Role of Dietary Fibre in Managing Periodontal Diseases—A Systematic Review and Meta-Analysis of Human Intervention Studies
Abstract
1. Introduction
2. Methodology
2.1. Inclusion and Exclusion Criteria
- Population (P): Studies with participants having the following periodontal diseases according to the American Academy of Periodontology (AAP) 1999 and 2017 classifications were included: gingivitis (dental biofilm-induced), necrotizing periodontal disease, periodontitis as a manifestation of systemic diseases and periodontitis. Studies where participants reported any habits such as smoking, tobacco chewing, use of the smokeless or chewing form of tobacco, areca nut or supari were excluded. Studies with subjects undergoing chemotherapy, or radiation therapy and pregnant or lactating females were also excluded.
- Intervention (I)/Exposure (E): The intervention was dietary fibre (roughage or bulk), defined as parts of plant-derived food that the gut cannot absorb. Studies with any dose or type of dietary fibre in any form (part of the diet or supplementary as liquid or capsule) administered via the oral cavity were included. Studies where dietary fibre was used as an adjunct to non-surgical treatment (scaling and root planning ([SRP])) were also considered.
- Comparator/Control (C): Studies where the comparator was as follows: SRP alone, SRP + low fibre, low fibre alone, SRP + other soluble fibre, soluble fibre alone, or no fibre.
- Outcomes (O): The primary outcomes were changes in clinical Probing Depth/pocket Probing Depth (PD) and Clinical Attachment Level/loss (CAL). Other clinical parameters, Bleeding on Probing (BOP), Gingival Index (GI), sulcus bleeding index, Plaque Index (PI), gingival recession, Periodontal Inflamed Surface Area index (PISA), oral microbial outcomes or inflammatory biomarkers and metabolites in blood, Gingival Crevicular Fluid (GCF), saliva or oral tissues, were included as secondary outcomes. If the studies reported any other parameters, these were also included.
- Types of study: Randomised controlled trials (RCTs), quasi-randomised trials, and pre-clinical trials (e.g., cross-over studies, sequential feeding trials ([SFT]), parallel studies) evaluating the efficacy of fibre on periodontal diseases for any study duration were included following the PICO strategy. Observational studies (e.g., cohort, case-control, and cross-sectional studies), in-vitro models, case reports, case series, letters to the editor, reviews, unpublished data, and secondary literature on the effect of dietary fibre and periodontal diseases were excluded.
2.2. Information Sources and Search Strategies
