scholar] [CrossRef]
- Shock, N.W.; Greulick, R.C.; Andres, R.; Arenberg, D.; Costa, P.; Lakatta, E.; Tobin, J.D. Normal Human Aging: The Baltimore Study of Aging; No.84-2450; NIH Publication: Washington, DC, USA, 1984.
- Talegawkar, S.A.; Tanaka, T.; Maras, J.E.; Ferrucci, L.; Tucker, K.L. Validation of Nutrient Intake Estimates Derived Using a Semi-Quantitative FFQ against 3 Day Diet Records in the Baltimore Longitudinal Study of Aging. J. Nutr. Health Aging 2015, 19, 994–1002. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef]
- Searle, S.D.; Mitnitski, A.; Gahbauer, E.A.; Gill, T.M.; Rockwood, K. A standard procedure for creating a frailty index. BMC Geriatr. 2008, 8, 24. [Google Scholar] [CrossRef]
- Ware, J., Jr.; Kosinski, M.; Keller, S.D. A 12-Item Short-Form Health Survey: Construction of scales and preliminary tests of reliability and validity. Med. Care 1996, 34, 220–233. [Google Scholar] [CrossRef]
- Irwin, M.; Artin, K.H.; Oxman, M.N. Screening for depression in the older adult: Criterion validity of the 10-item Center for Epidemiological Studies Depression Scale (CES-D). Arch. Intern. Med. 1999, 159, 1701–1704. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Bartali, B.; Frongillo, E.A.; Stipanuk, M.H.; Bandinelli, S.; Salvini, S.; Palli, D.; Morais, J.A.; Volpato, S.; Guralnik, J.M.; Ferrucci, L. Protein intake and muscle strength in older persons: Does inflammation matter? J. Am. Geriatr. Soc. 2012, 60, 480–484. [Google Scholar] [CrossRef]
- Bartali, B.; Frongillo, E.A.; Bandinelli, S.; Lauretani, F.; Semba, R.D.; Fried, L.P.; Ferrucci, L. Low nutrient intake is an essential component of frailty in older persons. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 589–593. [Google Scholar] [CrossRef]
- Beasley, J.M.; LaCroix, A.Z.; Neuhouser, M.L.; Huang, Y.; Tinker, L.; Woods, N.; Michael, Y.; Curb, J.D.; Prentice, R.L. Protein intake and incident frailty in the Women’s Health Initiative observational study. J. Am. Geriatr. Soc. 2010, 58, 1063–1071. [Google Scholar] [CrossRef]
- Coelho-Junior, H.J.; Calvani, R.; Picca, A.; Tosato, M.; Landi, F.; Marzetti, E. Protein Intake and Frailty in Older Adults: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients 2022, 14, 2767. [Google Scholar] [CrossRef]
