Use of Fermented Red Clover Isoflavones in the Treatment of Overactive Bladder in Postmenopausal Women: A Randomized, Double-Blinded, Placebo-Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
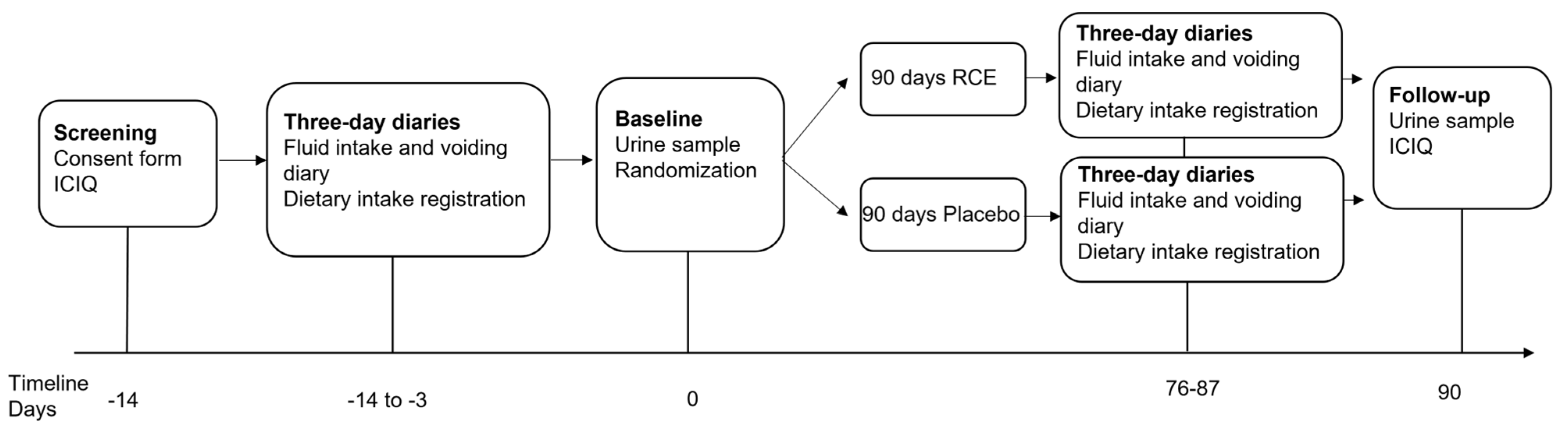
2.1. Study Design
Randomization and Double Blinding
2.2. Study Population
2.2.1. Sample Size
2.2.2. Participants
2.3. Questionnaires and Data Acquisition
2.3.1. Demographic and Clinical Data
2.3.2. ICIQ-OAB Questionnaire
2.3.3. ICIQ-UI-SF Questionnaire
2.3.4. Fluid Intake and Voiding Diary
2.3.5. Diet Intake Registration
2.4. Standard Urine Culture
2.5. Red Clover Extract and Placebo Formulations
2.6. Compliance and Adverse Events
2.7. Statistical Analysis
2.8. Ethics
3. Results
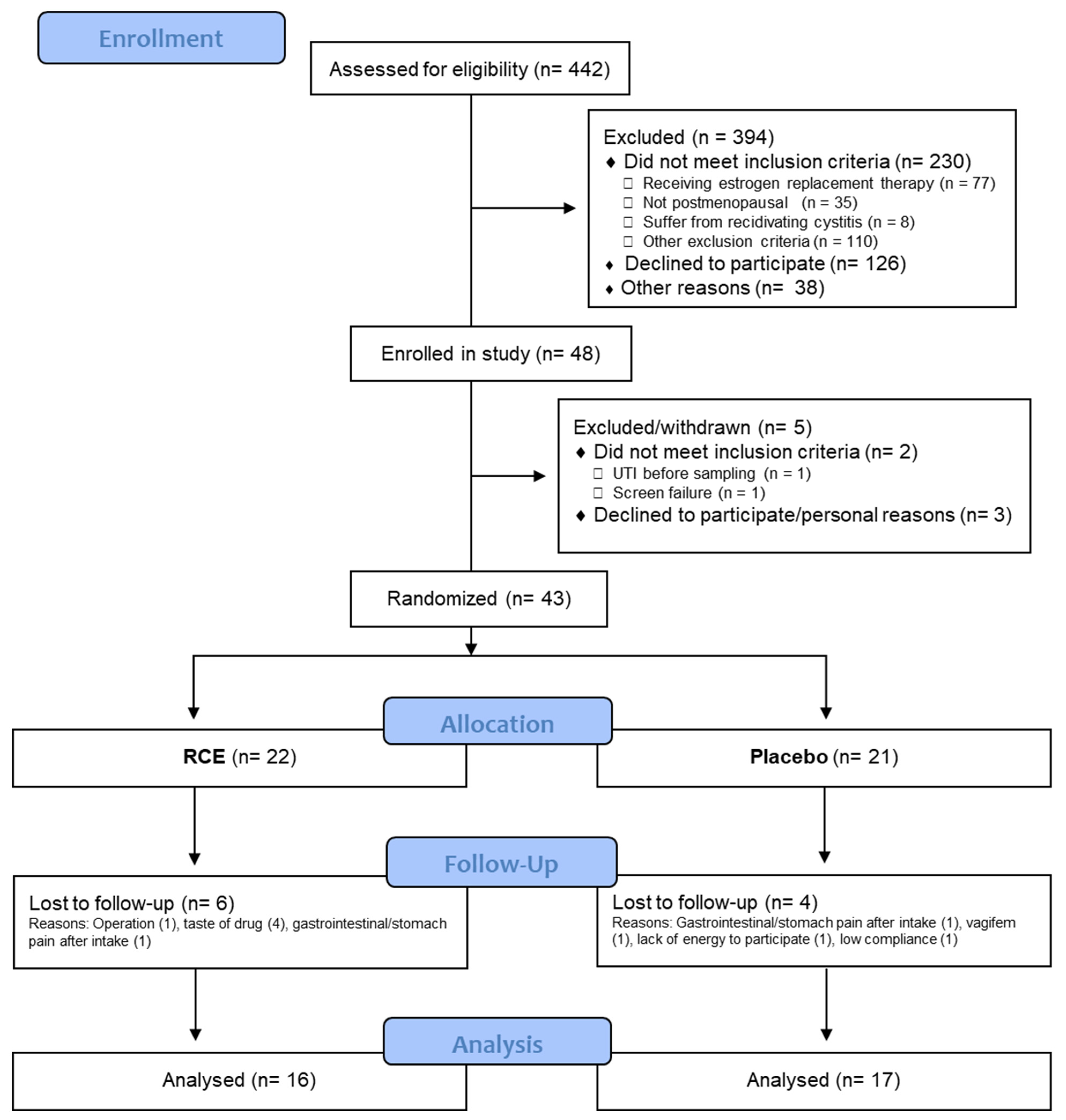
3.1. Study Participants
3.2. Baseline Characteristics
3.3. ICIQ-OAB Score
3.4. Individual ICIQ-OAB Items
3.5. Patient-Reported Symptom Bother (NRS Values) for Urinary Frequency, Nocturia, Urgency, and Urge Urinary Incontinence from the ICIQ-OAB Questionnaire
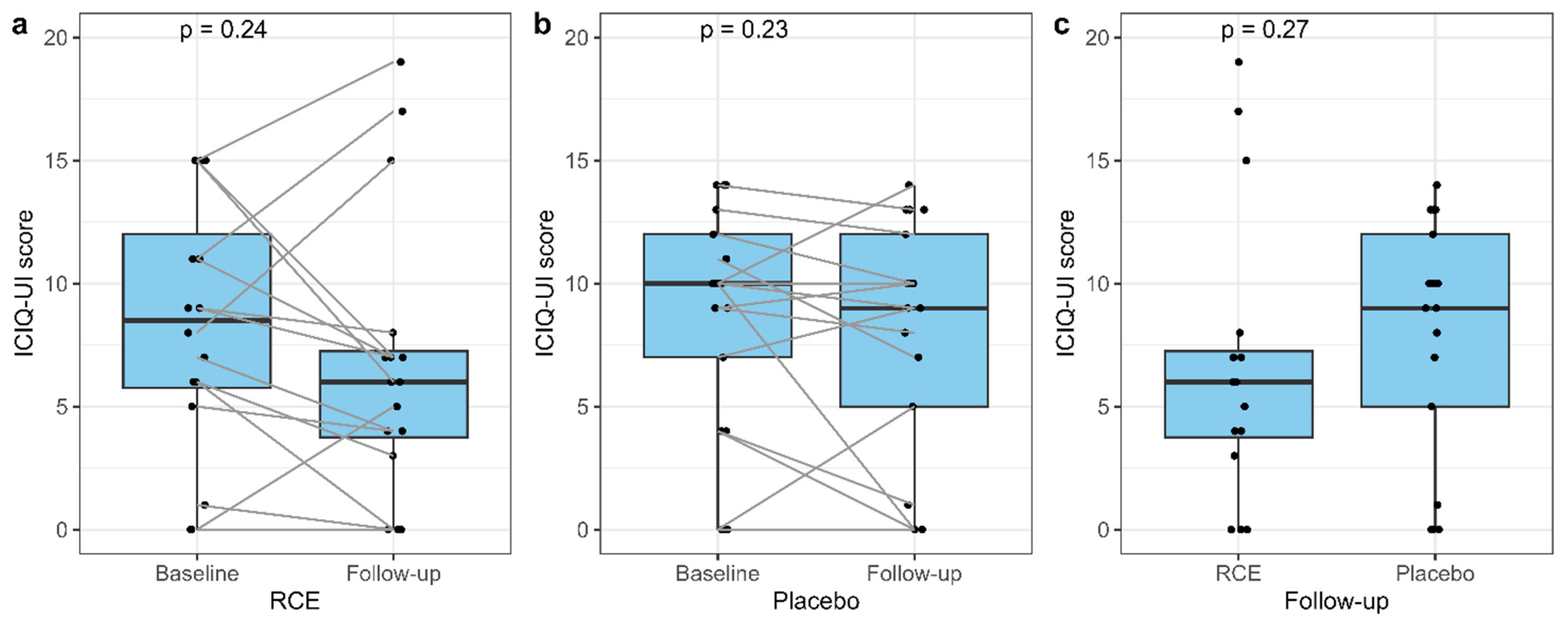
3.6. The ICIQ-UI Score
3.7. Fluid Intake and Voiding Diary
3.8. Compliance and Adverse Events
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coyne, K.S.; Sexton, C.C.; Irwin, D.E.; Kopp, Z.S.; Kelleher, C.J.; Milsom, I. The Impact of Overactive Bladder, Incontinence and Other Lower Urinary Tract Symptoms on Quality of Life, Work Productivity, Sexuality and Emotional Well-Being in Men and Women: Results from the EPIC Study. BJU Int. 2008, 101, 1388–1395. [Google Scholar] [CrossRef]
- Haylen, B.T.; de Ridder, D.; Freeman, R.M.; Swift, S.E.; Berghmans, B.; Lee, J.; Monga, A.; Petri, E.; Rizk, D.E.; Sand, P.K.; et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) Joint Report on the Terminology for Female Pelvic Floor Dysfunction. Neurourol. Urodyn. 2010, 29, 4–20. [Google Scholar] [CrossRef]
- Abrams, P.; Cardozo, L.; Fall, M.; Griffiths, D.; Rosier, P.; Ulmsten, U.; Van Kerrebroeck, P.; Victor, A.; Wein, A. The Standardisation of Terminology of Lower Urinary Tract Function: Report from the Standardisation Sub-Committee of the International Continence Society. Neurourol. Urodyn. 2002, 21, 167–178. [Google Scholar] [CrossRef]
- Irwin, D.E.; Milsom, I.; Hunskaar, S.; Reilly, K.; Kopp, Z.; Herschorn, S.; Coyne, K.; Kelleher, C.; Hampel, C.; Artibani, W.; et al. Population-Based Survey of Urinary Incontinence, Overactive Bladder, and Other Lower Urinary Tract Symptoms in Five Countries: Results of the EPIC Study. Eur. Urol. 2006, 50, 1306–1314, discussion 1314–1315. [Google Scholar] [CrossRef]
- Milsom, I.; Abrams, P.; Cardozo, L.; Roberts, R.G.; Thüroff, J.; Wein, A.J. How Widespread Are the Symptoms of an Overactive Bladder and How Are They Managed? A Population-Based Prevalence Study. BJU Int. 2001, 87, 760–766. [Google Scholar] [CrossRef]
- Stewart, W.F.; Van Rooyen, J.B.; Cundiff, G.W.; Abrams, P.; Herzog, A.R.; Corey, R.; Hunt, T.L.; Wein, A.J. Prevalence and Burden of Overactive Bladder in the United States. World J. Urol. 2003, 20, 327–336. [Google Scholar] [CrossRef]
- Robinson, D.; Cardozo, L.; Milsom, I.; Pons, M.E.; Kirby, M.; Koelbl, H.; Vierhout, M. Oestrogens and Overactive Bladder. Neurourol. Urodyn. 2014, 33, 1086–1091. [Google Scholar] [CrossRef]