2.3. Study Selection
2.4. Data Extraction
- (1)
- General study details (first author, country, year of publication);
- (2)
- Study design and intervention period;
- (3)
- Diagnostic criteria defining the periodontal diseases in the given study;
- (4)
- Participant characteristics (health status of the participants, sample size of intervention vs. control groups, male and female distribution of intervention vs. control groups, age range or mean age of intervention vs. control groups);
- (5)
- Characteristics of intervention and comparator (type of fibre, form of intake [as part of the diet or oral supplement], dosage [grams per day]);
- (6)
- Evaluated outcomes:
- Primary outcomes: PD (mm/tooth), CAL (mm/tooth)
- Secondary outcomes
- Other metabolic parameters;
- (7)
- Effect of oral fibre intake compared to the comparison group (p values were presented);
- (8)
- Comparisons of outcome measures.
2.5. Risk of Bias and Methodological Quality Assessment
- (1)
- Was the study referred to as being random?
- (2)
- Was the study referred to as being double-blind/single-blind?
- (3)
- Was there a description of dropouts and withdrawals?
- (4)
- Was the paper’s described randomization method appropriate or not?
- (5)
- Was the described and appropriate blinding technique used?
2.6. Quantitative Data Synthesis
3. Results
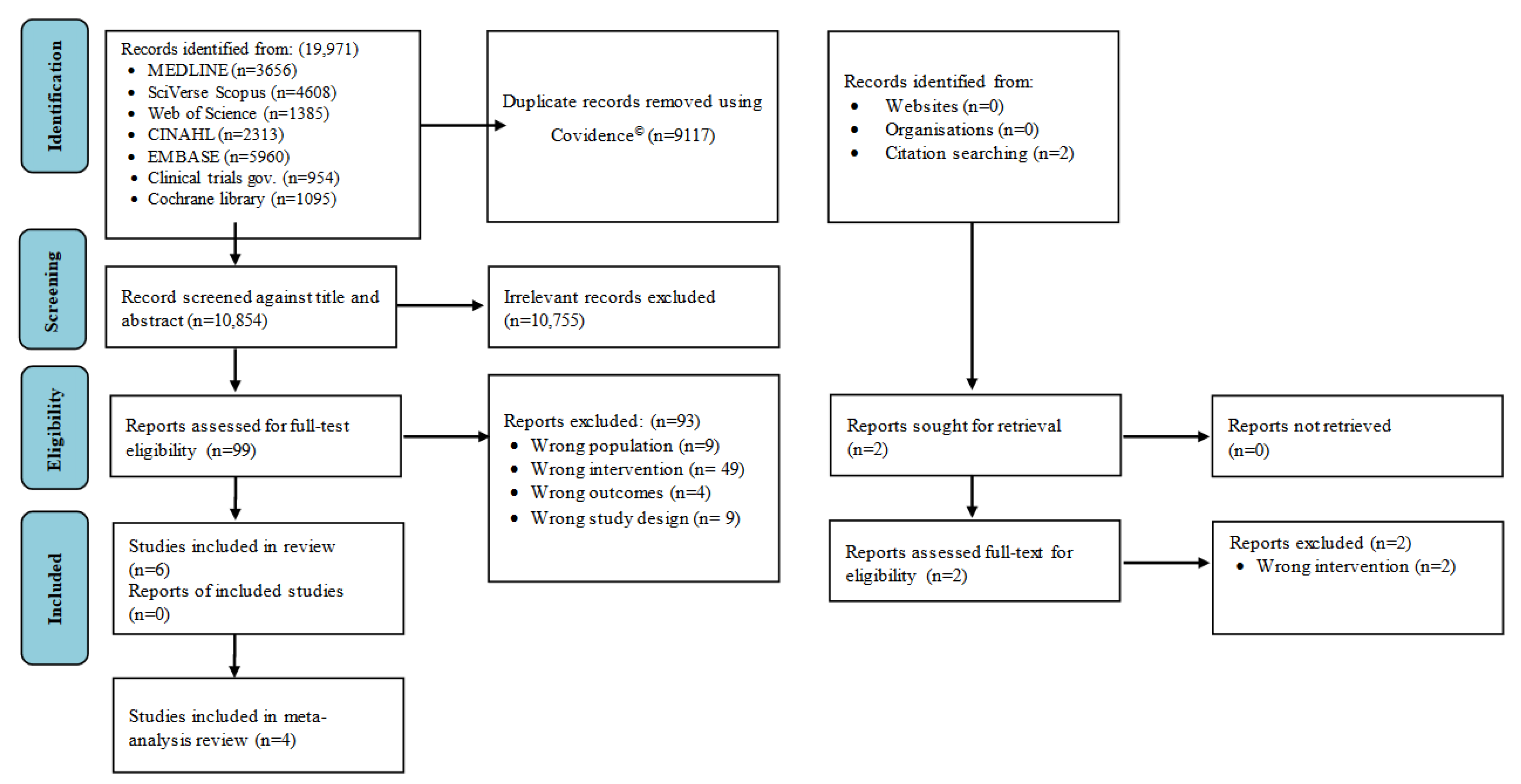
3.1. Results of the Search
3.2. Study Characteristics
3.3. Intervention Characteristics
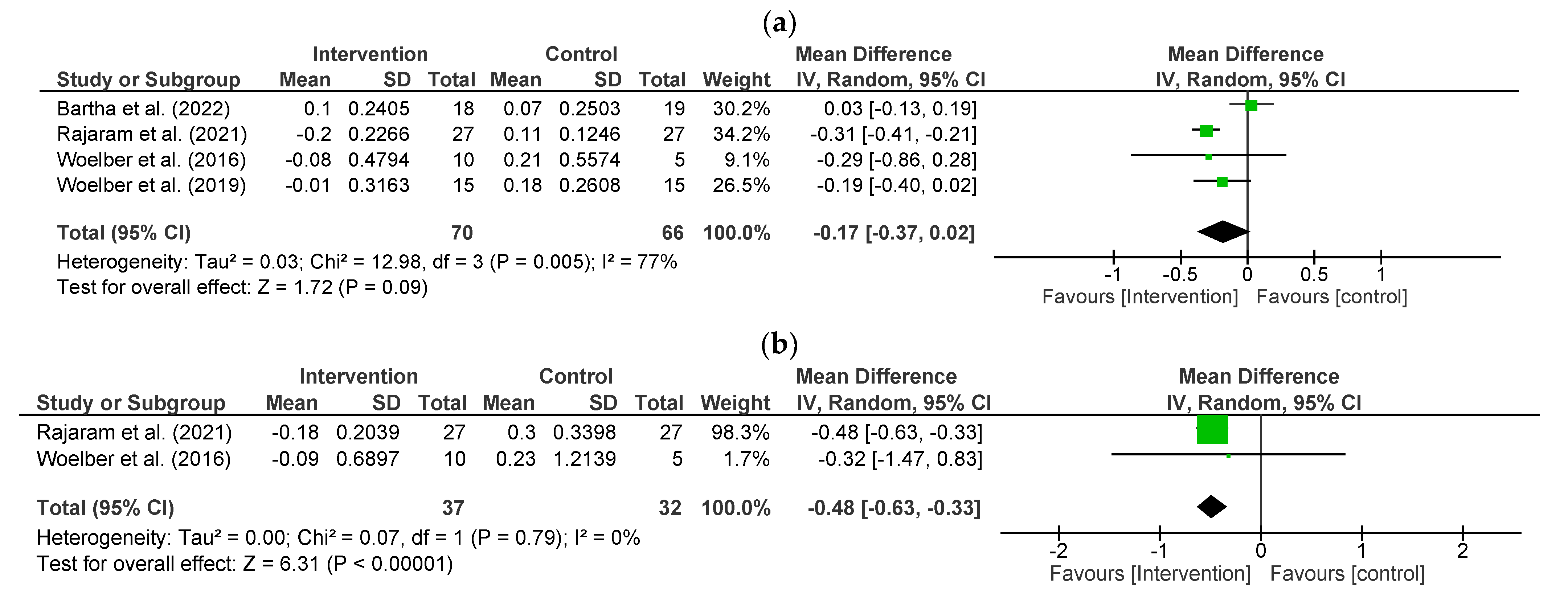
3.4. Effect of Fibre on Periodontal Status-Primary Outcomes
Probing Depth (PD)
3.5. Clinical Attachment Loss/Level (CAL)
3.6. Effect of Fibre on Periodontal Status- Secondary Outcomes
Bleeding on Probing (BOP)
3.7. Volume of Gingival Crevicular Fluid (GCF)
3.8. Periodontal Inflamed Surface Area (PISA)
3.9. Plaque Index (PI)
3.10. Gingival Index (GI)
3.11. Oral Microbiota Outcomes
3.12. Impact of Fibre on Inflammatory Markers and Metabolic Parameters
3.13. Degree of Compliance to Dietary Recommendation of Fibre Intake
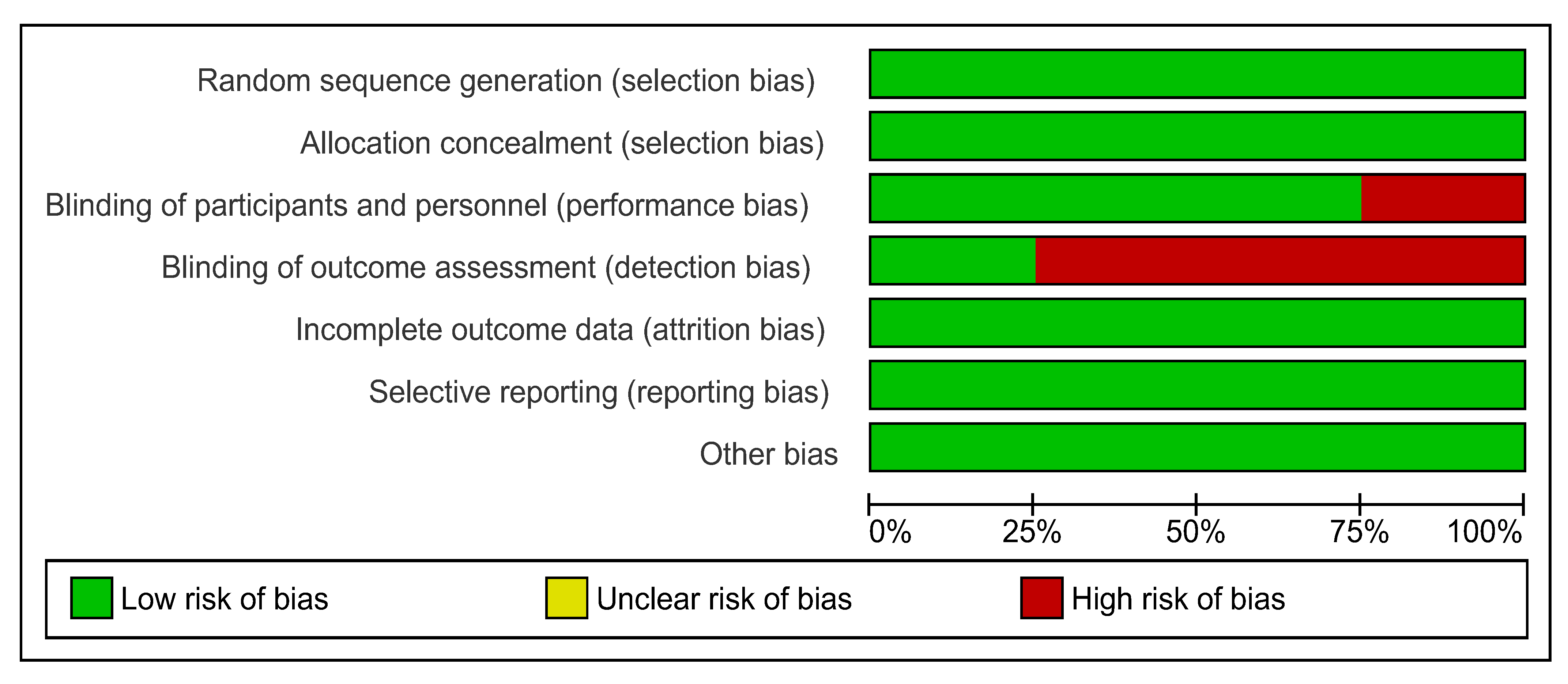
3.14. Methodological Quality and Risk of Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Pihlstrom, B.L.; Michalowicz, B.S.; Johnson, N.W. Periodontal diseases. Lancet 2005, 366, 1809–1820. [Google Scholar] [CrossRef]
- Chapple, I.; Wilson, N. Chronic non-communicable diseases. Br. Dent. J. 2014, 216, 487. [Google Scholar] [CrossRef][Green Version]
- Oral Health. Available online: https://www.who.int/news-room/fact-sheets/detail/oral-health (accessed on 23 March 2023).
