The Gluten-Free Diet for Celiac Disease: Critical Insights to Better Understand Clinical Outcomes
Abstract
:1. Introduction
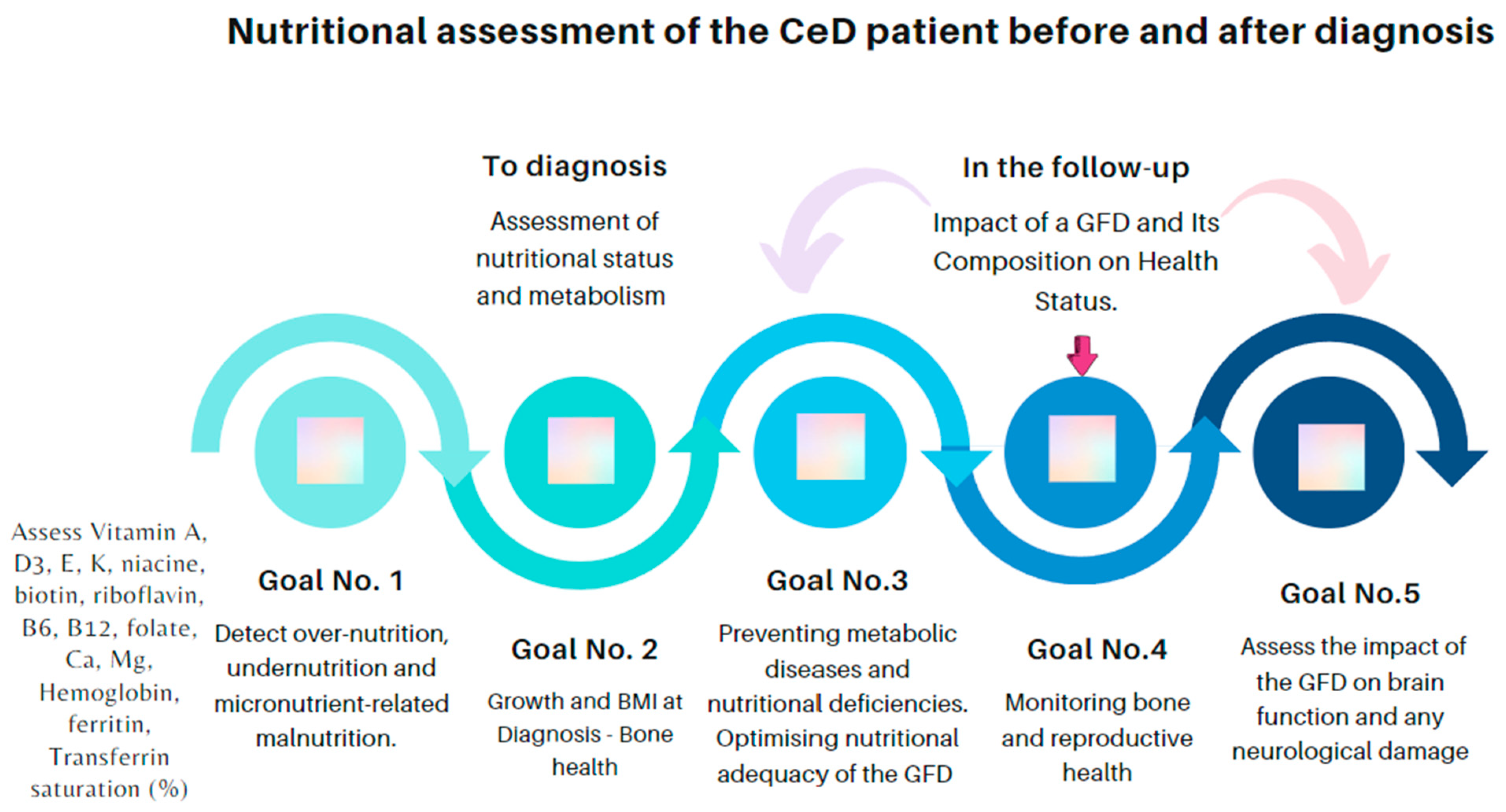
2. Celiac Disease Nutritional Status before Diagnosis
2.1. Nutritional Status
2.2. Anemia and Iron Deficiency
2.3. Bone Health
2.4. Other Micronutrient Deficiencies’ Consequences
3. Following a Gluten-Free Diet
3.1. Advantages and Nutritional Risks
- (1)
- Malnutrition, including over- and undernutrition, may be present in CeD, both at diagnosis and while under treatment. Underweight and growth retardation in children, which mostly reflect malabsorption, are not the rule. Nutritional deficiencies may be due to the poor absorption of amino acids and fats, as well as micronutrients, including calcium; iron; zinc; copper; vitamins A, D, E, and K; folate; and pyridoxine.
- (2)
- Malabsorption of calcium and vitamin D with a chronic inflammatory state affects bone health and may result in osteopenia or osteoporosis in CeD. Thus, it is recommended to measure calcium, alkaline phosphatase, and vitamin D at CeD diagnosis to assess bone health. Moreover, it is necessary to perform bone mineral density measurement with the dual X-ray absorptiometry (DEXA) scan in adults, not later than the age of 30–35 years, especially if there is a history of fractures and growth retardation in childhood. Surveillance of nutritional status during follow-up.
- (3)
- The nutritional composition of gluten-free rendered products (GFPs) can be unsatisfactory, and they are often not fortified with micronutrients. Therefore, the content of above-described vitamin and minerals in GFPs can be low, and these metabolic levels should be checked. In addition, eliminating gluten from the diet often impacts the proportion of nutrients consumed, leading to metabolic disorders. A critical point here is that GFPs are ultra-processed foods, which in the long term could be more detrimental to health. All this places the individual at risk for cardiovascular problems and metabolic-dysfunction-associated steatotic liver disease, the prevalence of which appears to be increased in this population when the gluten-free diet (GFD) has not been suitably advised.
- (4)
- A GFD should be balanced with proper iodine, iron, calcium, and vitamin D, among others, advised by a dietitian.
- (5)
- A GFD reduces or ameliorates some neurological symptoms, such as headache, ataxia, and epilepsy, in children and adults with CeD. In addition, a strict GFD can improve neuroimaging results in patients with gluten-sensitivity-related disorders.
3.2. Effects of the Gluten-Free Diet on Symptoms and Enteropathy
3.3. Adherence to GFD
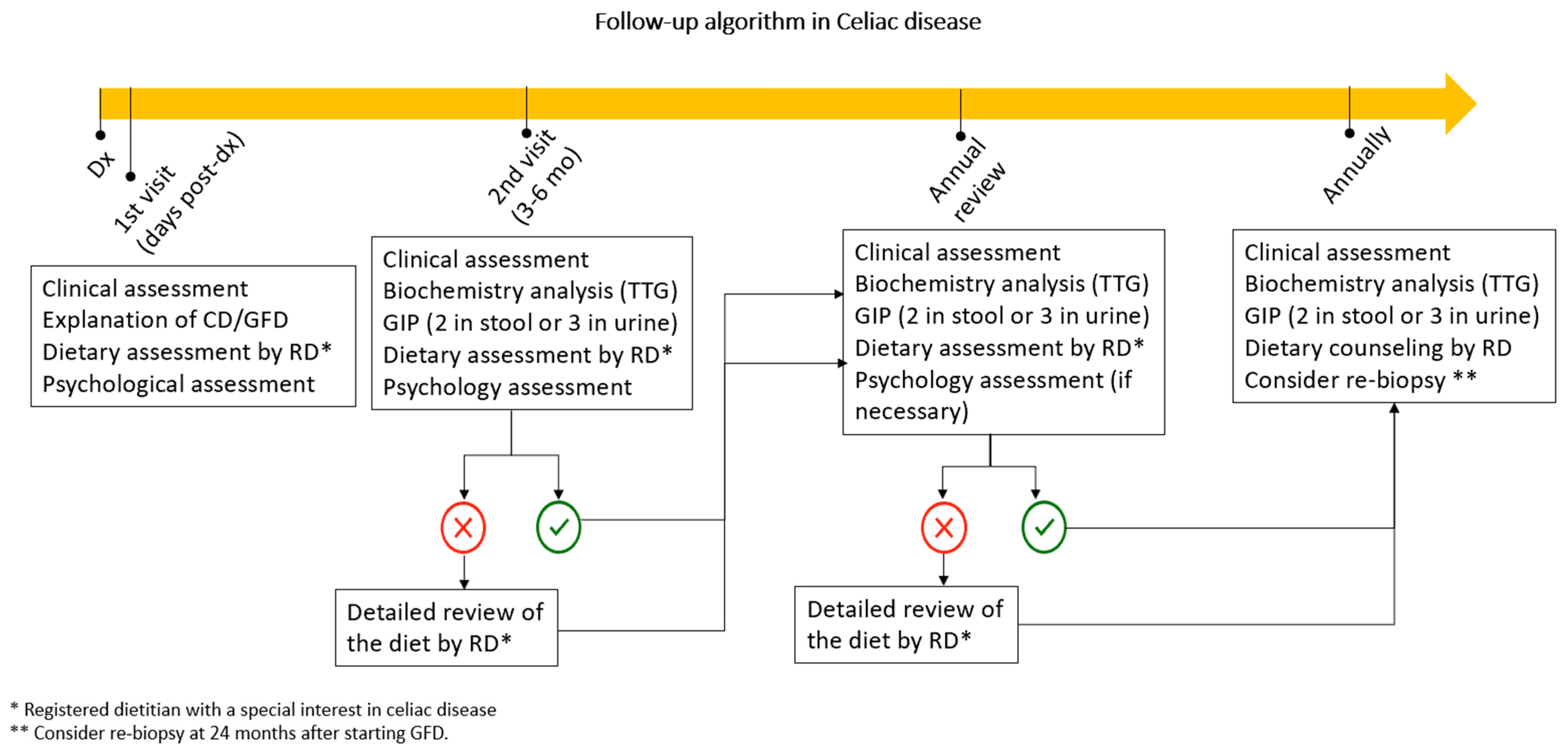
4. Patient Follow-Up
4.1. Tools to Monitor Adherence to the Gluten-Free Diet: Techniques and Procedures
4.1.1. Periodic Visits by Expert Dietitians and Dietary Records
4.1.2. Structured Questionnaires
4.1.3. Clinical Follow-Up: Recording of Symptoms
4.1.4. Serology: CeD-Specific Antibodies
4.1.5. Gluten Immunogenic Peptides (GIP) in Urine and Feces
4.1.6. Intestinal Biopsy
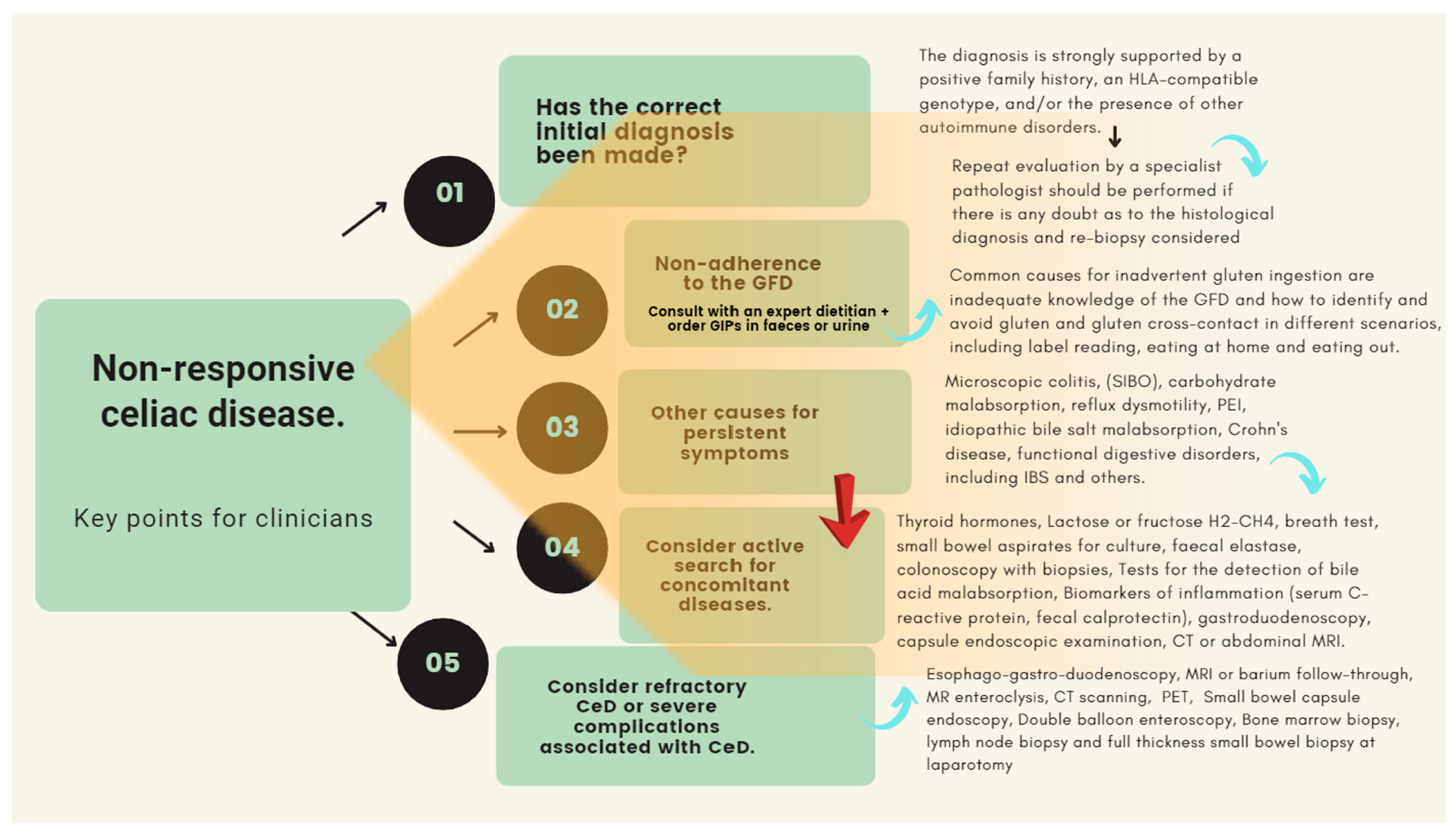
4.2. Non-Responsive Celiac Disease
- The clinical condition responsible for the enteropathy is not true celiac disease and there has been a misdiagnosis In cases of doubt, re-evaluation of the biopsy specimen by one or two expert pathologists is necessary to exclude other causes of enteropathy including, small bowel bacterial overgrowth (SIBO), Whipples’s disease, Crohn’s disease, adult autoimmune enteropathy, common variable immunodeficiency, AIDS enteropathy, collagenous sprue, giardiasis, tuberculosis, or drugs (e.g., olmesartan), among others [1,3,4,10,15].
- Once the initial diagnosis of CeD has been confirmed, the first step in the investigation of patients with ongoing symptoms is assessing for exposure to gluten. Patients will tend to overestimate their adherence to a gluten-free diet (GFD). The patient unintentionally or deliberately eats gluten or is extremely sensitive to minimal amounts of gluten (super sensitivity to gluten). In fact, some patients required as little as 10 mg of gluten per day to induce the development of intestinal mucosal abnormalities [135,146]. On the other hand, the persistence of symptoms is a severe problem in the presence of seronegative villous atrophy, where the resolution of symptoms and enteropathy is a mandatory requirement for diagnosis. In this context, dietary transgressions are an essential bias in interpreting the clinical course of these patients [12,16,138].