- Cardozo, L.; Lose, G.; McClish, D.; Versi, E. A Systematic Review of the Effects of Estrogens for Symptoms Suggestive of Overactive Bladder. Acta Obstet. Gynecol. Scand. 2004, 83, 892–897. [Google Scholar] [CrossRef]
- Hanna-Mitchell, A.T.; Robinson, D.; Cardozo, L.; Everaert, K.; Petkov, G. V Do We Need to Know More About the Effects of Hormones on Lower Urinary Tract Dysfunction? ICI-RS 2014 HHS Public Access. Neurourol. Urodyn. 2016, 35, 299–303. [Google Scholar] [CrossRef]
- Eriksen, P.S.; Rasmussen, H. Low-Dose 17β-Estradiol Vaginal Tablets in the Treatment of Atrophic Vaginitis: A Double-Blind Placebo Controlled Study. Eur. J. Obstet. Gynecol. Reprod. Biol. 1992, 44, 137–144. [Google Scholar] [CrossRef]
- Cardozo, L.D.; Wise, B.G.; Benness, C.J. Vaginal Oestradiol for the Treatment of Lower Urinary Tract Symptoms in Postmenopausal Women—A Double-Blind Placebo-Controlled Study. J. Obstet. Gynaecol. 2001, 21, 383–385. [Google Scholar] [CrossRef]
- Šimunić, V.; Banović, I.; Ciglar, S.; Jeren, L.; Pavičić Baldani, D.; Šprem, M. Local Estrogen Treatment in Patients with Urogenital Symptoms. Int. J. Gynecol. Obstet. 2003, 82, 187–197. [Google Scholar] [CrossRef]
- Long, C.Y.; Liu, C.M.; Hsu, S.C.; Chen, Y.H.; Wu, C.H.; Tsai, E.M. A Randomized Comparative Study of the Effects of Oral and Topical Estrogen Therapy on the Lower Urinary Tract of Hysterectomized Postmenopausal Women. Fertil. Steril. 2006, 85, 155–160. [Google Scholar] [CrossRef]
- Nelken, R.S.; Özel, B.Z.; Leegant, A.R.; Felix, J.C.; Mishell, D.R. Randomized Trial of Estradiol Vaginal Ring versus Oral Oxybutynin for the Treatment of Overactive Bladder. Menopause 2011, 18, 962–966. [Google Scholar] [CrossRef]
- Cardozo, L.; Rekers, H.; Tapp, A.; Barnick, C.; Shepherd, A.; Schussler, B.; Kerr-Wilson, R.; van Geelan, J.; Barlebo, H.; Walter, S. Oestriol in the Treatment of Postmenopausal Urgency: A Multicentre Study. Maturitas 1993, 18, 47–53. [Google Scholar] [CrossRef]
- Grady, D.; Brown, J.S.; Vittinghoff, E.; Applegate, W.; Varner, E.; Snyder, T.; HERS Research Group. Postmenopausal Hormones and Incontinence: The Heart and Estrogen/Progestin Replacement Study. Obstet. Gynecol. 2001, 97, 116–120. [Google Scholar] [CrossRef]
- Hendrix, S.L.; Cochrane, B.B.; Nygaard, I.E.; Handa, V.L.; Barnabei, V.M.; Iglesia, C.; Aragaki, A.; Naughton, M.J.; Wallace, R.B.; McNeeley, S.G. Effects of Estrogen with and without Progestin on Urinary Incontinence. Jama 2005, 293, 935–948. [Google Scholar] [CrossRef]
- Steinauer, J.E.; Waetjen, L.E.; Vittinghoff, E.; Subak, L.L.; Hulley, S.B.; Grady, D.; Lin, F.; Brown, J.S. Postmenopausal Hormone Therapy: Does It Cause Incontinence? Obstet. Gynecol. 2005, 106, 940–945. [Google Scholar] [CrossRef]
- Cody, J.D.; Jacobs, M.L.; Richardson, K.; Moehrer, B.; Hextall, A. Oestrogen Therapy for Urinary Incontinence in Post-menopausal Women. Cochrane Database Syst. Rev. 2012, 2012, CD001405. [Google Scholar] [CrossRef]
- Beck, V.; Rohr, U.; Jungbauer, A. Phytoestrogens Derived from Red Clover: An Alternative to Estrogen Replacement Therapy? J. Steroid Biochem. Mol. Biol. 2005, 94, 499–518. [Google Scholar] [CrossRef]