- Cote, S.E.; Singh, H. Dental Diseases and Disorders. In Immigrant Medicine: Text with CD-ROM; Elsevier: Amsterdam, The Netherlands, 2007; pp. 597–610. [Google Scholar] [CrossRef]
- Theilade, E. Factors Controlling the Microflora of the Healthy Mouth. In Human Microbial Ecology; Taylor & Francis, Milton Park, Abingdon, Oxfordshire, OX14 4RN, United Kingdom; 2020; pp. 1–56. [CrossRef]
- Saini, R.; Marawar, P.P.; Shete, S.; Saini, S. Periodontitis, A True Infection. J. Glob. Infect. Dis. 2009, 1, 149. [Google Scholar] [CrossRef] [PubMed]
- Genco, R.J.; Borgnakke, W.S. Risk factors for periodontal disease. Periodontol 2013, 62, 59–94. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Bhatia, S.; Sodhi, A.S.; Batra, N. Oral microbiome and health. AIMS Microbiol. 2018, 4, 42–66. [Google Scholar] [CrossRef] [PubMed]
- Sukumar, S.; Martin, F.E.; Hughes, T.E.; Adler, C.J. Think before you prescribe: How dentistry contributes to antibiotic resistance. Aust. Dent. J. 2019, 65, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Zohaib, S.; Almas, K. The Role of Nutrition in Periodontal Health: An Update. Nutrients 2016, 8, 530. [Google Scholar] [CrossRef]
- O’Connor, J.L.; Milledge, K.L.; O’Leary, F.; Cumming, R.; Eberhard, J.; Hirani, V. Poor dietary intake of nutrients and food groups are associated with increased risk of periodontal disease among community-dwelling older adults: A systematic literature review. Nutr. Rev. 2020, 78, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S.; Mealey, B.L.; Mariotti, A.; Chapple, I.L.C. Dental plaque-induced gingival conditions. J. Periodontol. 2018, 89 (Suppl. 1), S17–S27. [Google Scholar] [CrossRef] [PubMed]
- Cronin, P.; Joyce, S.A.; O’Toole, P.W.; O’Connor, E.M. Dietary Fibre Modulates the Gut Microbiota. Nutrients 2021, 13, 1655. [Google Scholar] [CrossRef]
- van Dam, R.M.; Rimm, E.B.; Willett, W.C.; Stampfer, M.J.; Hu, F.B. Dietary Patterns and Risk for Type 2 Diabetes Mellitus in U.S. Men. Ann. Intern. Med. 2002, 136, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Lutsey, P.L.; Steffen, L.M.; Stevens, J. Dietary intake and the development of the metabolic syndrome: The Atherosclerosis Risk in Communities study. Circulation 2008, 117, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Kendall, C.W.; Esfahani, A.; Jenkins, D.J. The link between dietary fibre and human health. Food Hydrocoll. 2010, 24, 42–48. [Google Scholar] [CrossRef]
- Nutrient Reference Values for Australia and New Zealand Including Recommended Dietary Intakes | NHMRC. Available online: https://www.nhmrc.gov.au/about-us/publications/nutrient-reference-values-australia-and-new-zealand-including-recommended-dietary-intakes (accessed on 27 February 2023).