- Kaimoto, K.; Yamashita, M.; Suzuki, T.; Makizako, H.; Koriyama, C.; Kubozono, T.; Takenaka, T.; Ohishi, M.; Kanouchi, H.; The Tarumizu Study Diet, G. Association of Protein and Magnesium Intake with Prevalence of Prefrailty and Frailty in Community-Dwelling Older Japanese Women. J. Nutr. Sci. Vitaminol. 2021, 67, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Shikany, J.M.; Barrett-Connor, E.; Ensrud, K.E.; Cawthon, P.M.; Lewis, C.E.; Dam, T.T.; Shannon, J.; Redden, D.T.; Osteoporotic Fractures in Men Research Group. Macronutrients, diet quality, and frailty in older men. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Berner, L.A.; Becker, G.; Wise, M.; Doi, J. Characterization of dietary protein among older adults in the United States: Amount, animal sources, and meal patterns. J. Acad. Nutr. Diet. 2013, 113, 809–815. [Google Scholar] [CrossRef]
- Keller, B.O.; Wu, B.T.; Li, S.S.; Monga, V.; Innis, S.M. Hypaphorine is present in human milk in association with consumption of legumes. J. Agric. Food Chem. 2013, 61, 7654–7660. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Talegawkar, S.A.; Jin, Y.; Candia, J.; Tian, Q.; Moaddel, R.; Simonsick, E.M.; Ferrucci, L. Metabolomic Profile of Different Dietary Patterns and Their Association with Frailty Index in Community-Dwelling Older Men and Women. Nutrients 2022, 14, 2237. [Google Scholar] [CrossRef]
- Playdon, M.C.; Moore, S.C.; Derkach, A.; Reedy, J.; Subar, A.F.; Sampson, J.N.; Albanes, D.; Gu, F.; Kontto, J.; Lassale, C.; et al. Identifying biomarkers of dietary patterns by using metabolomics. Am. J. Clin. Nutr. 2017, 105, 450–465. [Google Scholar] [CrossRef]
- Kim, H.; Appel, L.J.; Lichtenstein, A.H.; Wong, K.E.; Chatterjee, N.; Rhee, E.P.; Rebholz, C.M. Metabolomic Profiles Associated With Blood Pressure Reduction in Response to the DASH and DASH-Sodium Dietary Interventions. Hypertension 2023, 80, 1494–1506. [Google Scholar] [CrossRef]
- Kim, H.; Lichtenstein, A.H.; White, K.; Wong, K.E.; Miller, E.R., 3rd; Coresh, J.; Appel, L.J.; Rebholz, C.M. Plasma Metabolites Associated with a Protein-Rich Dietary Pattern: Results from the OmniHeart Trial. Mol. Nutr. Food Res. 2022, 66, e2100890. [Google Scholar] [CrossRef]
- Sun, H.; Cai, W.; Wang, X.; Liu, Y.; Hou, B.; Zhu, X.; Qiu, L. Vaccaria hypaphorine alleviates lipopolysaccharide-induced inflammation via inactivation of NFkappaB and ERK pathways in Raw 264.7 cells. BMC Complement. Altern. Med. 2017, 17, 120. [Google Scholar] [CrossRef]