- Okabe, Y.; Shimazu, T.; Tanimoto, H. Higher Bioavailability of Isoflavones after a Single Ingestion of Aglycone-Rich Fermented Soybeans Compared with Glucoside-Rich Non-Fermented Soybeans in Japanese Postmenopausal Women. J. Sci. Food Agric. 2011, 91, 658–663. [Google Scholar] [CrossRef]
- Vitale, D.C.; Piazza, C.; Melilli, B.; Drago, F.; Salomone, S. Isoflavones: Estrogenic Activity, Biological Effect and Bioavailability. Eur. J. Drug Metab. Pharmacokinet. 2013, 38, 15–25. [Google Scholar] [CrossRef]
- Poluzzi, E.; Piccinni, C.; Raschi, E.; Rampa, A.; Recanatini, M.; De Ponti, F. Phytoestrogens in Postmenopause: The State of the Art from a Chemical, Pharmacological and Regulatory Perspective. Curr. Med. Chem. 2014, 21, 417–436. [Google Scholar] [CrossRef]
- Manas, E.S.; Xu, Z.B.; Unwalla, R.J.; Somers, W.S.; Drive, C.P. Understanding the Selectivity of Genistein for Human Estrogen Receptor-β Using X-Ray Crystallography and Computational Methods. Structure 2004, 12, 2197–2207. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, H.; Yao, J.L.; Chaux, A.; Zheng, Y.; Hsu, I.; Izumi, K.; Chang, C.; Messing, E.M.; Netto, G.J.; Yeh, S. Expression of Androgen and Oestrogen Receptors and Its Prognostic Significance in Urothelial Neoplasm of the Urinary Bladder. BJU Int. 2012, 109, 1716–1726. [Google Scholar] [CrossRef] [PubMed]
- Kashiwagi, E.; Fujita, K.; Yamaguchi, S.; Fushimi, H.; Ide, H.; Inoue, S.; Mizushima, T.; Reis, L.O.; Sharma, R.; Netto, G.J.; et al. Expression of Steroid Hormone Receptors and Its Prognostic Significance in Urothelial Carcinoma of the Upper Urinary Tract. Cancer Biol. Ther. 2016, 17, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Miyamoto, H. The Role of Estrogen Receptors in Urothelial Cancer. Front. Endocrinol. 2021, 12, 643870. [Google Scholar] [CrossRef]
- Tuygun, C.; Kankaya, D.; Imamoglu, A.; Sertcelik, A.; Zengin, K.; Oktay, M.; Sertcelik, N. Sex-Specific Hormone Receptors in Urothelial Carcinomas of the Human Urinary Bladder: A Comparative Analysis of Clinicopathological Features and Survival Outcomes According to Receptor Expression. Urol. Oncol. Semin. Orig. Investig. 2011, 29, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Kontos, S.; Kominea, A.; Melachrinou, M.; Balampani, E.; Sotiropoulou-Bonikou, G. Inverse Expression of Estrogen Receptor-Beta and Nuclear Factor-KappaB in Urinary Bladder Carcinogenesis. Int. J. Urol. 2010, 17, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Teng, J.; Wang, Z.-Y.; Jarrard, D.F.; Bjorling, D.E. Roles of Estrogen Receptor and in Modulating Urothelial Cell Proliferation. Endocr. Relat. Cancer 2008, 15, 351–364. [Google Scholar] [CrossRef]
- Blakeman, P.J.; Hilton, P.; Bulmer, J.N. Oestrogen and Progesterone Receptor Expression in the Female Lower Urinary Tract, with Reference to Oestrogen Status. BJU Int. 2000, 86, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Iosif, C.S.; Batra, S.; Ek, A.; Astedt, B. Estrogen Receptors in the Human Female Lower Uninary Tract. Am. J. Obstet. Gynecol. 1981, 141, 817–820. [Google Scholar] [CrossRef] [PubMed]
- Saez, S.; Martin, P.M. Evidence of Estrogen Receptors in the Trigone Area of Human Urinary Bladder. J. Steroid Biochem. 1981, 15, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Batra, S.C.; Iosif, C.S. Female Urethra: A Target for Estrogen Action. J. Urol. 1983, 129, 418–420. [Google Scholar] [CrossRef]