- Slavin, J. Fiber and Prebiotics: Mechanisms and Health Benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef]
- Farmer, B.; Larson, B.T.; Fulgoni, V.L., III; Rainville, A.J.; Liepa, G.U. A vegetarian dietary pattern as a nutrient-dense approach to weight management: An analysis of the national health and nutrition examination survey 1999–2004. J. Am. Diet. Assoc. 2011, 111, 819–827. [Google Scholar] [CrossRef]
- Shay, C.M.; Van Horn, L.; Stamler, J.; Dyer, A.R.; Brown, I.J.; Chan, Q.; Miura, K.; Zhao, L.; Okuda, N.; Daviglus, M.L.; et al. Food and nutrient intakes and their associations with lower BMI in middle-aged US adults: The International Study of Macro-/Micronutrients and Blood Pressure (INTERMAP). Am. J. Clin. Nutr. 2012, 96, 483–491. [Google Scholar] [CrossRef]
- Schwartz, N.; Kaye, E.K.; Nunn, M.E.; Spiro, A.; Garcia, R.I. High-Fiber Foods Reduce Periodontal Disease Progression in Men Aged 65 and Older: The Veterans Affairs Normative Aging Study/Dental Longitudinal Study. J. Am. Geriatr. Soc. 2012, 60, 676–683. [Google Scholar] [CrossRef]
- Nielsen, S.J.; Trak-Fellermeier, M.A.; Joshipura, K.; Dye, B.A. Dietary Fiber Intake Is Inversely Associated with Periodontal Disease among US Adults. J. Nutr. 2016, 146, 2530–2536. [Google Scholar] [CrossRef] [PubMed]
- Niedzielska, I. Differential diagnosis of gingival hyperplasia based on IFN-gamma-stimulated gene expression using oligonucleotide microarrays. Folic Biol. 2007, 53, 189–192. [Google Scholar]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Healthcare Interventions: Explanation and Elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [PubMed]
- Chapter 8: Assessing Risk of Bias in a Randomized Trial | Cochrane Training. Available online: https://training.cochrane.org/handbook/current/chapter-08 (accessed on 24 April 2023).
- Table—PMC. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3311794/table/Tab1/ (accessed on 4 April 2023).
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.; Gavaghan, D.J.; McQuay, H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control. Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Woelber, J.P.; Bremer, K.; Vach, K.; König, D.; Hellwig, E.; Ratka-Krüger, P.; Al-Ahmad, A.; Tennert, C. An oral health optimized diet can reduce gingival and periodontal inflammation in humans—A randomized controlled pilot study. BMC Oral Health 2016, 17, 28. [Google Scholar] [CrossRef]
- Woelber, J.P.; Gärtner, M.; Breuninger, L.; Anderson, A.; König, D.; Hellwig, E.; Al-Ahmad, A.; Vach, K.; Dötsch, A.; Ratka-Krüger, P.; et al. The influence of an anti-inflammatory diet on gingivitis. A randomized controlled trial. J. Clin. Periodontol. 2019, 46, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Bartha, V.; Exner, L.; Schweikert, D.; Woelber, J.P.; Vach, K.; Meyer, A.; Basrai, M.; Bischoff, S.C.; Meller, C.; Wolff, D. Effect of the Mediterranean diet on gingivitis: A randomized controlled trial. J. Clin. Periodontol. 2021, 49, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Kondo, K.; Ishikado, A.; Morino, K.; Nishio, Y.; Ugi, S.; Kajiwara, S.; Kurihara, M.; Iwakawa, H.; Nakao, K.; Uesaki, S.; et al. A high-fiber, low-fat diet improves periodontal disease markers in high-risk subjects: A pilot study. Nutr. Res. 2014, 34, 491–498. [Google Scholar] [CrossRef]
- Tennert, C.; Reinmuth, A.; Bremer, K.; Al-Ahmad, A.; Karygianni, L.; Hellwig, E.; Vach, K.; Ratka-Krüger, P.; Wittmer, A.; Woelber, J.P. An oral health optimized diet reduces the load of potential cariogenic and periodontal bacterial species in the supragingival oral plaque: A randomized controlled pilot study. MicrobiologyOpen 2020, 9, e1056. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.; Rajaram, S.; Nisha, S.; Ali, N.; Shashikumar, P.; Karmakar, S. Influence of a low-carbohydrate and rich in Omega-3 fatty acids, ascorbic acid, antioxidants, and fiber diet on clinical outcomes in patients with chronic gingivitis: A randomized controlled trial. J. Int. Soc. Prev. Community Dent. 2021, 11, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Chiang, N.; Dalli, J. The resolution code of acute inflammation: Novel pro-resolving lipid mediators in resolution. Semin. Immunol. 2015, 27, 200–215. [Google Scholar] [CrossRef]
- van Woudenbergh, G.J.; Theofylaktopoulou, D.; Kuijsten, A.; Ferreira, I.; van Greevenbroek, M.M.; van der Kallen, C.J.; Schalkwijk, C.G.; DA Stehouwer, C.; Ocké, M.C.; Nijpels, G.; et al. Adapted dietary inflammatory index and its association with a summary score for low-grade inflammation and markers of glucose metabolism: The Cohort study on Diabetes and Atherosclerosis Maastricht (CODAM) and the Hoorn study. Am. J. Clin. Nutr. 2013, 98, 1533–1542. [Google Scholar] [CrossRef] [PubMed]
- Hujoel, P. Dietary Carbohydrates and Dental-Systemic Diseases. J. Dent. Res. 2009, 88, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Van Der Velden, U.; Kuzmanova, D.; Chapple, I.L.C. Micronutritional approaches to periodontal therapy. J. Clin. Periodontol. 2011, 38 (Suppl. 11), 142–158. [Google Scholar] [CrossRef] [PubMed]
- Periodontal Disease and Non-Communicable Diseases. Strength of bidirectional Associations. Available online: http://www.scielo.org.za/scielo.php?script=sci_arttext&pid=S0011-85162016000900006 (accessed on 15 November 2022).