- Ding, Y.H.; Miao, R.X.; Zhang, Q. Hypaphorine exerts anti-inflammatory effects in sepsis induced acute lung injury via modulating DUSP1/p38/JNK pathway. Kaohsiung J. Med. Sci. 2021, 37, 883–893. [Google Scholar] [CrossRef]
- Bhave, V.M.; Ament, Z.; Patki, A.; Gao, Y.; Kijpaisalratana, N.; Guo, B.; Chaudhary, N.S.; Guarniz, A.G.; Gerszten, R.; Correa, A.; et al. Plasma Metabolites Link Dietary Patterns to Stroke Risk. Ann. Neurol. 2023, 93, 500–510. [Google Scholar] [CrossRef]
- Peron, G.; Merono, T.; Gargari, G.; Hidalgo-Liberona, N.; Minarro, A.; Lozano, E.V.; Castellano-Escuder, P.; Gonzalez-Dominguez, R.; Del Bo, C.; Bernardi, S.; et al. A Polyphenol-Rich Diet Increases the Gut Microbiota Metabolite Indole 3-Propionic Acid in Older Adults with Preserved Kidney Function. Mol. Nutr. Food Res. 2022, 66, e2100349. [Google Scholar] [CrossRef]
- Karbownik, M.; Stasiak, M.; Zygmunt, A.; Zasada, K.; Lewinski, A. Protective effects of melatonin and indole-3-propionic acid against lipid peroxidation, caused by potassium bromate in the rat kidney. Cell Biochem. Funct. 2006, 24, 483–489. [Google Scholar] [CrossRef]
- Bendheim, P.E.; Poeggeler, B.; Neria, E.; Ziv, V.; Pappolla, M.A.; Chain, D.G. Development of indole-3-propionic acid (OXIGON) for Alzheimer’s disease. J. Mol. Neurosci. 2002, 19, 213–217. [Google Scholar] [CrossRef]
- Aoki, R.; Aoki-Yoshida, A.; Suzuki, C.; Takayama, Y. Indole-3-Pyruvic Acid, an Aryl Hydrocarbon Receptor Activator, Suppresses Experimental Colitis in Mice. J. Immunol. 2018, 201, 3683–3693. [Google Scholar] [CrossRef]
- Xu, X.; Sun, S.; Liang, L.; Lou, C.; He, Q.; Ran, M.; Zhang, L.; Zhang, J.; Yan, C.; Yuan, H.; et al. Role of the Aryl Hydrocarbon Receptor and Gut Microbiota-Derived Metabolites Indole-3-Acetic Acid in Sulforaphane Alleviates Hepatic Steatosis in Mice. Front Nutr. 2021, 8, 756565. [Google Scholar] [CrossRef]
- de Mello, V.D.; Paananen, J.; Lindstrom, J.; Lankinen, M.A.; Shi, L.; Kuusisto, J.; Pihlajamaki, J.; Auriola, S.; Lehtonen, M.; Rolandsson, O.; et al. Indolepropionic acid and novel lipid metabolites are associated with a lower risk of type 2 diabetes in the Finnish Diabetes Prevention Study. Sci. Rep. 2017, 7, 46337. [Google Scholar] [CrossRef]