- Wolf, H.; Wandt, H.; Jonat, W. Immunohistochemical Evidence of Estrogen and Progesterone Receptors in the Female Lower Urinary Tract and Comparison with the Vagina. Gynecol. Obstet. Investig. 1991, 32, 227–231. [Google Scholar] [CrossRef]
- Taylor, A.H.; Al-Azzawi, F. Immunolocalisation of Oestrogen Receptor Beta in Human Tissues. J. Mol. Endocrinol. 2000, 24, 145–155. [Google Scholar] [CrossRef]
- Kanadys, W.; Barańska, A.; Błaszczuk, A.; Polz-Dacewicz, M.; Drop, B.; Kanecki, K.; Malm, M. Evaluation of Clinical Meaningfulness of Red Clover (Trifolium pratense L.) Extract to Relieve Hot Flushes and Menopausal Symptoms in Peri-and Post-Menopausal Women: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2021, 13, 1258. [Google Scholar] [CrossRef]
- Lambert, M.N.T.; Thorup, A.C.; Hansen, E.S.S.; Jeppesen, P.B. Combined Red Clover Isoflavones and Probiotics Potently Reduce Menopausal Vasomotor Symptoms. PLoS ONE 2017, 12, e0176590. [Google Scholar] [CrossRef]
- Chen, M.N.; Lin, C.C.; Liu, C.F. Efficacy of Phytoestrogens for Menopausal Symptoms: A Meta-Analysis and Systematic Review. Climacteric 2015, 18, 260–269. [Google Scholar] [CrossRef]
- Ghazanfarpour, M.; Sadeghi, R.; Roudsari, R.L.; Khorsand, I.; Khadivzadeh, T.; Muoio, B. Red Clover for Treatment of Hot Flashes and Menopausal Symptoms: A Systematic Review and Meta-Analysis. J. Obstet. Gynaecol. 2016, 36, 301–311. [Google Scholar] [CrossRef]
- Lambert, M.N.T.; Thybo, C.B.; Lykkeboe, S.; Rasmussen, L.M.; Frette, X.; Christensen, L.P.; Jeppesen, P.B. Combined Bioavailable Isoflavones and Probiotics Improve Bone Status and Estrogen Metabolism in Postmenopausal Osteopenic Women: A Randomized Controlled Trial. Am. J. Clin. Nutr. 2017, 106, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Lambert, M.N.T.; Hu, L.M.; Jeppesen, P.B. A Systematic Review and Meta-Analysis of the Effects of Isoflavone Formulations against Estrogen-Deficient Bone Resorption in Peri- and Postmenopausal Women. Am. J. Clin. Nutr. 2017, 106, ajcn151464. [Google Scholar] [CrossRef] [PubMed]
- Panel, E.; Additives, F.; Sources, N. Risk Assessment for Peri- and Post-Menopausal Women Taking Food Supplements Containing Isolated Isoflavones. EFSA J. 2015, 13, 4246. [Google Scholar] [CrossRef]
- Cardenas-Trowers, O.; Meyer, I.; Markland, A.D.; Richter, H.E.; Addis, I. A Review of Phytoestrogens and Their Association with Pelvic Floor Conditions. Female Pelvic Med. Reconstr. Surg. 2018, 24, 193–202. [Google Scholar] [CrossRef]
- Gratzke, C.; Jarajapu, Y.P.R.; Christ, G.J.; Kaplan, J.R.; Williams, J.K.; Andersson, K.-E.; Badlani, G. Effects of Long-Term Dietary Soy Treatment on Female Urethral Morphology and Function in Ovariectomized Nonhuman Primates. J. Urol. 2008, 180, 2247–2253. [Google Scholar] [CrossRef]
- Owen, S.J.; Rose’meyer, R.B.; Massa, H.M. Dietary Phytoestrogens Maintain Contractile Responses to Carbachol with Age in the Female Rat Isolated Bladder. Life Sci. 2011, 89, 213–220. [Google Scholar] [CrossRef]
- Turgut, A.; Goruk, N.Y.; Sak, M.E.; Deveci, E.; Akdemir, F.; Keles, A.N.; Nergiz, Y.; Gul, T. Effects of Genistein, Estrogen and Progesterone Therapies on Bladder Morphology and M2, M3 Receptor Expressions in Oophorectomized Rats. Acta Medica Mediterr. 2014, 30, 907–916. [Google Scholar]