- Van Altena, F.; Beynen, A.C.; Altena, F.V.; Visser, E.A. Beneficial Effect of a Cellulose-Containing Chew Treat on Canine Periodontal Disease in a Double-Blind, Placebo-Controlled Trial. Am. J. Anim. Vet. Sci. 2010, 5, 192–195. [Google Scholar] [CrossRef]
- Logan, E.I.; Finney, O.; Hefferren, J.J. Effects of a Dental Food on Plaque Accumulation and Gingival Health in Dogs. J. Veter. Dent. 2002, 19, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Sedghi, L.; Byron, C.; Jennings, R.; Chlipala, G.E.; Green, S.J.; Silo-Suh, L. Effect of Dietary Fiber on the Composition of the Murine Dental Microbiome. Dent. J. 2019, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- The Best and Worst Foods for Your Teeth—Health Encyclopedia—University of Rochester Medical Center. Available online: https://www.urmc.rochester.edu/encyclopedia/content.aspx?contenttypeid=1&contentid=4062 (accessed on 21 January 2023).
- Inoue, M.; Ono, T.; Kameo, Y.; Sasaki, F.; Ono, T.; Adachi, T.; Nakashima, T. Forceful mastication activates osteocytes and builds a stout jawbone. Sci. Rep. 2019, 9, 4404. [Google Scholar] [CrossRef]
- Dahiya, P.; Kamal, R.; Gupta, R.; Bhardwaj, R.; Chaudhary, K.; Kaur, S. Reactive oxygen species in periodontitis. J. Indian Soc. Periodontol. 2013, 17, 411–416. [Google Scholar] [CrossRef]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef]
- Muniz, F.W.M.G.; Nogueira, S.B.; Mendes, F.L.V.; Rösing, C.K.; Moreira, M.M.S.M.; de Andrade, G.M.; Carvalho, R.d.S. The impact of antioxidant agents complimentary to periodontal therapy on oxidative stress and periodontal outcomes: A systematic review. Arch. Oral Biol. 2015, 60, 1203–1214. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kang, S.J.; Kim, J.W.; Cho, H.R.; Moon, S.B.; Kim, K.Y.; Lee, H.S.; Han, C.H.; Ku, S.K.; Lee, Y.J. Effects of Polycan, a β-glucan, on experimental periodontitis and alveolar bone loss in Sprague-Dawley rats. J. Periodontal Res. 2012, 47, 800–810. [Google Scholar] [CrossRef]
- Ioniță-Mîndrican, C.-B.; Ziani, K.; Mititelu, M.; Oprea, E.; Neacșu, S.M.; Moroșan, E.; Dumitrescu, D.-E.; Roșca, A.C.; Drăgănescu, D.; Negrei, C. Therapeutic Benefits and Dietary Restrictions of Fiber Intake: A State of the Art Review. Nutrients 2022, 14, 2641. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, S.J. The association between dietary fibre deficiency and high-income lifestyle-associated diseases: Burkitt’s hypothesis revisited. Lancet Gastroenterol. Hepatol. 2019, 4, 984–996. [Google Scholar] [CrossRef]
- Martínez-García, M.; Hernández-Lemus, E. Periodontal Inflammation and Systemic Diseases: An Overview. Front. Physiol. 2021, 12, 709438. [Google Scholar] [CrossRef] [PubMed]
- Olsen, I.; Yamazaki, K. Can oral bacteria affect the microbiome of the gut? J. Oral Microbiol. 2019, 11, 1586422. [Google Scholar] [CrossRef] [PubMed]
- Opinion: Understanding the Link between Oral and Gut Health—University of Birmingham. Available online: https://www.birmingham.ac.uk/news/2022/opinion-understanding-the-link-between-oral-and-gut-health (accessed on 26 March 2023).
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L., Jr. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef]
- Bui, F.Q.; Almeida-Da-Silva, C.L.C.; Huynh, B.; Trinh, A.; Liu, J.; Woodward, J.; Asadi, H.; Ojcius, D.M. Association between periodontal pathogens and systemic disease. Biomed. J. 2019, 42, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Zheng, Y.; Gao, Y.; Xu, W. Dietary Fibre Intake and Gut Microbiota in Human Health. Microorganism 2022, 10, 2507. [Google Scholar] [CrossRef]
- Ajani, U.A.; Ford, E.S.; Mokdad, A.H. Dietary Fiber and C-Reactive Protein: Findings from National Health and Nutrition Examination Survey Data. J. Nutr. 2004, 134, 1181–1185. [Google Scholar] [CrossRef] [PubMed]
- Mealey, B.L.; Ocampo, G.L. Diabetes mellitus and periodontal disease. Periodontology 2007, 44, 127–153. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.; West, M. Increasing Evidence for an Association Between Periodontitis and Cardiovascular Disease. Circulation 2016, 133, 549–551. [Google Scholar] [CrossRef]
- Pischon, N.; Heng, N.; Bernimoulin, J.P.; Kleber, B.M.; Willich, S.N.; Pischon, T. Obesity, inflammation, and periodontal disease. J. Dent. Res. 2007, 86, 400–409. [Google Scholar] [CrossRef]
- Park, Y.; Subar, A.F.; Hollenbeck, A.; Schatzkin, A. Dietary Fiber Intake and Mortality in the NIH-AARP Diet and Health Study. Arch. Intern. Med. 2011, 171, 1061–1068. [Google Scholar] [CrossRef]