- An associated pathology is the actual cause of the ongoing symptoms: microscopic colitis [147,148,149,150], SIBO [151,152,153,154], malabsorption of simple carbohydrates (e.g., lactose, fructose, or sorbitol) [155,156,157], and others such as reflux dysmotility [135], PEI [158,159], idiopathic bile salt malabsorption [160,161,162], Crohn’s disease, [163,164,165,166,167,168,169,170,171,172,173], and other functional digestive disorders, including irritable bowel syndrome [174,175,176]. In such patients, it is obvious that the nature of the symptoms is due to a different cause that overlaps with that of CeD itself. The active search for clinical conditions associated with CeD should be based on a judicious and cost-effective clinical assessment. For example, microscopic colitis must be ruled out if the dominant symptom is watery diarrhea in a woman who smokes cigarettes and takes regularly nonsteroidal anti-inflammatory drugs or omeprazole. Likewise, if the predominant symptom is flatulence and abdominal distension, or “explosive diarrhea” (mixed with abundant gas), it is a priority to rule out carbohydrate malabsorption, SIBO, or both. Finally, the GFD is often low in fiber, which may exacerbate constipation causing pain and bloating symptoms. Many patients with CeD will also fulfil the Rome IV criteria for irritable bowel syndrome (IBS) and in cases where other causes of symptoms have been excluded, this may be the most likely cause
- The patient developed RCD. Once other causes of ongoing symptoms have been ruled out, a diagnosis of true refractory celiac disease (RCD) can be considered. True RCD is a rare condition defined as persistent malabsorptive symptoms and villous atrophy despite strict adherence to a GFD with negative serology for anti-tissue transglutaminase or anti-endomysial antibodies; [135].
5. Psychological Considerations and Time-Related Variables
6. Cost-Efficacy of Optimal GFD Adherence Monitoring
- Increased education.
- Increased knowledge of a GFD.
- Increased intention/self-regulatory efficacy.
7. Follow-Up Algorithm in CeD
8. Gluten-Free Diet in CeD: Conclusions and Highlights
- In comparison with gluten-containing food, gluten-free alternative grains can render a lower amount of protein, dietary fiber and certain vitamins and minerals. Excessive consumption of ultra-processed foods such as many GFPs could have negative effects on health due to their inadequate nutritional composition (e.g., high sugar and saturated fat content).
- Following a GFD also entails significant challenges beyond avoiding gluten. It is also essential to correct nutritional deficits and achieve dietary balance. In addition, substituting manufactured GFPs with naturally gluten-free alternatives is essential to reduce the risk of metabolic disorders associated with high consumption of ultra-processed foods. In both cases, the role of a dietitian with specific training and expertise in this area is essential.
- Nutritional advice and counseling by an experienced dietitian can reduce the costs associated with long-term follow-up of the CeD patient. It should be noted that non-responsive CeD is a complex entity in which numerous causes may be involved (see Figure 2). Its investigation can be time-consuming, challenging, and cumbersome. In this regard, it should be borne in mind that inadequate adherence to the GFD remains the most frequent cause.
- Celiac patients report difficulties when eating out, constant worry about gluten, continuous planning, feeling different, emotional pressure, or coping with symptoms. Right after diagnosis, they feel anger, fear, shame, rage, and grief, but after some time in GFD, the situation normalizes, and their HRQoL improves. Psychological interventions are crucial in these scenarios.