- Kreydin, E.I.; Kim, M.M.; Barrisford, G.W.; Rodriguez, D.; Sanchez, A.; Santiago-Lastra, Y.; Ko, D.S.C. Urinary Lignans Are Associated with Decreased Incontinence in Postmenopausal Women. Urology 2015, 86, 716–720. [Google Scholar] [CrossRef]
- Juliato, C.R.T.; Baccaro, L.F.; Pedro, A.O.; Gabiatti, J.R.E.; Lui-Filho, J.F.; Costa-Paiva, L. Factors Associated with Urinary Incontinence in Middle-Aged Women: A Population-Based Household Survey. Int. Urogynecol. J. 2017, 28, 423–429. [Google Scholar] [CrossRef]
- Waetjen, L.E.; Leung, K.; Crawford, S.L.; Huang, M.-H.; Gold, E.B.; Greendale, G.A.; Study of Women’s Health Across the Nation. Relationship between Dietary Phytoestrogens and Development of Urinary Incontinence in Midlife Women. Menopause 2013, 20, 428–436. [Google Scholar] [CrossRef][Green Version]
- Manonai, J.; Songchitsomboon, S.; Chanda, K.; Hong, J.H.; Komindr, S. The Effect of a Soy-Rich Diet on Urogenital Atrophy: A Randomized, Cross-over Trial. Maturitas 2006, 54, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Marañón, J.A. Clinical Study: Effect of Supplementation with High Genistein Soybean Isoflavones and Pumpkin Standardized Extract on Urinary Incontinence in Western Perimenopausal Women. J. Gynecol. Womens Health 2017, 4, 8–11. [Google Scholar] [CrossRef][Green Version]
- Shim, B.; Jeong, H.; Lee, S.; Hwang, S.; Moon, B.; Storni, C. A Randomized Double-Blind Placebo-Controlled Clinical Trial of a Product Containing Pumpkin Seed Extract and Soy Germ Extract to Improve Overactive Bladder-Related Voiding Dysfunction and Quality of Life. J. Funct. Foods 2014, 8, 111–117. [Google Scholar] [CrossRef]
- Nishimura, M.; Ohkawara, T.; Sato, H.; Takeda, H.; Nishihira, J. Pumpkin Seed Oil Extracted From Cucurbita Maxima Improves Urinary Disorder in Human Overactive Bladder. J. Tradit. Complement. Med. 2014, 4, 72–74. [Google Scholar] [CrossRef]
- Jackson, S.; Donovan, J.; Brookes, S.; Eckford, S.; Swithinbank, L.; Abrams, P. The Bristol Female Lower Urinary Tract Symptoms Questionnaire: Development and Psychometric Testing. Br. J. Urol. 1996, 77, 805–812. [Google Scholar] [CrossRef]
- Avery, K.; Donovan, J.; Peters, T.J.; Shaw, C.; Gotoh, M.; Abrams, P. ICIQ: A Brief and Robust Measure for Evaluating the Symptoms and Impact of Urinary Incontinence. Neurourol. Urodyn. 2004, 23, 322–330. [Google Scholar] [CrossRef]
- Clausen, J.; Gimbel, H.; Arenholt, L.T.S.; Løwenstein, E. Validity and Reliability of Two Danish Versions of the ICIQ-UI SF. Int. Urogynecol. J. 2021, 32, 3223–3233. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research Electronic Data Capture (REDCap)—A Metadata-Driven Methodology and Workflow Process for Providing Translational Research Informatics Support. J. Biomed. Inform. 2009, 42, 377. [Google Scholar] [CrossRef]
- Petros, P.E.P. A Comprehensive Review of Overactive Bladder Pathophysiology: On the Way to Tailored Treatment. Eur. Urol. 2020, 77, 134–135. [Google Scholar] [CrossRef]
- Alcántara Montero, A. Long-Term Safety and Efficacy of Mirabegron and Solifenacin in Combination Compared with Monotherapy in Patients with Overactive Bladder: SYNERGY II Study. Actas Urol. Esp. 2019, 43, 51–52. [Google Scholar] [CrossRef]