- Chuang, S.-C.; Norat, T.; Murphy, N.; Olsen, A.; Tjønneland, A.; Overvad, K.; Boutron-Ruault, M.C.; Perquier, F.; Dartois, L.; Kaaks, R.; et al. Fiber intake and total and cause-specific mortality in the European Prospective Investigation into Cancer and Nutrition cohort. Am. J. Clin. Nutr. 2012, 96, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Liu, S.; Buring, E.J.; Sesso, H.D.; Rimm, E.B.; Willett, W.C.; Manson, E.J. A prospective study of dietary fiber intake and risk of cardiovascular disease among women. J. Am. Coll. Cardiol. 2002, 39, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Dietary fiber and risk of coronary heart disease: A pooled analysis of cohort studies. Arch. Intern. Med. 2004, 164, 370–376. [Google Scholar]
- Ma, Y.; Hébert, J.R.; Li, W.; Bertone-Johnson, E.R.; Olendzki, B.; Pagoto, S.L.; Tinker, L.; Rosal, M.C.; Ockene, I.S.; Ockene, J.K.; et al. Association between dietary fiber and markers of systemic inflammation in the Women’s Heath Initiative Observational Study. Nutrition 2008, 24, 941. [Google Scholar] [CrossRef]
- King, D.E. Dietary fiber, inflammation, and cardiovascular disease. Mol. Nutr. Food Res. 2005, 49, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Marangoni, F.; Poli, A. The glycemic index of bread and biscuits is markedly reduced by the addition of a proprietary fiber mixture to the ingredients. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 602–605. [Google Scholar] [CrossRef] [PubMed]
- Lattimer, J.M.; Haub, M.D. Effects of dietary fiber and its components on metabolic health. Nutrients 2010, 2, 1266–1289. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, S.; Nitta, H.; Nagasawa, T.; Uchimura, I.; Izumiyama, H.; Inagaki, K.; Kikuchi, T.; Noguchi, T.; Kanazawa, M.; Matsuo, A.; et al. Multi-center intervention study on glycohemoglobin (HbA1c) and serum, high-sensitivity CRP (hs-CRP) after local anti-infectious periodontal treatment in type 2 diabetic patients with periodontal disease. Diabetes Res. Clin. Pract. 2009, 83, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Correa, F.O.B.; Gonã§Alves, D.; Figueredo, C.M.S.; Bastos, A.S.; Gustafsson, A.; Orrico, S.R.P. Effect of periodontal treatment on metabolic control, systemic inflammation and cytokines in patients with type 2 diabetes. J. Clin. Periodontol. 2009, 37, 53–58. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, P.A.; Taba, M.; Nomizo, A.; Freitas, M.C.F.; Suaid, F.A.; Uyemura, S.A.; Trevisan, G.L.; Novaes, A.B.; Souza, S.L.; Palioto, D.B.; et al. Effects of Periodontal Therapy on Glycemic Control and Inflammatory Markers. J. Periodontol. 2008, 79, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Preshaw, P.M.; Alba, A.L.; Herrera, D.; Jepsen, S.; Konstantinidis, A.; Makrilakis, K.; Taylor, R. Periodontitis and diabetes: A two-way relationship. Diabetologia 2012, 55, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Sáenz-Ravello, G.; Matamala, L.; dos Santos, N.C.; Cisternas, P.; Gamonal, J.; Fernandez, A.; Bello-Escamilla, N.; Hernandez, M.; Baeza, M. Healthy Dietary Patterns on Clinical Periodontal Parameters: A GRADE Compliant Systematic Review and Meta-analysis. Curr. Oral Health Rep. 2022, 9, 32–55. [Google Scholar] [CrossRef]
- Stienstra, R.; Tack, C.J.; Kanneganti, T.-D.; Joosten, L.A.; Netea, M.G. The Inflammasome Puts Obesity in the Danger Zone. Cell Metab. 2012, 15, 10–18. [Google Scholar] [CrossRef]
- Fresno, M.; Alvarez, R.; Cuesta, N. Toll-like receptors, inflammation, metabolism and obesity. Arch. Physiol. Biochem. 2011, 117, 151–164. [Google Scholar] [CrossRef]
- Haque, M.; Yerex, K.; Kelekis-Cholakis, A.; Duan, K. Advances in novel therapeutic approaches for periodontal diseases. BMC Oral Health 2022, 22, 492. [Google Scholar] [CrossRef]
- Merchant, A.T.; Pitiphat, W.; Franz, M.; Joshipura, K.J. Whole-grain and fiber intakes and periodontitis risk in men. Am. J. Clin. Nutr. 2006, 83, 1395–1400. [Google Scholar] [CrossRef] [PubMed]
- Dodington, D.W.; Fritz, P.C.; Sullivan, P.J.; Ward, W.E. Higher Intakes of Fruits and Vegetables, β-Carotene, Vitamin C, α-Tocopherol, EPA, and DHA Are Positively Associated with Periodontal Healing after Nonsurgical Periodontal Therapy in Nonsmokers but Not in Smokers. J. Nutr. 2015, 145, 2512–2519. [Google Scholar] [CrossRef] [PubMed]