- For all the above reasons, the short- and long-term follow-up of celiac patients should preferably be performed by a gastroenterology, hepatology, and nutrition unit with well-defined quality standards and the multidisciplinary involvement of physicians, nurses, dieticians, and psychologists (see Figure 3). This approach has been shown to reduce the costs associated with their health care.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Rubio-Tapia, A.; Hill, I.D.; Semrad, C.; Kelly, C.P.; Greer, K.B.; Limketkai, B.N.; Lebwohl, B. American College of Gastroenterology Guidelines Update: Diagnosis and Management of Celiac Disease. Am. J. Gastroenterol. 2023, 118, 59–76. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Gillett, P.M.; Le Huray, V.; Bremner, G.; Paul, S.P. Updated European Guidelines for Coeliac Disease in Children. Nurs. Child. Young People 2023, 35, 16–21. [Google Scholar] [CrossRef]
- Lebwohl, B.; Sanders, D.S.; Green, P.H.R. Coeliac Disease. Lancet 2018, 391, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Al-Toma, A.; Volta, U.; Auricchio, R.; Castillejo, G.; Sanders, D.S.; Cellier, C.; Mulder, C.J.; Lundin, K.E.A. European Society for the Study of Coeliac Disease (ESsCD) Guideline for Coeliac Disease and Other Gluten-related Disorders. United Eur. Gastroenterol. J. 2019, 7, 583–613. [Google Scholar] [CrossRef]
- Uche-Anya, E.; Lebwohl, B. Celiac Disease: Clinical Update. Curr. Opin. Gastroenterol. 2021, 37, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Bañares, F.; Beltrán, B.; Salas, A.; Comino, I.; Ballester-Clau, R.; Ferrer, C.; Molina-Infante, J.; Rosinach, M.; Modolell, I.; Rodríguez-Moranta, F.; et al. Persistent Villous Atrophy in De Novo Adult Patients With Celiac Disease and Strict Control of Gluten-Free Diet Adherence: A Multicenter Prospective Study (CADER Study). Am. J. Gastroenterol. 2021, 116, 1036–1043. [Google Scholar] [CrossRef]
- Fernández-Bañares, F.; Crespo, L.; Núñez, C.; López-Palacios, N.; Tristán, E.; Vivas, S.; Farrais, S.; Arau, B.; Vidal, J.; Roy, G.; et al. Gamma Delta+ Intraepithelial Lymphocytes and Coeliac Lymphogram in a Diagnostic Approach to Coeliac Disease in Patients with Seronegative Villous Atrophy. Aliment. Pharmacol. Ther. 2020, 51, 699–705. [Google Scholar] [CrossRef]
- Aziz, I.; Peerally, M.F.; Barnes, J.-H.; Kandasamy, V.; Whiteley, J.C.; Partridge, D.; Vergani, P.; Cross, S.S.; Green, P.H.; Sanders, D.S. The Clinical and Phenotypical Assessment of Seronegative Villous Atrophy; a Prospective UK Centre Experience Evaluating 200 Adult Cases over a 15-Year Period (2000–2015). Gut 2017, 66, 1563–1572. [Google Scholar] [CrossRef] [PubMed]
- Jansson-Knodell, C.L.; Murray, J.A.; Rubio-Tapia, A. Management of Small Bowel Villous Atrophy in Patients Seronegative for Celiac Disease. Am. J. Gastroenterol. 2020, 115, 492–497. [Google Scholar] [CrossRef]
- Leonard, M.M.; Lebwohl, B.; Rubio-Tapia, A.; Biagi, F. AGA Clinical Practice Update on the Evaluation and Management of Seronegative Enteropathies: Expert Review. Gastroenterology 2021, 160, 437–444. [Google Scholar] [CrossRef]
- Paavola, S.; Kurppa, K.; Huhtala, H.; Saavalainen, P.; Lindfors, K.; Kaukinen, K. Coeliac Disease Re-screening among Once Seronegative At-risk Relatives: A Long-term Follow-up Study. UEG J. 2022, 10, 585–593. [Google Scholar] [CrossRef]
- Schiepatti, A.; Cincotta, M.; Biagi, F.; Sanders, D.S. Enteropathies with Villous Atrophy but Negative Coeliac Serology in Adults: Current Issues. BMJ Open Gastroenterol. 2021, 8, e000630. [Google Scholar] [CrossRef]
- Schiepatti, A.; Sanders, D.S.; Zuffada, M.; Luinetti, O.; Iraqi, A.; Biagi, F. Overview in the Clinical Management of Patients with Seronegative Villous Atrophy. Eur. J. Gastroenterol. Hepatol. 2019, 31, 409–417. [Google Scholar] [CrossRef]
- Schiepatti, A.; Savioli, J.; Vernero, M.; Borrelli De Andreis, F.; Perfetti, L.; Meriggi, A.; Biagi, F. Pitfalls in the Diagnosis of Coeliac Disease and Gluten-Related Disorders. Nutrients 2020, 12, 1711. [Google Scholar] [CrossRef] [PubMed]
- Schiepatti, A.; Sanders, D.S.; Baiardi, P.; Caio, G.; Ciacci, C.; Kaukinen, K.; Lebwohl, B.; Leffler, D.; Malamut, G.; Murray, J.A.; et al. Nomenclature and Diagnosis of Seronegative Coeliac Disease and Chronic Non-Coeliac Enteropathies in Adults: The Paris Consensus. Gut 2022, 71, 2218–2225. [Google Scholar] [CrossRef] [PubMed]
- Schiepatti, A.; Sanders, D.S.; Biagi, F. Seronegative Coeliac Disease: Clearing the Diagnostic Dilemma. Curr. Opin. Gastroenterol. 2018, 34, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Schiepatti, A.; Rej, A.; Maimaris, S.; Cross, S.S.; Porta, P.; Aziz, I.; Key, T.; Goodwin, J.; Therrien, A.; Yoosuf, S.; et al. Clinical Classification and Long-term Outcomes of Seronegative Coeliac Disease: A 20-year Multicentre Follow-up Study. Aliment. Pharmacol. Ther. 2021, 54, 1278–1289. [Google Scholar] [CrossRef]
- Szaflarska-Popławska, A. The Role of the Gluten-Free Diet in the Management of Seronegative Enteropathy. Nutrients 2021, 13, 4027. [Google Scholar] [CrossRef] [PubMed]
- Volta, U.; Caio, G.; Boschetti, E.; Giancola, F.; Rhoden, K.J.; Ruggeri, E.; Paterini, P.; De Giorgio, R. Seronegative Celiac Disease: Shedding Light on an Obscure Clinical Entity. Dig. Liver Dis. 2016, 48, 1018–1022. [Google Scholar] [CrossRef] [PubMed]
- Schiepatti, A.; Maimaris, S.; Raju, S.A.; Green, O.L.; Mantica, G.; Therrien, A.; Flores-Marin, D.; Linden, J.; Fernández-Bañares, F.; Esteve, M.; et al. Persistent Villous Atrophy Predicts Development of Complications and Mortality in Adult Patients with Coeliac Disease: A Multicentre Longitudinal Cohort Study and Development of a Score to Identify High-Risk Patients. Gut 2023. [Google Scholar] [CrossRef]
- Abdi, F.; Zuberi, S.; Blom, J.-J.; Armstrong, D.; Pinto-Sanchez, M.I. Nutritional Considerations in Celiac Disease and Non-Celiac Gluten/Wheat Sensitivity. Nutrients 2023, 15, 1475. [Google Scholar] [CrossRef]
- Montoro-Huguet, M.A.; Belloc, B.; Domínguez-Cajal, M. Small and Large Intestine (I): Malabsorption of Nutrients. Nutrients 2021, 13, 1254. [Google Scholar] [CrossRef] [PubMed]
- Kreutz, J.M.; Adriaanse, M.P.M.; van der Ploeg, E.M.C.; Vreugdenhil, A.C.E. Narrative Review: Nutrient Deficiencies in Adults and Children with Treated and Untreated Celiac Disease. Nutrients 2020, 12, 500. [Google Scholar] [CrossRef] [PubMed]
- Melini, V.; Melini, F. Gluten-Free Diet: Gaps and Needs for a Healthier Diet. Nutrients 2019, 11, 170. [Google Scholar] [CrossRef] [PubMed]
- Ahlawat, R.; Weinstein, T.; Markowitz, J.; Kohn, N.; Pettei, M.J. Should We Assess Vitamin D Status in Pediatric Patients with Celiac Disease? J. Pediatr. Gastroenterol. Nutr. 2019, 69, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Churruca, I.; Miranda, J.; Lasa, A.; Bustamante, M.; Larretxi, I.; Simon, E. Analysis of Body Composition and Food Habits of Spanish Celiac Women. Nutrients 2015, 7, 5515–5531. [Google Scholar] [CrossRef]
- Kabbani, T.A.; Goldberg, A.; Kelly, C.P.; Pallav, K.; Tariq, S.; Peer, A.; Hansen, J.; Dennis, M.; Leffler, D.A. Body Mass Index and the Risk of Obesity in Coeliac Disease Treated with the Gluten-Free Diet. Lett. Ed. 2012, 35, 723–729. [Google Scholar] [CrossRef]
- De Giuseppe, R.; Bergomas, F.; Loperfido, F.; Giampieri, F.; Preatoni, G.; Calcaterra, V.; Cena, H. Could Celiac Disease and Overweight/Obesity Coexist in School-Aged Children and Adolescents? A Systematic Review. Child. Obes. 2023. [Google Scholar] [CrossRef]
- Valletta, E.; Fornaro, M.; Cipolli, M.; Conte, S.; Bissolo, F.; Danchielli, C. Celiac Disease and Obesity: Need for Nutritional Follow-up after Diagnosis. Eur. J. Clin. Nutr. 2010, 64, 1371–1372. [Google Scholar] [CrossRef]
- Wierdsma, N.; van Bokhorst-de van der Schueren, M.; Berkenpas, M.; Mulder, C.; van Bodegraven, A. Vitamin and Mineral Deficiencies Are Highly Prevalent in Newly Diagnosed Celiac Disease Patients. Nutrients 2013, 5, 3975–3992. [Google Scholar] [CrossRef]
- Seidita, A.; Mansueto, P.; Compagnoni, S.; Castellucci, D.; Soresi, M.; Chiarello, G.; Cavallo, G.; De Carlo, G.; Nigro, A.; Chiavetta, M.; et al. Anemia in Celiac Disease: Prevalence, Associated Clinical and Laboratory Features, and Persistence after Gluten-Free Diet. JPM 2022, 12, 1582. [Google Scholar] [CrossRef]