- Cason, C.A.; Dolan, K.T.; Sharma, G.; Tao, M.; Kulkarni, R.; Helenowski, I.B.; Doane, B.M.; Avram, M.J.; McDermott, M.M.; Chang, E.B.; et al. Plasma microbiome-modulated indole- and phenyl-derived metabolites associate with advanced atherosclerosis and postoperative outcomes. J. Vasc. Surg. 2018, 68, 1552–1562.e7. [Google Scholar] [CrossRef]
- Rebholz, C.M.; Lichtenstein, A.H.; Zheng, Z.; Appel, L.J.; Coresh, J. Serum untargeted metabolomic profile of the Dietary Approaches to Stop Hypertension (DASH) dietary pattern. Am. J. Clin. Nutr. 2018, 108, 243–255. [Google Scholar] [CrossRef]
- Ramirez-Velez, R.; Martinez-Velilla, N.; Correa-Rodriguez, M.; Saez de Asteasu, M.L.; Zambom-Ferraresi, F.; Palomino-Echeverria, S.; Garcia-Hermoso, A.; Izquierdo, M. Lipidomic signatures from physically frail and robust older adults at hospital admission. Geroscience 2022, 44, 1677–1688. [Google Scholar] [CrossRef]
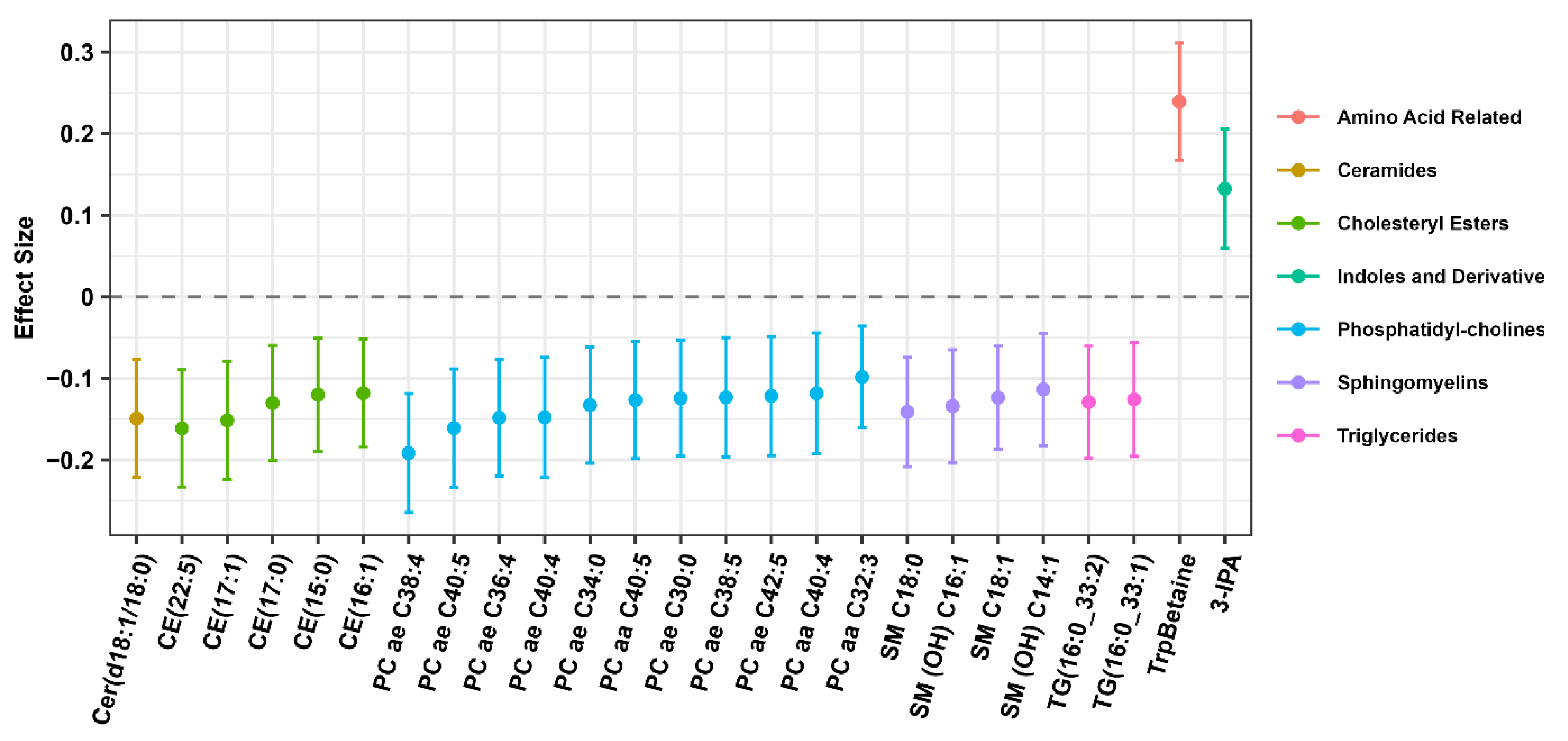
| Quartiles of %Animal Protein Intake (Median Value) | Quartiles of %Plant Protein Intake (Median Value) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Q1 (8%) | Q2 (11%) | Q3 (13%) | Q4 (16%) | p | Q1 (4%) | Q2 (5%) | Q3 (6%) | Q4 (7%) | p | |
| n | 184 | 184 | 184 | 183 | 184 | 184 | 184 | 183 | ||
| Age (y) | 78.1 (7.40) | 77.4 (7.34) | 76.3 (7.14) | 76.5 (7.83) | 0.07 | 76.9 (7.82) | 76.7 (7.27) | 77.0 (7.17) | 77.5 (7.56) | 0.76 |
| Sex (% male) | 0.51 (0.50) | 0.47 (0.50) | 0.53 (0.50) | 0.40 (0.49) | 0.09 | 0.53 (0.50) | 0.48 (0.50) | 0.43 (0.50) | 0.47 (0.50) | 0.31 |
| Self-reported Race | 0.08 | <0.001 | ||||||||
| White | 128 (69.6) | 137 (74.5) | 131 (71.2) | 134 (73.2) | 137 (74.5) | 136 (73.9) | 141 (76.6) | 116 (63.4) | ||
| Black | 40 (21.7) | 34 (18.5) | 47 (25.5) | 44 (24.0) | 44 (23.9) | 43 (23.4) | 37 (20.1) | 41 (22.4) | ||
| Other | 16 (8.7) | 13 (7.1) | 6 (3.3) | 5 (2.7) | 3 (1.6) | 5 (2.7) | 6 (3.3) | 26 (14.2) | ||
| Smoking | 0.74 | 0.01 | ||||||||
| Current (within 3 years) | 4 (2.2) | 4 (2.2) | 6 (3.3) | 4 (2.2) | 7 (3.8) | 7 (3.8) | 2 (1.1) | 2 (1.1) | ||
| Former | 78 (42.4) | 69 (37.5) | 81 (44.0) | 83 (45.4) | 93 (50.5) | 80 (43.5) | 71 (38.6) | 67 (36.6) | ||
| Non-smoker | 102 (55.4) | 111 (60.3) | 97 (52.7) | 96 (52.5) | 84 (45.7) | 97 (52.7) | 111 (60.3) | 114 (62.3) | ||
| BMI (kg/m2) | 26.7 (4.6) | 26.2 (4.1) | 28.1 (5.1) | 28.0 (4.9) | <0.001 | 28.0 (5.3) | 28.2 (4.7) | 26.8 (4.5) | 25.9 (4.1) | <0.001 |
| Year of education | 16.9 (2.6) | 17.1 (2.9) | 16.9 (2.6) | 16.9 (2.5) | 0.85 | 17.0 (2.5) | 16.9 (2.7) | 16.9 (2.6) | 17.0 (2.8) | 0.98 |