- Liao, L.; Zhou, Z.; Chen, G.; Xu, Z.; Huang, B.; Chong, T.; Chen, Q.; Wei, Z.; Shen, B.; Chen, Z.; et al. Sacral Neuromodulation Using a Novel Device with a Six–Contact-Point Electrode for the Treatment of Patients with Refractory Overactive Bladder: A Multicenter, Randomized, Single-Blind, Parallel-Control Clinical Trial. Eur. Urol. Focus 2022, 8, 1823–1830. [Google Scholar] [CrossRef] [PubMed]
- Sonmez, R.; Yildiz, N.; Alkan, H. Efficacy of Percutaneous and Transcutaneous Tibial Nerve Stimulation in Women with Idiopathic Overactive Bladder: A Prospective Randomised Controlled Trial. Ann. Phys. Rehabil. Med. 2022, 65, 101486. [Google Scholar] [CrossRef]
- Falcão Padilha, J.; Arias Avila, M.; Driusso, P. Parasacral versus Tibial Transcutaneous Electrical Stimulation to Treat Urinary Urgency in Adult Women: Randomized Controlled Clinical Trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 267, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Nambiar, A.K.; Arlandis, S.; Bø, K.; Cobussen-Boekhorst, H.; Costantini, E.; de Heide, M.; Farag, F.; Groen, J.; Karavitakis, M.; Lapitan, M.C.; et al. European Association of Urology Guidelines on the Diagnosis and Management of Female Non-Neurogenic Lower Urinary Tract Symptoms. Part 1: Diagnostics, Overactive Bladder, Stress Urinary Incontinence, and Mixed Urinary Incontinence. Eur. Urol. 2022, 82, 49–59. [Google Scholar] [CrossRef]
- Staskin, D.; Frankel, J.; Varano, S.; Shortino, D.; Jankowich, R.; Mudd, P.N. International Phase III, Randomized, Double-Blind, Placebo and Active Controlled Study to Evaluate the Safety and Efficacy of Vibegron in Patients with Symptoms of Overactive Bladder: EMPOWUR. J. Urol. 2020, 204, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Mueller, E.R.; van Maanen, R.; Chapple, C.; Abrams, P.; Herschorn, S.; Robinson, D.; Stoelzel, M.; Yoon, S.J.; Al-Shukri, S.; Rechberger, T.; et al. Long-Term Treatment of Older Patients with Overactive Bladder Using a Combination of Mirabegron and Solifenacin: A Prespecified Analysis from the Randomized, Phase III SYNERGY II Study. Neurourol. Urodyn. 2019, 38, 779–792. [Google Scholar] [CrossRef]
- Carmignani, L.O.; Pedro, A.O.; Montemor, E.B.; Arias, V.A.; Costa-Paiva, L.H.; Pinto-Neto, A.M. Effects of a Soy-Based Dietary Supplement Compared with Low-Dose Hormone Therapy on the Urogenital System: A Randomized, Double-Blind, Controlled Clinical Trial. Menopause 2015, 22, 741–749. [Google Scholar] [CrossRef]
- Ribeiro, A.E.; Monteiro, N.E.S.; De Moraes, A.V.G.; Costa-Paiva, L.H.; Pedro, A.O. Can the Use of Probiotics in Association with Isoflavone Improve the Symptoms of Genitourinary Syndrome of Menopause? Results from a Randomized Controlled Trial. Menopause 2019, 26, 643–652. [Google Scholar] [CrossRef]
- Bernardo, C.; Santos, J.; Costa, C.; Tavares, A.; Amaro, T.; Marques, I.; Gouveia, M.J.; Félix, V.; Afreixo, V.; Brindley, P.J.; et al. Estrogen Receptors in Urogenital Schistosomiasis and Bladder Cancer: Estrogen Receptor Alpha-Mediated Cell Proliferation. Urol. Oncol. Semin. Orig. Investig. 2020, 38, 738.e23–738.e35. [Google Scholar] [CrossRef]
- Shen, S.S.; Smith, C.L.; Hsieh, J.-T.; Yu, J.; Kim, I.Y.; Jian, W.; Sonpavde, G.; Ayala, G.E.; Younes, M.; Lerner, S.P. Expression of Estrogen Receptors-Alpha and -Beta in Bladder Cancer Cell Lines and Human Bladder Tumor Tissue. Cancer 2006, 106, 2610–2616. [Google Scholar] [CrossRef]