- Halfdanarson, T.R.; Litzow, M.R.; Murray, J.A. Hematologic Manifestations of Celiac Disease. Blood 2007, 109, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Talarico, V.; Giancotti, L.; Mazza, G.A.; Miniero, R.; Bertini, M. Iron Deficiency Anemia in Celiac Disease. Nutrients 2021, 13, 1695. [Google Scholar] [CrossRef]
- Annibale, B.; Lahner, E.; Fave, G.D. Efficacy of Gluten-Free Diet Alone on Recovery from Iron Deficiency Anemia in Adult Celiac Patients. Am. J. Gastroenterol. 2001, 96, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Montoro-Huguet, M.A.; Santolaria-Piedrafita, S.; Cañamares-Orbis, P.; García-Erce, J.A. Iron Deficiency in Celiac Disease: Prevalence, Health Impact, and Clinical Management. Nutrients 2021, 13, 3437. [Google Scholar] [CrossRef] [PubMed]
- Shiha, M.G.; Marks, L.J.; Sanders, D.S. Diagnosing Coeliac Disease in the Elderly: A United Kingdom Cohort Study. Gastroenterol. Hepatol. Bed Bench. 2020, 13, 37–43. [Google Scholar]
- Oxentenko, A.S.; Murray, J.A. Celiac Disease: Ten Things That Every Gastroenterologist Should Know. Clin. Gastroenterol. Hepatol. 2015, 13, 1396–1404. [Google Scholar] [CrossRef] [PubMed]
- Moya, D.A.; Nugent, C.A.; Baker, R.D.; Baker, S.S. Celiac Disease Nutritional Status and Poor Adherence to Follow-Up. Clin. Pediatr. (Phila.) 2020, 59, 649–655. [Google Scholar] [CrossRef]
- Jivraj, A.; Hutchinson, J.M.; Ching, E.; Marwaha, A.; Verdu, E.F.; Armstrong, D.; Pinto-Sanchez, M.I. Micronutrient Deficiencies Are Frequent in Adult Patients with and without Celiac Disease on a Gluten-Free Diet, Regardless of Duration and Adherence to the Diet. Nutrition 2022, 103–104, 111809. [Google Scholar] [CrossRef] [PubMed]
- McGrogan, L.; Mackinder, M.; Stefanowicz, F.; Aroutiounova, M.; Catchpole, A.; Wadsworth, J.; Buchanan, E.; Cardigan, T.; Duncan, H.; Hansen, R.; et al. Micronutrient Deficiencies in Children with Coeliac Disease; a Double-Edged Sword of Both Untreated Disease and Treatment with Gluten-Free Diet. Clin. Nutr. 2021, 40, 2784–2790. [Google Scholar] [CrossRef]
- Laurikka, P.; Kivelä, L.; Kurppa, K.; Kaukinen, K. Review Article: Systemic Consequences of Coeliac Disease. Aliment. Pharmacol. Ther. 2022, 56, S64–S72. [Google Scholar] [CrossRef] [PubMed]
- Webster, J.; Vajravelu, M.E.; Choi, C.; Zemel, B.; Verma, R. Prevalence of and Risk Factors for Low Bone Mineral Density in Children with Celiac Disease. Clin. Gastroenterol. Hepatol. 2019, 17, 1509–1514. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.D.; Williams, J.; Lewis, S.K.; Bai, J.C.; Lebwohl, B.; Green, P.H.R. Measurement of Forearm Bone Density by Dual Energy X-Ray Absorptiometry Increases the Prevalence of Osteoporosis in Men with Celiac Disease. Clin. Gastroenterol. Hepatol. 2020, 18, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Zanchetta, M.B.; Longobardi, V.; Bai, J.C. Bone and Celiac Disease. Curr. Osteoporos. Rep. 2016, 14, 43–48. [Google Scholar] [CrossRef]
- Zingone, F.; Secchettin, E.; Marsilio, I.; Valiante, F.; Zorzetto, V.; Cataudella, G.; D’Odorico, A.; Canova, C. Clinical Features and Psychological Impact of Celiac Disease at Diagnosis. Dig. Liver Dis. 2021, 53, 1565–1570. [Google Scholar] [CrossRef]
- Zanchi, C.; Di Leo, G.; Ronfani, L.; Martelossi, S.; Not, T.; Ventura, A. Bone Metabolism in Celiac Disease. J. Pediatr. 2008, 153, 262–265. [Google Scholar] [CrossRef]
- Henri-Bhargava, A.; Melmed, C.; Glikstein, R.; Schipper, H.M. Neurologic Impairment Due to Vitamin E and Copper Deficiencies in Celiac Disease. Neurology 2008, 71, 860–861. [Google Scholar] [CrossRef]
- Elfström, P.; Montgomery, S.M.; Kämpe, O.; Ekbom, A.; Ludvigsson, J.F. Risk of Thyroid Disease in Individuals with Celiac Disease. J. Clin. Endocrinol. Metab. 2008, 93, 3915–3921. [Google Scholar] [CrossRef]
- Sun, X.; Lu, L.; Yang, R.; Li, Y.; Shan, L.; Wang, Y. Increased Incidence of Thyroid Disease in Patients with Celiac Disease: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0168708. [Google Scholar] [CrossRef]
- Stazi, A.V.; Trinti, B. Selenium deficiency in celiac disease: Risk of autoimmune thyroid diseases. Minerva Med. 2008, 99, 643–653. [Google Scholar]
- Delvecchio, M.; Bizzoco, F.; Lapolla, R.; Gentile, A.; Carrozza, C.; Barone, M.; Simonetti, S.; Giordano, P.; Dargenio, V.N.; Cristofori, F.; et al. Iodine Absorption in Celiac Children: A Longitudinal Pilot Study. Nutrients 2021, 13, 808. [Google Scholar] [CrossRef] [PubMed]
- Cardo, A.; Churruca, I.; Lasa, A.; Navarro, V.; Vázquez-Polo, M.; Perez-Junkera, G.; Larretxi, I. Nutritional Imbalances in Adult Celiac Patients Following a Gluten-Free Diet. Nutrients 2021, 13, 2877. [Google Scholar] [CrossRef] [PubMed]
- Vici, G.; Belli, L.; Biondi, M.; Polzonetti, V. Gluten Free Diet and Nutrient Deficiencies: A Review. Clin. Nutr. 2016, 35, 1236–1241. [Google Scholar] [CrossRef]
- Babio, N.; Alcázar, M.; Castillejo, G.; Recasens, M.; Martínez-Cerezo, F.; Gutiérrez-Pensado, V.; Masip, G.; Vaqué, C.; Vila-Martí, A.; Torres-Moreno, M.; et al. Patients With Celiac Disease Reported Higher Consumption of Added Sugar and Total Fat Than Healthy Individuals. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 63–69. [Google Scholar] [CrossRef]
- Hopman, E.G.D.; Le Cessie, S.; Von Blomberg, B.M.E.; Mearin, M.L. Nutritional Management of the Gluten-Free Diet in Young People with Celiac Disease in The Netherlands. J. Pediatr. Gastroenterol. Nutr. 2006, 43, 102–108. [Google Scholar] [CrossRef]
- Larretxi, I.; Txurruka, I.; Navarro, V.; Lasa, A.; Bustamante, M.Á.; Fernández-Gil, M.D.P.; Simón, E.; Miranda, J. Micronutrient Analysis of Gluten-Free Products: Their Low Content Is Not Involved in Gluten-Free Diet Imbalance in a Cohort of Celiac Children and Adolescent. Foods 2019, 8, 321. [Google Scholar] [CrossRef]
- Kulai, T.; Rashid, M. Assessment of Nutritional Adequacy of Packaged Gluten-Free Food Products. Can. J. Diet. Pract. Res. 2014, 75, 186–190. [Google Scholar] [CrossRef]
- Cyrkot, S.; Anders, S.; Kamprath, C.; Liu, A.; Mileski, H.; Dowhaniuk, J.; Nasser, R.; Marcon, M.; Brill, H.; Turner, J.M.; et al. Folate Content of Gluten-Free Food Purchases and Dietary Intake Are Low in Children with Coeliac Disease. Int. J. Food Sci. Nutr. 2020, 71, 863–874. [Google Scholar] [CrossRef]
- Nestares, T.; Martín-Masot, R.; Flor-Alemany, M.; Bonavita, A.; Maldonado, J.; Aparicio, V.A. Influence of Ultra-Processed Foods Consumption on Redox Status and Inflammatory Signaling in Young Celiac Patients. Nutrients 2021, 13, 156. [Google Scholar] [CrossRef] [PubMed]
- Raiteri, A.; Granito, A.; Giamperoli, A.; Catenaro, T.; Negrini, G.; Tovoli, F. Current Guidelines for the Management of Celiac Disease: A Systematic Review with Comparative Analysis. World J. Gastroenterol. 2022, 28, 154–175. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A Multi-Society Delphi Consensus Statement on New Fatty Liver Disease Nomenclature. J. Hepatol. 2023, 101133. [Google Scholar] [CrossRef]
- Mármol-Soler, C.; Matias, S.; Miranda, J.; Larretxi, I.; Fernández-Gil, M.D.P.; Bustamante, M.Á.; Churruca, I.; Martínez, O.; Simón, E. Gluten-Free Products: Do We Need to Update Our Knowledge? Foods 2022, 11, 3839. [Google Scholar] [CrossRef]
- De Las Heras-Delgado, S.; Alías-Guerrero, A.D.L.N.; Cendra-Duarte, E.; Salas-Salvadó, J.; Vilchez, E.; Roger, E.; Hernández-Alonso, P.; Babio, N. Assessment of Price and Nutritional Quality of Gluten-Free Products versus Their Analogues with Gluten through the Algorithm of the Nutri-Score Front-of-Package Labeling System. Food Funct. 2021, 12, 4424–4433. [Google Scholar] [CrossRef]
- González, T.; Larretxi, I.; Vitoria, J.; Castaño, L.; Simón, E.; Churruca, I.; Navarro, V.; Lasa, A. Celiac Male’s Gluten-Free Diet Profile: Comparison to That of the Control Population and Celiac Women. Nutrients 2018, 10, 1713. [Google Scholar] [CrossRef]
- Kautto, E.; Ivarsson, A.; Norström, F.; Högberg, L.; Carlsson, A.; Hörnell, A. Nutrient Intake in Adolescent Girls and Boys Diagnosed with Coeliac Disease at an Early Age Is Mostly Comparable to Their Non-Coeliac Contemporaries. J. Hum. Nutr. Diet. 2014, 27, 41–53. [Google Scholar] [CrossRef]