| Mediterranean diet score | 4.49 (1.62) | 4.51 (1.58) | 3.97 (1.72) | 3.68 (1.61) | <0.001 | 3.30 (1.45) | 3.85 (1.60) | 4.33 (1.55) | 5.17 (1.49) | <0.001 |
| % Energy from carbohydrates | 51.4 (8.5) | 45.4 (6.4) | 44.1 (6.48) | 39.8 (7.1) | <0.001 | 40.5 (9.3) | 43.7 (6.8) | 47.1 (7.0) | 49.6 (6.8) | <0.001 |
| % Energy from fat | 32.0 (7.1) | 35.3 (5.3) | 36.4 (5.90) | 37.4 (6.4) | <0.001 | 36.9 (7.3) | 36.6 (6.4) | 34.8 (5.6) | 32.7 (5.8) | <0.001 |
| % Energy from protein | 14.4 (2.1) | 16.7 (1.48) | 18.2 (1.2) | 21.6 (2.8) | <0.001 | 17.6 (4.1) | 18.0 (3.4) | 17.5 (2.7) | 17.8 (2.7) | 0.57 |
| % Energy from animal protein | 7.97 (1.59) | 10.8 (0.6) | 12.9 (0.7) | 16.7 (2.8) | <0.001 | 13.6 (4.0) | 12.9 (3.4) | 11.7 (2.8) | 10.1 (3.0) | <0.001 |
| % Energy from plant protein | 6.45 (2.03) | 5.84 (1.39) | 5.28 (1.04) | 4.93 (1.10) | <0.001 | 3.99 (0.59) | 5.03 (0.19) | 5.86 (0.29) | 7.60 (1.47) | <0.001 |
| Total energy (kcal/d) | 1971 (846) | 1945 (648) | 1921 (780) | 1694 (679) | <0.001 | 1943 (698) | 1905 (776) | 1836 (756) | 1849 (765) | 0.48 |
| Alcohol intake (g/d) | 14.9 (34.1) | 12.8 (18.2) | 8.21 (13.4) | 6.05 (9.34) | <0.001 | 18.8 (33.5) | 9.71 (17.6) | 7.42 (11.9) | 6.02 (11.8) | <0.001 |
| Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|
| β | SE | p | β | SE | p | |
| Animal protein intake | ||||||
| Per % increase | −0.001 | 0.001 | 0.327 | −0.001 | 0.001 | 0.336 |
| Q1 | ref | ref | ||||
| Q2 | −0.008 | 0.007 | 0.216 | −0.007 | 0.007 | 0.275 |
| Q3 | −0.006 | 0.007 | 0.398 | −0.006 | 0.007 | 0.409 |
| Q4 | −0.008 | 0.007 | 0.252 | −0.008 | 0.007 | 0.272 |
| Plant protein intake | ||||||
| Per % increase | −0.008 | 0.002 | 0.001 | −0.006 | 0.003 | 0.034 |
| Q1 | ref | ref | ||||
| Q2 | −0.004 | 0.007 | 0.576 | −0.002 | 0.007 | 0.812 |
| Q3 | −0.013 | 0.007 | 0.053 | −0.010 | 0.007 | 0.180 |
| Q4 | −0.023 | 0.008 | 0.003 | −0.016 | 0.008 | 0.053 |
| Average Direct Effect | Average Causal Mediated Effect | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Metabolite | β | LCI | UCI | p | β | LCI | UCI | p | % Mediation |
| 3-IPA | −0.0068 | −0.0108 | −0.0018 | 0.004 | −0.001 | −0.0021 | −0.0002 | <0.001 | 12.89 |
| CE(15:0) | −0.0087 | −0.0137 | −0.0044 | <0.001 | 0.0009 | 0.0003 | 0.0018 | <0.001 | 11.85 |
| CE(17:0) | −0.0087 | −0.0136 | −0.0039 | <0.001 | 0.001 | 0.0002 | 0.0019 | 0.004 | 12.72 |