- Kandwal, A.; Chatterjee, A.; Bhattacharya, H. Probiotics in periodontal health and disease. J. Indian Soc. Periodontol. 2011, 15, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Butera, A.; Gallo, S.; Pascadopoli, M.; Maiorani, C.; Milone, A.; Alovisi, M.; Scribante, A. Paraprobiotics in Non-Surgical Periodontal Therapy: Clinical and Microbiological Aspects in a 6-Month Follow-Up Domiciliary Protocol for Oral Hygiene. Microorganisms 2022, 10, 337. [Google Scholar] [CrossRef] [PubMed]


| Study Details | Study Design; Duration | Diagnosis Criteria Defining the Periodontal Diseases | Study Population; Sample Size (I:C); Mean Age (I:C) | Form of Giving of Dietary Fiber; Dietary Fiber Intake/Day (g) | Outcomes Evaluated |
|---|---|---|---|---|---|
| Bartha et al. Germany; (2022) [30] | RCT, SB; 6 weeks | Generalized gingivitis (BOP > 30%) | Patients with gingivitis; 37 (18:19); 32.71 ± 8.87:29.21 ± 7.17 years | Mediterranean diet including fibre rich foods; 22.10–26.06 g | Periodontal outcomes: PD, GI, BOP, PI, PISA Physical outcomes: BW, BMI, WC |
| Kondo et al. Japan; (2014) [31] | SFT; 8 weeks | No specific periodontal diseases criteria mention. | High-risk subjects (BMI of at least 25.0 kg/m2 or impaired glucose tolerance); 17 (17:17); 45.0 ± 6.5:45.0 ± 6.5 years | Recommended diet including fibre rich foods; 30.3 g | Periodontal outcomes: PD, CAL, BOP, GCF Inflammatory markers: hs-CRP Physicaloutcomes: BMI, WC Other serum parameters: Lipid profile, plasma glucose, serum leptin |
| Rajaram et al. India; (2021) [33] | RCT, DB; 4 weeks | Gingivitis (GI > 0.5 and ≤3) | Patients with gingivitis; 54 (27:27); 36.1 ± 8.3:35.2 ± 7.2 years | Recommended diet including fibre rich foods(from fruits and vegetables); Amount not specified | Periodontal outcomes: PD, CAL, PI, GI, BOP% |
| Woelber et al. Germany; (2016) [28] | RCT; 8 weeks | Mild gingivitis (GI = 1.10) | Patients with gingivitis; 15 (10/5); 34.4 ± 14.1:34.0 ± 16.5 years | Recommended diet including fibre rich foods(from fruits and vegetables); Amount not specified | Periodontal outcomes: PD, CAL, BOP, PISA, PI, GI |
| Woelber et al. Germany; (2019) [29] | RCT, SB; 8 weeks | Gingivitis (GI ≥ 0.5) | Patients with gingivitis; 30 (15:15); 27.2 ± 4.7:33.7 ± 13.1 years | Recommended diet including fibre rich foods (from vegetables, fruits, legumes, bran); Amount not specified | Periodontal outcomes: PD, PI, GI, BOP, PISA Inflammatory markers: hs-CRP, IL-6, TNF-α Physicaloutcomes: BW, BMI, WC |
| Tennert et al. Switzerland; (2020) [32] | RCT; 4 weeks | Gingivitis (GI > 0.5) | Patients with gingivitis; 14 (9:5); 34.0 (24–63) years | Oral health optimized diet including fibre rich foods (fruits and vegetables) Amount not specified | Only Oral microbiota outcomes |
| Parameter | Bartha et al. (2022) [30] | Kondo et al. (2014) [31] | Rajaram et al. (2021) [33] | Woelber et al.(2016) [28] | Woelber et al. (2019) [29] | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention (I) Mean ± SD | Control (C) Mean ± SD | Inter-p (I vs. C) | Intervention (I) Mean ± SD | Control (C) Mean ± SD | Inter-P (I vs. C) | Intervention (I) Mean ± SD | Control (C) Mean ± SD | Inter-P (I vs. C) | Intervention (I) Mean ± SD | Control (C) Mean ± SD | Inter-P (I vs. C) | Intervention (I) Mean ± SD | Control (C) Mean ± SD | Inter-P (I vs. C) | ||
| Mean ±SD | ||||||||||||||||
| PD (mm/tooth) | Baseline (BL) | 2.26 ± 0.18 | 2.29 ± 0.18 | 0.616 | 2.28 ± 0.74 | - | - | 2.43 ± 0.07 | 2.22 ± 0.01 | <0.001 | 2.19 ± 0.34 | 2.31 ± 0.43 | 0.564 | 1.85 ± 0.27 | 1.82 ± 0.24 | 0.750 |
| Follow-up (FU) | 2.36 ± 0.17 | 2.36 ± 0.18 | 1.000 | 2.21 ± 0.77 | - | - | 2.23 ± 0.01 | 2.33 ± 0.03 | <0.001 | 2.11 ± 0.35 | 2.52 ± 0.40 | 0.062 | 1.84 ± 0.17 | 2.00 ± 0.14 | 0.009 | |
| Intra-P (BL vs. FU) | 0.096 | 0.239 | 0.789 | - | <0.001 | <0.001 | 0.611 | 0.447 | 0.940 | 0.018 | ||||||
| CAL (mm) | Baseline (BL) | - | - | - | 6.11 ± 1.39 | - | - | 2.35 ± 0.06 | 2.49 ± 0.10 | <0.001 | 2.31± 0.52 | 2.53 ± 0.90 | 0.554 | - | - | - |
| Follow-up (FU) | - | - | - | 6.06 ± 1.39 | - | - | 2.17 ± 0.05 | 2.79 ± 0.13 | <0.001 | 2.22 ± 0.47 | 2.76 ± 0.88 | 0.139 | - | - | - | |
| Intra-P (BL vs. FU) | - | - | 0.9171 | - | <0.001 | <0.001 | 0.670 | 0.694 | - | - | ||||||
| %BOP (% sites/tooth) | Baseline (BL) | 51.00 ± 14.65 | 43.21 ± 14.25 | 0.110 | 16.20 ± 22.3 | - | - | 53.88 ± 1.75 | 43.62 ± 2.49 | <0.001 | 53.57 ± 18.65 | 46.46 ± 15.61 | 0.478 | 30.35 ± 11.07 | 28.39 ± 13.32 | 0.665 |
| Follow-up (FU) | 39.93 ± 13.74 | 39.74 ± 11.0 | 0.963 | 13.20 ± 20.3 | - | - | 23.55 ± 1.79 | 68.34 ± 0.88 | <0.001 | 24.17 ± 11.57 | 64.06 ± 11.27 | <0.001 | 23.55 ± 13.61 | 27.09 ± 10.03 | 0.424 | |
| Intra-P (BL vs. FU) | 0.025 | 0.406 | 0.684 | - | <0.001 | <0.001 | <0.001 | 0.075 | 0.145 | 0.765 | ||||||