- Suárez-González, M.; Bousoño García, C.; Jiménez Treviño, S.; Iglesias Cabo, T.; Díaz Martín, J.J. Influence of Nutrition Education in Paediatric Coeliac Disease: Impact of the Role of the Registered Dietitian: A Prospective, Single-arm Intervention Study. J. Hum. Nutr. Diet. 2020, 33, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.R.; Ng, D.L.; Dave, E.; Ciaccio, E.J.; Green, P.H.R. The Effect of Substituting Alternative Grains in the Diet on the Nutritional Profile of the Gluten-Free Diet. J. Hum. Nutr. Diet. 2009, 22, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Niro, S.; D’Agostino, A.; Fratianni, A.; Cinquanta, L.; Panfili, G. Gluten-Free Alternative Grains: Nutritional Evaluation and Bioactive Compounds. Foods 2019, 8, 208. [Google Scholar] [CrossRef]
- Agostoni, C.; Bresson, J.L.; Fairweather-Tait, S.; Flynn, A.; Golly, I.; Korhonen, H.; Lagiou, P.; Løvik, M.; Marchelli, R.; Martin, A.; et al. EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific Opinion on establishing Food-Based Dietary Guidelines. EFSA J. 2010, 8, 1460–1502. [Google Scholar] [CrossRef]
- Larussa, T.; Suraci, E.; Imeneo, M.; Marasco, R.; Luzza, F. Normal Bone Mineral Density Associates with Duodenal Mucosa Healing in Adult Patients with Celiac Disease on a Gluten-Free Diet. Nutrients 2017, 9, 98. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Tapia, A.; Rahim, M.W.; See, J.A.; Lahr, B.D.; Wu, T.-T.; Murray, J.A. Mucosal Recovery and Mortality in Adults with Celiac Disease After Treatment with a Gluten-Free Diet. Am. J. Gastroenterol. 2010, 105, 1412–1420. [Google Scholar] [CrossRef] [PubMed]
- Laurikka, P.; Salmi, T.; Collin, P.; Huhtala, H.; Mäki, M.; Kaukinen, K.; Kurppa, K. Gastrointestinal Symptoms in Celiac Disease Patients on a Long-Term Gluten-Free Diet. Nutrients 2016, 8, 429. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.A.; Watson, T.; Clearman, B.; Mitros, F. Effect of a Gluten-Free Diet on Gastrointestinal Symptoms in Celiac Disease. Am. J. Clin. Nutr. 2004, 79, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Pulido, O.; Zarkadas, M.; Dubois, S.; MacIsaac, K.; Cantin, I.; La Vieille, S.; Godefroy, S.; Rashid, M. Clinical Features and Symptom Recovery on a Gluten-Free Diet in Canadian Adults with Celiac Disease. Can. J. Gastroenterol. 2013, 27, 449–453. [Google Scholar] [CrossRef]
- Rubio-Tapia, A.; Hill, I.D.; Kelly, C.P.; Calderwood, A.H.; Murray, J.A. ACG Clinical Guidelines: Diagnosis and Management of Celiac Disease. Am. J. Gastroenterol. 2013, 108, 656–676. [Google Scholar] [CrossRef] [PubMed]
- Wahab, P.J.; Meijer, J.W.R.; Mulder, C.J.J. Histologic Follow-up of People with Celiac Disease on a Gluten-Free Diet: Slow and Incomplete Recovery. Am. J. Clin. Pathol. 2002, 118, 459–463. [Google Scholar] [CrossRef]
- Belei, O.; Dobrescu, A.; Heredea, R.; Iacob, E.R.; David, V.; Marginean, O. Histologic Recovery among Children with Celiac Disease on a Gluten-Free Diet. A Long-Term Follow-Up Single-Center Experience. AOMS 2018, 1, 94–100. [Google Scholar] [CrossRef]
- Carroccio, A.; Brusca, I.; Iacono, G.; Alessio, M.G.; Sonzogni, A.; Di Prima, L.; Barrale, M.; Ottomano, C.; Ambrosiano, G.; Teresi, S.; et al. IgA Anti-Actin Antibodies ELISA in Coeliac Disease: A Multicentre Study. Dig. Liver Dis. 2007, 39, 818–823. [Google Scholar] [CrossRef]
- Silvester, J.A.; Kurada, S.; Szwajcer, A.; Kelly, C.P.; Leffler, D.A.; Duerksen, D.R. Tests for Serum Transglutaminase and Endomysial Antibodies Do Not Detect Most Patients with Celiac Disease and Persistent Villous Atrophy on Gluten-Free Diets: A Meta-Analysis. Gastroenterology 2017, 153, 689–701.e1. [Google Scholar] [CrossRef]
- Stefanelli, G.; Navisse, S.; Valvano, M.; Vernia, F.; Ciccone, A.; Melideo, D.; Necozione, S.; Calvisi, G.; Coletti, G.; Viscido, A.; et al. Serum Transglutaminase Antibodies Do Not Always Detect the Persistent Villous Atrophy in Patients with Celiac Disease on a Gluten-Free Diet. Eur. J. Gastroenterol. Hepatol. 2021, 33, e650. [Google Scholar] [CrossRef]
- Adriaanse, M.P.M.; Tack, G.J.; Passos, V.L.; Damoiseaux, J.G.M.C.; Schreurs, M.W.J.; van Wijck, K.; Riedl, R.G.; Masclee, A.a.M.; Buurman, W.A.; Mulder, C.J.J.; et al. Serum I-FABP as Marker for Enterocyte Damage in Coeliac Disease and Its Relation to Villous Atrophy and Circulating Autoantibodies. Aliment. Pharmacol. Ther. 2013, 37, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Lomash, A.; Prasad, A.; Singh, R.; Kumar, S.; Gupta, R.; Dholakia, D.; Kumar, P.; Batra, V.V.; Puri, A.S.; Kapoor, S. Evaluation of the Utility of Amino Acid Citrulline as a Surrogate Metabolomic Biomarker for the Diagnosis of Celiac Disease. Nutr. Metab. Insights 2021, 14, 11786388211060603. [Google Scholar] [CrossRef]
- Kozioł-Kozakowska, A.; Salamon, D.; Grzenda-Adamek, Z.; Krawczyk, A.; Duplaga, M.; Gosiewski, T.; Kowalska-Duplaga, K. Changes in Diet and Anthropometric Parameters in Children and Adolescents with Celiac Disease—One Year of Follow-Up. Nutrients 2021, 13, 4306. [Google Scholar] [CrossRef] [PubMed]
- Nestares, T.; Martín-Masot, R.; Labella, A.; Aparicio, V.A.; Flor-Alemany, M.; López-Frías, M.; Maldonado, J. Is a Gluten-Free Diet Enough to Maintain Correct Micronutrients Status in Young Patients with Celiac Disease? Nutrients 2020, 12, 844. [Google Scholar] [CrossRef]
- Siniscalchi, M.; Iovino, P.; Tortora, R.; Forestiero, S.; Somma, A.; Capuano, L.; Franzese, M.D.; Sabbatini, F.; Ciacci, C. Fatigue in Adult Coeliac Disease. Aliment. Pharmacol. Ther. 2005, 22, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Skjellerudsveen, B.M.; Omdal, R.; Grimstad, T. Fatigue in Celiac Disease: A Review of the Literature. JGH Open 2019, 3, 242–248. [Google Scholar] [CrossRef]
- Abu-Janb, N.; Jaana, M. Facilitators and Barriers to Adherence to Gluten-Free Diet among Adults with Celiac Disease: A Systematic Review. J. Hum. Nutr. Diet. 2020, 33, 786–810. [Google Scholar] [CrossRef]
- Muhammad, H.; Reeves, S.; Jeanes, Y.M. Identifying and Improving Adherence to the Gluten-Free Diet in People with Coeliac Disease. Proc. Nutr. Soc. 2019, 78, 418–425. [Google Scholar] [CrossRef]
- Wieser, H.; Ruiz-Carnicer, Á.; Segura, V.; Comino, I.; Sousa, C. Challenges of Monitoring the Gluten-Free Diet Adherence in the Management and Follow-up of Patients with Celiac Disease. Nutrients 2021, 13, 2274. [Google Scholar] [CrossRef]
- Hall, N.J.; Rubin, G.; Charnock, A. Systematic Review: Adherence to a Gluten-Free Diet in Adult Patients with Coeliac Disease. Aliment. Pharmacol. Ther. 2009, 30, 315–330. [Google Scholar] [CrossRef]
- Myléus, A.; Reilly, N.R.; Green, P.H.R. Rate, Risk Factors, and Outcomes of Nonadherence in Pediatric Patients with Celiac Disease: A Systematic Review. Clin. Gastroenterol. Hepatol. 2020, 18, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Schiepatti, A.; Maimaris, S.; Nicolardi, M.L.; Alimenti, E.; Vernero, M.; Costetti, M.; Costa, S.; Biagi, F. Determinants and Trends of Adherence to a Gluten-Free Diet in Adult Celiac Patients on a Long-Term Follow-up (2000–2020). Clin. Gastroenterol. Hepatol. 2022, 20, e741–e749. [Google Scholar] [CrossRef]
- Czaja-Bulsa, G.; Bulsa, M. Adherence to Gluten-Free Diet in Children with Celiac Disease. Nutrients 2018, 10, 1424. [Google Scholar] [CrossRef] [PubMed]
- Kori, M.; Goldstein, S.; Hofi, L.; Topf-Olivestone, C. Adherence to Gluten-Free Diet and Follow-up of Pediatric Celiac Disease Patients, during Childhood and after Transition to Adult Care. Eur. J. Pediatr. 2021, 180, 1817–1823. [Google Scholar] [CrossRef] [PubMed]
- Comino, I.; Fernández-Bañares, F.; Esteve, M.; Ortigosa, L.; Castillejo, G.; Fambuena, B.; Ribes-Koninckx, C.; Sierra, C.; Rodríguez-Herrera, A.; Salazar, J.C.; et al. Fecal Gluten Peptides Reveal Limitations of Serological Tests and Food Questionnaires for Monitoring Gluten-Free Diet in Celiac Disease Patients. Am. J. Gastroenterol. 2016, 111, 1456–1465. [Google Scholar] [CrossRef]
- Schiepatti, A.; Maimaris, S.; De Queiros Mattoso Archela Dos Sant, C.; Rusca, G.; Costa, S.; Biagi, F. Long-Term Adherence to a Gluten-Free Diet and Quality of Life of Celiac Patients After Transition to an Adult Referral Center. Dig. Dis. Sci. 2022, 67, 3955–3963. [Google Scholar] [CrossRef] [PubMed]