| CE(17:1) | −0.0089 | −0.0133 | −0.0042 | <0.001 | 0.0011 | 0.0003 | 0.002 | <0.001 | 14.36 |
| CE(22:5) | −0.0090 | −0.0137 | −0.004 | <0.001 | 0.0012 | 0.0004 | 0.0022 | 0.004 | 15.8 |
| PC aa C32:3 | −0.0084 | −0.0126 | −0.0037 | <0.001 | 0.0007 | 0.0001 | 0.0015 | 0.036 | 8.54 |
| PC ae C30:0 | −0.0085 | −0.0132 | −0.0039 | <0.001 | 0.0008 | 0.0002 | 0.0015 | <0.001 | 9.77 |
| PC ae C36:4 | −0.0086 | −0.0133 | −0.0042 | <0.001 | 0.0009 | 0.0002 | 0.0018 | 0.02 | 10.99 |
| PC ae C38:5 | −0.0084 | −0.0126 | −0.004 | <0.001 | 0.0007 | 0.0001 | 0.0016 | 0.044 | 8.45 |
| PC ae C40:5 | −0.0087 | −0.0128 | −0.0043 | <0.001 | 0.001 | 0.0001 | 0.0021 | 0.008 | 12.41 |
| PC ae C42:5 | −0.0084 | −0.0126 | −0.004 | <0.001 | 0.0006 | 0.0001 | 0.0014 | 0.016 | 8.35 |
| SM (OH) C14:1 | −0.0088 | −0.0134 | −0.0044 | <0.001 | 0.0011 | 0.0003 | 0.002 | <0.001 | 13.85 |
| SM (OH) C16:1 | −0.0089 | −0.0138 | −0.0045 | <0.001 | 0.0012 | 0.0004 | 0.0023 | <0.001 | 15.27 |
| SM C18:0 | −0.0090 | −0.0138 | −0.0044 | <0.001 | 0.0013 | 0.0005 | 0.0024 | <0.001 | 16.61 |
| SM C18:1 | −0.0089 | −0.0134 | −0.0046 | <0.001 | 0.0012 | 0.0004 | 0.0022 | 0.004 | 15.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, T.; Das, J.K.; Jin, Y.; Tian, Q.; Moaddel, R.; Moore, A.Z.; Tucker, K.L.; Talegawkar, S.A.; Ferrucci, L. Plant Protein but Not Animal Protein Consumption Is Associated with Frailty through Plasma Metabolites. Nutrients 2023, 15, 4193. https://doi.org/10.3390/nu15194193
Tanaka T, Das JK, Jin Y, Tian Q, Moaddel R, Moore AZ, Tucker KL, Talegawkar SA, Ferrucci L. Plant Protein but Not Animal Protein Consumption Is Associated with Frailty through Plasma Metabolites. Nutrients. 2023; 15(19):4193. https://doi.org/10.3390/nu15194193
Chicago/Turabian StyleTanaka, Toshiko, Jayanta K. Das, Yichen Jin, Qu Tian, Ruin Moaddel, Ann Zenobia Moore, Katherine L. Tucker, Sameera A. Talegawkar, and Luigi Ferrucci. 2023. "Plant Protein but Not Animal Protein Consumption Is Associated with Frailty through Plasma Metabolites" Nutrients 15, no. 19: 4193. https://doi.org/10.3390/nu15194193
APA StyleTanaka, T., Das, J. K., Jin, Y., Tian, Q., Moaddel, R., Moore, A. Z., Tucker, K. L., Talegawkar, S. A., & Ferrucci, L. (2023). Plant Protein but Not Animal Protein Consumption Is Associated with Frailty through Plasma Metabolites. Nutrients, 15(19), 4193. https://doi.org/10.3390/nu15194193