| GCF (µL/tooth) | Baseline (BL) | - | - | - | 0.57 ± 0.28 | - | - | - | - | - | - | - | - | - | - | |
| Follow-up (FU) | - | - | - | 0.52 ± 0.26 | - | - | - | - | - | - | - | - | - | - | ||
| Intra-P (BL vs. FU) | - | - | 0.593 | - | - | - | - | - | - | - | ||||||
| PISA (mm2) | Baseline (BL) | 616.33 ± 201.39 | 528.94 ± 173.48 | 0.165 | - | - | - | - | - | - | 638.88 ± 305.41 | 662.24 ± 420.05 | 0.903 | 315.20 ± 148.68 | 270.50 ± 140.97 | 0.405 |
| Follow-up (FU) | 512.02 ± 205.83 | 514.26 ±148.79 | 0.970 | - | - | - | - | - | - | 284.83 ± 174.14 | 963.24 ± 373.78 | <0.001 | 252.37 ± 151.78 | 286.00 ± 114.02 | 0.498 | |
| Intra-P (BL vs. FU) | 0.134 | 0.781 | - | - | - | - | 0.005 | 0.266 | 0.261 | 0.273 | ||||||
| PI | Baseline (BL) | 1.51 ± 0.21 | 1.37 ± 0.38 | 0.178 | - | - | - | 0.84 ± 0.01 | 0.87 ± 0.01 | <0.001 | 0.77± 0.52 | 0.75± 0.63 | 0.949 | 0.56 ± 0.27 | 0.57 ± 0.19 | 0.908 |
| Follow-up (FU) | 1.49 ± 0.24 | 1.39 ± 0.24 | <0.001 | - | - | - | 0.86 ± 0.02 | 0.91 ± 0.01 | <0.001 | 0.84 ± 0.47 | 0.97 ± 0.70 | 0.674 | 0.48 ± 0.13 | 0.48 ± 0.12 | 1.000 | |
| Intra-P (BL vs. FU) | 0.792 | <0.001 | - | - | <0.001 | <0.001 | 0.756 | 0.616 | 0.310 | 0.132 | ||||||
| GI | Baseline (BL) | 1.30 ± 0.25 | 1.11 ± 0.42 | 0.143 | - | - | - | 1.39 ± 0.03 | 1.51 ± 0.12 | <0.001 | 1.10 ± 0.51 | 1.01 ± 0.14 | 0.709 | 0.92 ± 0.14 | 0.83 ± 0.22 | 0.192 |
| Follow-up (FU) | 0.99 ± 0.22 | 0.97 ± 0.27 | 0.826 | - | - | - | 0.80 ± 0.11 | 1.49 ± 0.03 | <0.001 | 0.54 ± 0.30 | 1.22 ± 0.17 | <0.001 | 0.61 ± 0.29 | 0.74 ± 0.18 | 0.151 | |
| Intra-P (BL vs. FU) | <0.001 | 0.286 | - | - | <0.001 | 0.405 | 0.008 | 0.066 | <0.001 | 0.230 | ||||||
| Periodontal Outcome (Unit) | All Trials * | ||||
|---|---|---|---|---|---|
| N | n | Pooled Estimate (95% CI) | p Value (Pooled Estimate) | I2 (%), p Value of I2 | |
| Probing Depth (mm/tooth) | 4 | 136 | −0.17 (−0.37, 0.02) | 0.09 | 77, 0.005 |
| Clinical Attachment Loss (mm/tooth) | 2 | 69 | −0.48 (−0.63, −0.33) | <0.001 | 0, 0.79 |
| Bleeding on Probingm (% sites/tooth) | 4 | 136 | −27.57(−50.40, −4.74) | 0.02 | 94, <0.001 |
| Periodontal Inflamed Surface Area (mm2) | 3 | 82 | −173.88 (−288.06, −59.69) | 0.003 | 91, <0.001 |
| Plaque Index | 4 | 136 | −0.02 (−0.04, −0.00) | 0.04 | 0, 0.98 |
| Gingival Index | 4 | 136 | −0.41 (−0.67, −0.16) | 0.002 | 73, 0.01 |
| Characteristics of Modified Jadad Scale | Bartha et al. (2022) [30] | Kondo et al. (2014) [31] | Rajaram et al. (2021) [33] | Woelber et al. (2016) [28] | Woelber et al. (2019) [29] | Tennert et al. (2020) [32] |
|---|---|---|---|---|---|---|
| Was the study characterized as being random? (1 or 0) | 1 | 0 | 1 | 1 | 1 | 1 |
| Was the study referred to as being double-blind? (1 if double-blind, 0.5 if single blind or 0 if no-blinded) | 0.5 | 0 | 1 | 0 | 0.5 | 0 |
| Was a description given of dropouts and withdrawals? (1 or 0) | 1 | 1 | 1 | 1 | 1 | 1 |
| Was the randomization technique outlined in the paper appropriate? (1 or 0) | 1 | 0 | 1 | 1 | 1 | 1 |
| Was the described and appropriate blinding technique used? (1 or 0) | 1 | 0 | 1 | 0 | 1 | 0 |
| Was the paper’s description of the randomization method inappropriate? (0 or −1) | 0 | 0 | 0 | 0 | 0 | 0 |
| Was the described blinding technique inappropriate? (0 or −1) | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 4.5 | 1 | 5 | 3 | 4.5 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swarnamali, H.; Medara, N.; Chopra, A.; Spahr, A.; Jayasinghe, T.N. Role of Dietary Fibre in Managing Periodontal Diseases—A Systematic Review and Meta-Analysis of Human Intervention Studies. Nutrients 2023, 15, 4034. https://doi.org/10.3390/nu15184034
Swarnamali H, Medara N, Chopra A, Spahr A, Jayasinghe TN. Role of Dietary Fibre in Managing Periodontal Diseases—A Systematic Review and Meta-Analysis of Human Intervention Studies. Nutrients. 2023; 15(18):4034. https://doi.org/10.3390/nu15184034
Chicago/Turabian StyleSwarnamali, Hasinthi, Nidhi Medara, Aditi Chopra, Axel Spahr, and Thilini N. Jayasinghe. 2023. "Role of Dietary Fibre in Managing Periodontal Diseases—A Systematic Review and Meta-Analysis of Human Intervention Studies" Nutrients 15, no. 18: 4034. https://doi.org/10.3390/nu15184034
APA StyleSwarnamali, H., Medara, N., Chopra, A., Spahr, A., & Jayasinghe, T. N. (2023). Role of Dietary Fibre in Managing Periodontal Diseases—A Systematic Review and Meta-Analysis of Human Intervention Studies. Nutrients, 15(18), 4034. https://doi.org/10.3390/nu15184034