- Mearin, M.L.; Agardh, D.; Antunes, H.; Al-toma, A.; Auricchio, R.; Castillejo, G.; Catassi, C.; Ciacci, C.; Discepolo, V.; Dolinsek, J.; et al. ESPGHAN Position Paper on Management and Follow-up of Children and Adolescents With Celiac Disease. J. Pediatr. Gastroenterol. Nutr. 2022, 75, 369–386. [Google Scholar] [CrossRef]
- Ludvigsson, J.F.; Bai, J.C.; Biagi, F.; Card, T.R.; Ciacci, C.; Ciclitira, P.J.; Green, P.H.R.; Hadjivassiliou, M.; Holdoway, A.; van Heel, D.A.; et al. Diagnosis and Management of Adult Coeliac Disease: Guidelines from the British Society of Gastroenterology. Gut 2014, 63, 1210–1228. [Google Scholar] [CrossRef]
- Gładyś, K.; Dardzińska, J.; Guzek, M.; Adrych, K.; Małgorzewicz, S. Celiac Dietary Adherence Test and Standardized Dietician Evaluation in Assessment of Adherence to a Gluten-Free Diet in Patients with Celiac Disease. Nutrients 2020, 12, 2300. [Google Scholar] [CrossRef]
- Atsawarungruangkit, A.; Silvester, J.A.; Weiten, D.; Green, K.L.; Wilkey, K.E.; Rigaux, L.N.; Bernstein, C.N.; Graff, L.A.; Walker, J.R.; Duerksen, D.R. Development of the Dietitian Integrated Evaluation Tool for Gluten-Free Diets (DIET-GFD). Nutrition 2020, 78, 110819. [Google Scholar] [CrossRef]
- Gładyś, K.; Dardzińska, J.; Guzek, M.; Adrych, K.; Kochan, Z.; Małgorzewicz, S. Expanded Role of a Dietitian in Monitoring a Gluten-Free Diet in Patients with Celiac Disease: Implications for Clinical Practice. Nutrients 2021, 13, 1859. [Google Scholar] [CrossRef] [PubMed]
- Wessels, M.M.S.; Te Lintelo, M.; Vriezinga, S.L.; Putter, H.; Hopman, E.G.; Mearin, M.L. Assessment of Dietary Compliance in Celiac Children Using a Standardized Dietary Interview. Clin. Nutr. 2018, 37, 1000–1004. [Google Scholar] [CrossRef]
- Sharkey, L.M.; Corbett, G.; Currie, E.; Lee, J.; Sweeney, N.; Woodward, J.M. Optimising Delivery of Care in Coeliac Disease—Comparison of the Benefits of Repeat Biopsy and Serological Follow-Up. Aliment. Pharmacol. Ther. 2013, 38, 1278–1291. [Google Scholar] [CrossRef] [PubMed]
- DiGiacomo, D.V.; Tennyson, C.A.; Green, P.H.; Demmer, R.T. Prevalence of Gluten-Free Diet Adherence among Individuals without Celiac Disease in the USA: Results from the Continuous National Health and Nutrition Examination Survey 2009–2010. Scand. J. Gastroenterol. 2013, 48, 921–925. [Google Scholar] [CrossRef]
- Mehta, P.; Pan, Z.; Riley, M.D.; Liu, E. Adherence to a Gluten-Free Diet: Assessment by Dietician Interview and Serology. J. Pediatr. Gastroenterol. Nutr. 2018, 66, e67–e70. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, L.; Pérez-Martinez, I.; Lauret-Braña, E.; Suárez-González, A. Descriptive Study of the Different Tools Used to Evaluate the Adherence to a Gluten-Free Diet in Celiac Disease Patients. Nutrients 2018, 10, 1777. [Google Scholar] [CrossRef]
- Ortega, R.M.; Pérez-Rodrigo, C.; López-Sobaler, A.M. Dietary Assessment Methods: Dietary Records. Nutr. Hosp. 2015, 31 (Suppl. S3), 38–45. [Google Scholar] [CrossRef]
- Shim, J.-S.; Oh, K.; Kim, H.C. Dietary Assessment Methods in Epidemiologic Studies. Epidemiol. Health 2014, 36, e2014009. [Google Scholar] [CrossRef]
- Leffler, D.A.; Dennis, M.; Edwards George, J.; Jamma, S.; Cook, E.F.; Schuppan, D.; Kelly, C.P. A Validated Disease-Specific Symptom Index for Adults with Celiac Disease. Clin. Gastroenterol. Hepatol. 2009, 7, 1328–1334.e3. [Google Scholar] [CrossRef] [PubMed]
- Biagi, F.; Bianchi, P.I.; Marchese, A.; Trotta, L.; Vattiato, C.; Balduzzi, D.; Brusco, G.; Andrealli, A.; Cisarò, F.; Astegiano, M.; et al. A Score That Verifies Adherence to a Gluten-Free Diet: A Cross-Sectional, Multicentre Validation in Real Clinical Life. Br. J. Nutr. 2012, 108, 1884–1888. [Google Scholar] [CrossRef]
- Silvester, J.A.; Weiten, D.; Graff, L.A.; Walker, J.R.; Duerksen, D.R. Is It Gluten-Free? Relationship between Self-Reported Gluten-Free Diet Adherence and Knowledge of Gluten Content of Foods. Nutrition 2016, 32, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Silvester, J.A.; Graff, L.A.; Rigaux, L.; Bernstein, C.N.; Leffler, D.A.; Kelly, C.P.; Walker, J.R.; Duerksen, D.R. Symptoms of Functional Intestinal Disorders Are Common in Patients with Celiac Disease Following Transition to a Gluten-Free Diet. Dig. Dis. Sci. 2017, 62, 2449–2454. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.J.; Heaton, K.W. Stool Form Scale as a Useful Guide to Intestinal Transit Time. Scand J. Gastroenterol. 1997, 32, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Sansotta, N.; Amirikian, K.; Guandalini, S.; Jericho, H. Celiac Disease Symptom Resolution: Effectiveness of the Gluten-Free Diet. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 48–52. [Google Scholar] [CrossRef]
- Galli, G.; Carabotti, M.; Pilozzi, E.; Lahner, E.; Annibale, B.; Conti, L. Relationship between Persistent Gastrointestinal Symptoms and Duodenal Histological Findings after Adequate Gluten-Free Diet: A Gray Area of Celiac Disease Management in Adult Patients. Nutrients 2021, 13, 600. [Google Scholar] [CrossRef] [PubMed]
- Wessels, M.; Dolinsek, J.; Castillejo, G.; Donat, E.; Riznik, P.; Roca, M.; Valitutti, F.; Veenvliet, A.; Mearin, M.L. Follow-up Practices for Children and Adolescents with Celiac Disease: Results of an International Survey. Eur. J. Pediatr. 2022, 181, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Giersiepen, K.; Lelgemann, M.; Stuhldreher, N.; Ronfani, L.; Husby, S.; Koletzko, S.; Korponay-Szabó, I.R. ESPGHAN Working Group on Coeliac Disease Diagnosis Accuracy of Diagnostic Antibody Tests for Coeliac Disease in Children: Summary of an Evidence Report. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Husby, S.; Koletzko, S.; Korponay-Szabó, I.R.; Mearin, M.L.; Phillips, A.; Shamir, R.; Troncone, R.; Giersiepen, K.; Branski, D.; Catassi, C.; et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition Guidelines for the Diagnosis of Coeliac Disease. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 136–160. [Google Scholar] [CrossRef] [PubMed]
- Husby, S.; Koletzko, S.; Korponay-Szabó, I.; Kurppa, K.; Mearin, M.L.; Ribes-Koninckx, C.; Shamir, R.; Troncone, R.; Auricchio, R.; Castillejo, G.; et al. European Society Paediatric Gastroenterology, Hepatology and Nutrition Guidelines for Diagnosing Coeliac Disease 2020. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 141–156. [Google Scholar] [CrossRef]
- Gould, M.J.; Brill, H.; Marcon, M.A.; Munn, N.J.; Walsh, C.M. In Screening for Celiac Disease, Deamidated Gliadin Rarely Predicts Disease When Tissue Transglutaminase Is Normal. J. Pediatr. Gastroenterol. Nutr. 2019, 68, 20–25. [Google Scholar] [CrossRef]
- Ribes-Koninckx, C.; Roca, M.; Donat, E. Value and Use of Serologic Markers of Celiac Disease. In Advances in Celiac Disease: Improving Paediatric and Adult Care, 1st ed.; Amil-Dias, J., Polanco, I., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 63–78. [Google Scholar]
- Di Tola, M.; Marino, M.; Goetze, S.; Casale, R.; Di Nardi, S.; Borghini, R.; Donato, G.; Tiberti, A.; Picarelli, A. Identification of a Serum Transglutaminase Threshold Value for the Noninvasive Diagnosis of Symptomatic Adult Celiac Disease Patients: A Retrospective Study. J. Gastroenterol. 2016, 51, 1031–1039. [Google Scholar] [CrossRef]
- Sansotta, N.; Alessio, M.G.; Norsa, L.; Previtali, G.; Ferrari, A.; Guerra, G.; D’Antiga, L. Trend of Antitissue Transglutaminase Antibody Normalization in Children with Celiac Disease Started on Gluten-Free Diet: A Comparative Study Between Chemiluminescence and ELISA Serum Assays. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 37. [Google Scholar] [CrossRef]