- An, J.; Tzagarakis-Foster, C.; Scharschmidt, T.C.; Lomri, N.; Leitman, D.C. Estrogen Receptor Î2-Selective Transcriptional Activity and Recruitment of Coregulators by Phytoestrogens*. J. Biol. Chem. 2001, 276, 17808–17814. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.D.; Oliver, R.H.; Leung, B.S.; Lin, L.Y.; Yeh, J. Estrogen Receptor Alpha and Beta Expression in the Vaginal Walls and Uterosacral Ligaments of Premenopausal and Postmenopausal Women. Fertil. Steril. 1999, 71, 1099–1102. [Google Scholar] [CrossRef] [PubMed]
- Lima, S.M.R.R.; Yamada, S.S.; Reis, B.F.; Postigo, S.; Galvão da Silva, M.A.L.; Aoki, T. Effective Treatment of Vaginal Atrophy with Isoflavone Vaginal Gel. Maturitas 2013, 74, 252–258. [Google Scholar] [CrossRef] [PubMed]


| Parameters | Total (n = 33) | RCE (n = 16) | Placebo (n = 17) | p |
|---|---|---|---|---|
| Age (years), mean ± SD | 62.8 ± 6.2 | 62.9 ± 6.1 | 62.8 ± 6.5 | 0.938 a |
| Body mass index (kg/m2) *, median (QR1–QR3) | 25.0 (22.0–30.9) | 25.6 (20.7–31.0) | 24.4 (22.7–28.6) | 0.909 b |
Smoking status, n (%)
| 15 (45.5) 11 (33.3) 7 (21.2) | 8 (50.0) 5 (31.2) 3 (18.8) | 7 (41.2) 6 (35.3) 4 (23.5) | 0.874 c |
Number of cigarettes/day, mean ± SD
| 12.9 ± 6.8 6.0 ± 1.7 | 12.6 ± 8.1 6.3 ± 1.2 | 13.2 ± 6.3 5.8 ± 2.2 | 0.902 a 0.672 a |
| Number of births, median (QR1–QR3) | 2.0 (2.0–2.0) | 2.0 (2.0–2.25) | 2.0 (2.0–2.0) | 0.704 c |
| Number of cesareans ◊, median (QR1–QR3) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0.570 c |
OAB type, n (%)
| 18 (54.5) 15 (45.5) | 7 (43.8) 9 (56.2) | 11 (64.7) 6 (35.3) | 0.391 c |
Urinary incontinence type, n (%)
| 4 (12.1) 14 (42.4) 5 (15.1) 10 (30.3) | 2 (12.5) 5 (31.2) 3 (18.8) 6 (37.5) | 2 (11.8) 9 (52.9) 2 (11.8) 4 (23.5) | 0.684 d |
| ICIQ-UI score (0–21), median (QR1–QR3) | 9.0 (6.0–12.0) | 8.5 (5.8–12.0) | 10.0 (7.0–12.0) | 0.800 b |
| ICIQ-OAB score (0–16), median (QR1–QR3) | 7.0 (6.0–8.0) | 6.5 (5.0–8.25) | 7.0 (6.0–8.0) | 0.728 b |
| Selected diseases ⁰, self-reported, n (%) Urogenital
| 1 (3.1) 1 (3.1) 1 (3.1) 2 (6.3) | 1 (6.25) 1 (6.25) 1 (6.25) 1 (6.25) | 0 (0.0) 0 (0.0) 0 (0.0) 1 (6.25) | 1 1 1 1 |
| OAB NRS Values | RCE (n = 16) | Placebo (n = 17) | Difference between Groups | ||||
|---|---|---|---|---|---|---|---|
| Baseline | Follow-Up | p | Baseline | Follow-Up | p | p a | |
| Urinary frequency bother (0–10) | 5.0 (1.5–6.3) | 1.0 (0.0–8.3) | 0.263 | 5.0 (4.0–6.0) | 5.0 (3.0–6.0) | 0.245 | 0.19 |
| Nocturia bother (0–10) | 3.0 (0.8–7.0) | 2.0 (0.0–4.3) | 0.248 | 5.0 (1.0–6.0) | 5.0 (2.0–6.0) | 0.633 | 0.11 |
| Urgency bother (0–10) | 7.0 (4.0–8.3) | 4.5 (0.8–8.0) | 0.033 | 7.0 (5.0–7.0) | 5.0 (4.0–7.0) | 0.138 | 0.44 |
| Urge urinary incontinence bother (0–10) | 4.5 (0.0–7.5) | 3.5 (0.0–8.0) | 0.501 | 6.0 (3.0–8.0) | 5.0 (1.0–7.0) | 0.403 | 0.7 |
| Fluid Intake and Voiding Diary | RCE (n = 15 *) | Placebo (n = 17) | Difference between Groups | ||||
|---|---|---|---|---|---|---|---|
| Baseline | Follow-Up | p | Baseline | Follow-Up | p | p a | |
| Fluid intake 24-h median (QR1–QR3) | 1897 (1498–2022) | 1750 (1438–2087) | 0.359 | 1883 (1617–2033) | 1513 (1417–1967) | 0.145 | 0.929 |