- Sbravati, F.; Cosentino, A.; Lenzi, J.; Fiorentino, M.; Ambrosi, F.; Salerno, A.; Di Biase, A.; Righi, B.; Brusa, S.; Valin, P.S.; et al. Antitissue Transglutaminase Antibodies’ Normalization after Starting a Gluten-Free Diet in a Large Population of Celiac Children-a Real-Life Experience. Dig. Liver Dis. 2022, 54, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Sbravati, F.; Pagano, S.; Retetangos, C.; Spisni, E.; Bolasco, G.; Labriola, F.; Filardi, M.C.; Grondona, A.G.; Alvisi, P. Adherence to Gluten-Free Diet in a Celiac Pediatric Population Referred to the General Pediatrician After Remission. J. Pediatr. Gastroenterol. Nutr. 2020, 71, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; King, K.S.; Larson, J.J.; Snyder, M.R.; Wu, T.T.; Gandhi, M.J.; Murray, J.A. Undetectable Negative Tissue Transglutaminase IgA Antibodies Predict Mucosal Healing in Treated Coeliac Disease Patients. Aliment. Pharmacol. Ther. 2017, 46, 681–687. [Google Scholar] [CrossRef]
- Husby, S.; Murray, J.A.; Katzka, D.A. AGA Clinical Practice Update on Diagnosis and Monitoring of Celiac Disease—Changing Utility of Serology and Histologic Measures: Expert Review. Gastroenterology 2019, 156, 885–889. [Google Scholar] [CrossRef]
- López, R.V.; Cid, C.M.; García, G.R.; Romero, R.G.; Cilleruelo, M.L.; Riechmann, E.R. Spanish Working Group on Coeliac Disease (Spanish Society of Paediatric Gastroenterology, Hepatology, Nutrition, SEGHNP) Influence of the 2012 European Guidelines in Diagnosis and Follow-up of Coeliac Children with Selective IgA Deficiency. J. Pediatr. Gastroenterol. Nutr. 2020, 71, 59–63. [Google Scholar] [CrossRef] [PubMed]
- de Lourdes Moreno, M.; Cebolla, Á.; Muñoz-Suano, A.; Carrillo-Carrion, C.; Comino, I.; Pizarro, Á.; León, F.; Rodríguez-Herrera, A.; Sousa, C. Detection of Gluten Immunogenic Peptides in the Urine of Patients with Coeliac Disease Reveals Transgressions in the Gluten-Free Diet and Incomplete Mucosal Healing. Gut 2017, 66, 250–257. [Google Scholar] [CrossRef]
- Coto, L.; Sousa, C.; Cebolla, A. Dynamics and Considerations in the Determination of the Excretion of Gluten Immunogenic Peptides in Urine: Individual Variability at Low Gluten Intake. Nutrients 2021, 13, 2624. [Google Scholar] [CrossRef]
- Costa, A.F.; Sugai, E.; de la Paz Temprano, M.; Niveloni, S.I.; Vázquez, H.; Moreno, M.L.; Domínguez-Flores, M.R.; Muñoz-Suano, A.; Smecuol, E.; Stefanolo, J.P.; et al. Gluten Immunogenic Peptide Excretion Detects Dietary Transgressions in Treated Celiac Disease Patients. WJG 2019, 25, 1409–1420. [Google Scholar] [CrossRef]
- Moreno, M.D.L.; Sánchez-Muñoz, D.; Sanders, D.; Rodríguez-Herrera, A.; Sousa, C. Verifying Diagnosis of Refractory Celiac Disease with Urine Gluten Immunogenic Peptides as Biomarker. Front. Med. 2021, 7, 601854. [Google Scholar] [CrossRef]
- Ruiz-Carnicer, Á.; Garzón-Benavides, M.; Fombuena, B.; Segura, V.; García-Fernández, F.; Sobrino-Rodríguez, S.; Gómez-Izquierdo, L.; Montes-Cano, M.A.; Rodríguez-Herrera, A.; Millán, R.; et al. Negative Predictive Value of the Repeated Absence of Gluten Immunogenic Peptides in the Urine of Treated Celiac Patients in Predicting Mucosal Healing: New Proposals for Follow-up in Celiac Disease. Am. J. Clin. Nutr. 2020, 112, 1240–1251. [Google Scholar] [CrossRef] [PubMed]
- Garzón-Benavides, M.; Ruiz-Carnicer, Á.; Segura, V.; Fombuena, B.; García-Fernandez, F.; Sobrino-Rodriguez, S.; Gómez-Izquierdo, L.; Montes-Cano, M.A.; Millan-Domínguez, R.; Del Carmen Rico, M.; et al. Clinical Utility of Urinary Gluten Immunogenic Peptides in the Follow-up of Patients with Coeliac Disease. Aliment. Pharmacol. Ther. 2023, 57, 993–1003. [Google Scholar] [CrossRef]
- Penny, H.A.; Rej, A.; Baggus, E.M.; Coleman, S.H.; Ward, R.; Wild, G.; Bouma, G.; Trott, N.; Snowden, J.A.; Wright, J.; et al. Non-Responsive and Refractory Coeliac Disease: Experience from the NHS England National Centre. Nutrients 2022, 14, 2776. [Google Scholar] [CrossRef] [PubMed]
- Coto, L.; Mendia, I.; Sousa, C.; Bai, J.C.; Cebolla, A. Determination of Gluten Immunogenic Peptides for the Management of the Treatment Adherence of Celiac Disease: A Systematic Review. WJG 2021, 27, 6306–6321. [Google Scholar] [CrossRef] [PubMed]
- Husby, S.; Bai, J.C. Follow-up of Celiac Disease. Gastroenterol. Clin. N. Am. 2019, 48, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Rahmanipour, E.; Ghorbani, M.; Ganji, A.; Mirzaei, Z.; Ghavami, V.; Shahbazkhani, B.; Attarian, F.; Amiri, M. Clinical profile of patients with seronegative celiac disease. Gastroenterol. Hepatol. Bed Bench 2023, 16, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Janczyk, W.; de Roo, J.H.C.; Schweizer, J.; Socha, J.; Socha, P.; Mearin, M.L. Coeliac Disease Not Responding to a Gluten-Free Diet in Children: Case Studies and Literature Review. Dev. Period Med. 2015, 19, 162–166. [Google Scholar] [PubMed]
- Malamut, G.; Afchain, P.; Verkarre, V.; Lecomte, T.; Amiot, A.; Damotte, D.; Bouhnik, Y.; Colombel, J.-F.; Delchier, J.-C.; Allez, M.; et al. Presentation and Long-Term Follow-up of Refractory Celiac Disease: Comparison of Type I with Type II. Gastroenterology 2009, 136, 81–90. [Google Scholar] [CrossRef]
- Mooney, P.D.; Evans, K.E.; Singh, S.; Sanders, D.S. Treatment Failure in Coeliac Disease: A Practical Guide to Investigation and Treatment of Non-Responsive and Refractory Coeliac Disease. J. Gastrointestin. Liver Dis. 2012, 21, 197–203. [Google Scholar]
- Abdulkarim, A.S.; Burgart, L.J.; See, J.; Murray, J.A. Etiology of Nonresponsive Celiac Disease: Results of a Systematic Approach. Am. J. Gastroenterol. 2002, 97, 2016–2021. [Google Scholar] [CrossRef]
- Fine, K.; Meyer, R.; Lee, E. The Prevalence and Causes of Chronic Diarrhea in Patients with Celiac Sprue Treated with a Gluten-Free Diet. Gastroenterology 1997, 112, 1830–1838. [Google Scholar] [CrossRef] [PubMed]
- Leffler, D.A.; Dennis, M.; Hyett, B.; Kelly, E.; Schuppan, D.; Kelly, C.P. Etiologies and Predictors of Diagnosis in Nonresponsive Celiac Disease. Clin. Gastroenterol. Hepatol. 2007, 5, 445–450. [Google Scholar] [CrossRef]
- Van Wanrooij, R.L.J.; Bouma, G.; Bontkes, H.J.; Neefjes-Borst, A.; Van Grieken, N.C.; Von Blomberg, B.M.E.; Mulder, C.J.J. Outcome of Referrals for Non-Responsive Celiac Disease in a Tertiary Center: Low Incidence of Refractory Celiac Disease in the Netherlands. Clin. Transl. Gastroenterol. 2017, 8, e218. [Google Scholar] [CrossRef]
- Akobeng, A.K.; Thomas, A.G. Systematic Review: Tolerable Amount of Gluten for People with Coeliac Disease. Aliment. Pharmacol. Ther. 2008, 27, 1044–1052. [Google Scholar] [CrossRef]
- Fernández-Bañares, F.; De Sousa, M.R.; Salas, A.; Beltrán, B.; Piqueras, M.; Iglesias, E.; Gisbert, J.P.; Lobo, B.; Puig-Diví, V.; García-Planella, E.; et al. Impact of Current Smoking on the Clinical Course of Microscopic Colitis. Inflamm. Bowel Dis. 2013, 19, 1470–1476. [Google Scholar] [CrossRef]
- Mellander, M.-R.; Ekbom, A.; Hultcrantz, R.; Löfberg, R.; Öst, Å.; Björk, J. Microscopic Colitis: A Descriptive Clinical Cohort Study of 795 Patients with Collagenous and Lymphocytic Colitis. Scand. J. Gastroenterol. 2016, 51, 556–562. [Google Scholar] [CrossRef]
- Nielsen, O.H.; Fernandez-Banares, F.; Sato, T.; Pardi, D.S. Microscopic Colitis: Etiopathology, Diagnosis, and Rational Management. eLife 2022, 11, e79397. [Google Scholar] [CrossRef]
- Rehde, A.; Hendel, S.K.; Juhl, C.B.; Gubatan, J.; Nielsen, O.H. Effectiveness of Non-Budesonide Therapies in Management of Microscopic Colitis: A Systematic Review and Meta-Analysis. Drugs 2023, 83, 1027–1038. [Google Scholar] [CrossRef] [PubMed]
- Efremova, I.; Maslennikov, R.; Poluektova, E.; Vasilieva, E.; Zharikov, Y.; Suslov, A.; Letyagina, Y.; Kozlov, E.; Levshina, A.; Ivashkin, V. Epidemiology of Small Intestinal Bacterial Overgrowth. World J. Gastroenterol. 2023, 29, 3400–3421. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, C.; Quigley, E.M.M. Small Bowel Bacterial Overgrowth, Celiac Disease, and IBS: What Are the Real Associations? Am. J. Gastroenterol. 2003, 98, 720–722. [Google Scholar] [CrossRef] [PubMed]
- Sroka, N.; Rydzewska-Rosołowska, A.; Kakareko, K.; Rosołowski, M.; Głowińska, I.; Hryszko, T. Show Me What You Have Inside—The Complex Interplay between SIBO and Multiple Medical Conditions—A Systematic Review. Nutrients 2022, 15, 90. [Google Scholar] [CrossRef] [PubMed]
- Tursi, A.; Brandimarte, G.; Giorgetti, G. High Prevalence of Small Intestinal Bacterial Overgrowth in Celiac Patients with Persistence of Gastrointestinal Symptoms after Gluten Withdrawal. Am. J. Gastroenterol. 2003, 98, 839–843. [Google Scholar] [CrossRef]