| Fluid intake night median (QR1–QR3) | 0 (0–62.5) | 33.3 (0–100) | 0.824 | 0 (0–0) | 0 (0–0) | 0.834 | 0.106 |
| 24-h urine volume mean ± SD | 1768 ± 502 | 1820 ±449 | 0.738 | 1780 ± 503 | 1684 ± 465 | 0.268 | 0.408 |
| Nocturnal urine volume median (QR1–QR3) | 410 (274–612) | 400 (282–715) | 0.890 | 450 (335–550) | 425 (400–630) | 0.636 | 0.734 |
| Average voided volume mean ± SD | 198 ± 71.2 | 217 ± 75.7 | 0.072 | 206 ± 62.7 | 221 ± 53.6 | 0.237 | 0.875 |
| 24-h frequency mean ± SD median (QR1–QR3) | 9.33 ± 1.98 8.67 (8.17–11.0) | 8.93 ± 2.59 8.67 (7.5–11.3) | 0.422 | 8.94 ± 2.23 8.33 (7.67–9.33) | 7.90 ± 2.43 7 (6.33–8.33) | 0.012 | 0.108 |
| Nocturnal frequency median (QR1–QR3) | 1.67 (0.67–2.33) | 1 (0.67–1.33) | 0.349 | 0.67 (0.33–1.33) | 1 (0.33–1.33) | 0.861 | 0.659 |
| Daytime urinary frequency mean ± SD median (QR1–QR3) | 7.89 ± 1.88 7.67 (6.83–9.17) | 7.69 ± 2.05 7.67 (6.83–9.17) | 0.578 | 7.98 ± 1.68 7.67 (7–9.33) | 6.98 ± 2.14 6 (5.67–7.67) | 0.009 | 0.173 |
| 24-h incontinence episodes ** median (QR1–QR3) | 0.33 (0.0–1.33) | 0.0 (0.0–0.67) | 0.612 | 0.67 (0.0–2.0) | 0.0 (0.0–1.0) | 0.089 | 0.627 |
| Incontinence episodes night ** mean ± SD median (QR1–QR3) | 0.17 ± 0.64 0 (0–0) | 0.10 ± 0.21 0 (0–0) | 0.854 | 0.15 ± 0.42 0 (0–0) | 0.09 0 (0–0) | 0.391 | 0.229 |
| 24-h max volume median (QR1–QR3) | 500 (300–585) | 460 (315–575) | 0.72 | 460 (350–500) | 500 (350–600) | 0.051 | 0.471 |
| Daytime max volume median (QR1–QR3) | 440 (285–500) | 450 (300–550) | 0.345 | 460 (300–500) | 400 (320–500) | 0.955 | 0.995 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villadsen, A.B.; Holm-Jacobsen, J.N.; Prabhala, B.K.; Bundgaard-Nielsen, C.; Huntjens, P.; Kornum, J.B.; Glavind, K.; Leutscher, P.D.C.; Christensen, L.P.; Jeppesen, P.B.; et al. Use of Fermented Red Clover Isoflavones in the Treatment of Overactive Bladder in Postmenopausal Women: A Randomized, Double-Blinded, Placebo-Controlled Trial. Nutrients 2023, 15, 4165. https://doi.org/10.3390/nu15194165
Villadsen AB, Holm-Jacobsen JN, Prabhala BK, Bundgaard-Nielsen C, Huntjens P, Kornum JB, Glavind K, Leutscher PDC, Christensen LP, Jeppesen PB, et al. Use of Fermented Red Clover Isoflavones in the Treatment of Overactive Bladder in Postmenopausal Women: A Randomized, Double-Blinded, Placebo-Controlled Trial. Nutrients. 2023; 15(19):4165. https://doi.org/10.3390/nu15194165
Chicago/Turabian StyleVilladsen, Annemarie B., Julie N. Holm-Jacobsen, Bala K. Prabhala, Caspar Bundgaard-Nielsen, Pam Huntjens, Jette B. Kornum, Karin Glavind, Peter D. C. Leutscher, Lars P. Christensen, Per B. Jeppesen, and et al. 2023. "Use of Fermented Red Clover Isoflavones in the Treatment of Overactive Bladder in Postmenopausal Women: A Randomized, Double-Blinded, Placebo-Controlled Trial" Nutrients 15, no. 19: 4165. https://doi.org/10.3390/nu15194165
APA StyleVilladsen, A. B., Holm-Jacobsen, J. N., Prabhala, B. K., Bundgaard-Nielsen, C., Huntjens, P., Kornum, J. B., Glavind, K., Leutscher, P. D. C., Christensen, L. P., Jeppesen, P. B., Sørensen, S., & Arenholt, L. T. S. (2023). Use of Fermented Red Clover Isoflavones in the Treatment of Overactive Bladder in Postmenopausal Women: A Randomized, Double-Blinded, Placebo-Controlled Trial. Nutrients, 15(19), 4165. https://doi.org/10.3390/nu15194165