- Fernández-Bañares, F. Carbohydrate Maldigestion and Intolerance. Nutrients 2022, 14, 1923. [Google Scholar] [CrossRef]
- Fernández-Bañares, F.; Esteve, M.; Salas, A.; Alsina, M.; Farré, C.; González, C.; Buxeda, M.; Forné, M.; Rosinach, M.; Espinós, J.C.; et al. Systematic Evaluation of the Causes of Chronic Watery Diarrhea With Functional Characteristics. Am J Gastroenterology 2007, 102, 2520–2528. [Google Scholar] [CrossRef]
- Fernández-Bañares, F.; Esteve, M.; Viver, J.M. Fructose-Sorbitol Malabsorption. Curr. Gastroenterol. Rep. 2009, 11, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Barkin, J.A.; Barkin, J.S. Exocrine Pancreatic Insufficiency Is Common in Celiac Disease: A Systematic Review and Meta-Analysis. Dig. Dis. Sci. 2023, 68, 3421–3427. [Google Scholar] [CrossRef] [PubMed]
- Yoosuf, S.; Barrett, C.G.; Papamichael, K.; Madoff, S.E.; Kurada, S.; Hansen, J.; Silvester, J.A.; Therrien, A.; Singh, P.; Dennis, M.; et al. Pancreatic Enzyme Supplementation versus Placebo for Improvement of Gastrointestinal Symptoms in Non-Responsive Celiac Disease: A Cross-over Randomized Controlled Trial. Front. Med. 2023, 9, 1001879. [Google Scholar] [CrossRef]
- BouSaba, J.; Camilleri, M. Bile Acid Diarrhea—As Bad as It Gets? Curr. Opin. Gastroenterol. 2023, 39, 184–191. [Google Scholar] [CrossRef]
- Camilleri, M.; Nurko, S. Bile Acid Diarrhea in Adults and Adolescents. Neurogastroenterol. Motil. 2022, 34, e14287. [Google Scholar] [CrossRef]
- Camilleri, M.; Vijayvargiya, P. The Role of Bile Acids in Chronic Diarrhea. Am. J. Gastroenterol. 2020, 115, 1596–1603. [Google Scholar] [CrossRef] [PubMed]
- Aziz, I.; Branchi, F.; Pearson, K.; Priest, J.; Sanders, D.S. A Study Evaluating the Bidirectional Relationship Between Inflammatory Bowel Disease and Self-Reported Non-Celiac Gluten Sensitivity. Inflamm. Bowel Dis. 2015, 21, 847–853. [Google Scholar] [CrossRef]
- Casella, G.; D’Incà, R.; Oliva, L.; Daperno, M.; Saladino, V.; Zoli, G.; Annese, V.; Fries, W.; Cortellezzi, C. Prevalence of Celiac Disease in Inflammatory Bowel Diseases: An IG-IBD Multicentre Study. Dig. Liver Dis. 2010, 42, 175–178. [Google Scholar] [CrossRef]
- Cenni, S.; Sesenna, V.; Boiardi, G.; Casertano, M.; Russo, G.; Reginelli, A.; Esposito, S.; Strisciuglio, C. The Role of Gluten in Gastrointestinal Disorders: A Review. Nutrients 2023, 15, 1615. [Google Scholar] [CrossRef]
- Cottone, M.; Marrone, C.; Casà, A.; Oliva, L.; Orlando, A.; Calabrese, E.; Martorana, G.; Pagliaro, L. Familial Occurrence of Inflammatory Bowel Disease in Celiac Disease. Inflamm. Bowel Dis. 2003, 9, 321–323. [Google Scholar] [CrossRef]
- Halling, M.L.; Kjeldsen, J.; Knudsen, T.; Nielsen, J.; Hansen, L.K. Patients with Inflammatory Bowel Disease Have Increased Risk of Autoimmune and Inflammatory Diseases. WJG 2017, 23, 6137–6146. [Google Scholar] [CrossRef]
- Leeds, J.S.; Höroldt, B.S.; Sidhu, R.; Hopper, A.D.; Robinson, K.; Toulson, B.; Dixon, L.; Lobo, A.J.; McAlindon, M.E.; Hurlstone, D.P.; et al. Is There an Association between Coeliac Disease and Inflammatory Bowel Diseases? A Study of Relative Prevalence in Comparison with Population Controls. Scand. J. Gastroenterol. 2007, 42, 1214–1220. [Google Scholar] [CrossRef]
- Pinto-Sanchez, M.I.; Seiler, C.L.; Santesso, N.; Alaedini, A.; Semrad, C.; Lee, A.R.; Bercik, P.; Lebwohl, B.; Leffler, D.A.; Kelly, C.P.; et al. Association Between Inflammatory Bowel Diseases and Celiac Disease: A Systematic Review and Meta-Analysis. Gastroenterology 2020, 159, 884–903.e31. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Sillke, Y.; Schumann, M.; Lissner, D.; Branchi, F.; Proft, F.; Steinhoff, U.; Siegmund, B.; Glauben, R. Analysis of Circulating Food Antigen-Specific T-Cells in Celiac Disease and Inflammatory Bowel Disease. IJMS 2023, 24, 8153. [Google Scholar] [CrossRef]
- Tursi, A.; Giorgetti, G.M.; Brandimarte, G.; Elisei, W. High Prevalence of Celiac Disease among Patients Affected by Crohn’s Disease. Inflamm. Bowel Dis. 2005, 11, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Weaver, K.N.; Herfarth, H. Gluten-Free Diet in IBD: Time for a Recommendation? Mol. Nutr. Food Res. 2021, 65, 1901274. [Google Scholar] [CrossRef]
- Yang, A.; Chen, Y.; Scherl, E.; Neugut, A.I.; Bhagat, G.; Green, P.H.R. Inflammatory Bowel Disease in Patients with Celiac Disease. Inflamm. Bowel Dis. 2005, 11, 528–532. [Google Scholar] [CrossRef]
- Aziz, I.; Törnblom, H.; Simrén, M. Small Intestinal Bacterial Overgrowth as a Cause for Irritable Bowel Syndrome: Guilty or Not Guilty? Curr. Opin. Gastroenterol. 2017, 33, 196–202. [Google Scholar] [CrossRef]
- Fiori Nastro, F.; Serra, M.R.; Cenni, S.; Pacella, D.; Martinelli, M.; Miele, E.; Staiano, A.; Tolone, C.; Auricchio, R.; Strisciuglio, C. Prevalence of Functional Gastrointestinal Disorders in Children with Celiac Disease on Different Types of Gluten-Free Diets. World J. Gastroenterol. 2022, 28, 6589–6598. [Google Scholar] [CrossRef]
- Schiepatti, A.; Maimaris, S.; Lusetti, F.; Scalvini, D.; Minerba, P.; Cincotta, M.; Fazzino, E.; Biagi, F. High Prevalence of Functional Gastrointestinal Disorders in Celiac Patients with Persistent Symptoms on a Gluten-Free Diet: A 20-Year Follow-Up Study. Dig. Dis. Sci. 2023, 68, 3374–3382. [Google Scholar] [CrossRef]
- Evans, K.E.; Sanders, D.S. Symposium 1: Joint BAPEN and British Society of Gastroenterology Symposium on ‘Coeliac Disease: Basics and Controversies’ Coeliac Disease: Optimising the Management of Patients with Persisting Symptoms? Conference on ‘Malnutrition Matters’. Proc. Nutr. Soc. 2009, 68, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Barrio, J.; Román, E.; Cilleruelo, M.; Márquez, M.; Mearin, M.; Fernández, C. Health-Related Quality of Life in Spanish Children with Coeliac Disease. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Nikniaz, Z.; Abbasalizad Farhangi, M.; Nikniaz, L. Systematic Review with Meta-Analysis of the Health-Related Quality of Life in Children with Celiac Disease. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Bacigalupe, G.; Plocha, A. Celiac Is a Social Disease: Family Challenges and Strategies. Fam. Syst. Health 2015, 33, 46–54. [Google Scholar] [CrossRef]
- Nordyke, K.; Myléus, A.; Ivarsson, A.; Carlsson, A.; Danielsson, L.; Högberg, L.; Karlsson, E.; Emmelin, M. How Do Children Experience Participating in a Coeliac Disease Screening? A Qualitative Study Based on Children’s Written Narratives. Scand J. Public Health 2010, 38, 351–358. [Google Scholar] [CrossRef]
- Kurppa, K.; Collin, P.; Mäki, M.; Kaukinen, K. Celiac Disease and Health-Related Quality of Life. Expert Rev. Gastroenterol. Hepatol. 2011, 5, 83–90. [Google Scholar] [CrossRef]
- Nachman, F.; del Campo, M.P.; González, A.; Corzo, L.; Vázquez, H.; Sfoggia, C.; Smecuol, E.; Sánchez, M.I.P.; Niveloni, S.; Sugai, E.; et al. Long-Term Deterioration of Quality of Life in Adult Patients with Celiac Disease Is Associated with Treatment Noncompliance. Dig. Liver Dis. 2010, 42, 685–691. [Google Scholar] [CrossRef]
- van de Water, J.M.W.; Mulder, C.J.J. Assessment of Quality of Life. Nat. Rev. Gastroenterol. Hepatol. 2009, 6, 204–205. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.D.; Rodgers, C.; Watson, R.G. Quality of Life in Screen-Detected and Typical Coeliac Disease and the Effect of Excluding Dietary Gluten. Eur. J. Gastroenterol. Hepatol. 2004, 16, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Viljamaa, M.; Collin, P.; Huhtala, H.; Sievanen, H.; Maki, M.; Kaukinen, K. Is Coeliac Disease Screening in Risk Groups Justified? A Fourteen-Year Follow-up with Special Focus on Compliance and Quality of Life. Aliment. Pharmacol. Ther. 2005, 22, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Fueyo-Díaz, R.; Montoro, M.; Magallón-Botaya, R.; Gascón-Santos, S.; Asensio-Martínez, Á.; Palacios-Navarro, G.; Sebastián-Domingo, J.J. Influence of Compliance to Diet and Self-Efficacy Expectation on Quality of Life in Patients with Celiac Disease in Spain. Nutrients 2020, 12, 2672. [Google Scholar] [CrossRef]
- Sverker, A.; Hensing, G.; Hallert, C. ‘Controlled by Food’-Lived Experiences of Coeliac Disease. J. Hum. Nutr. Diet. 2005, 18, 171–180. [Google Scholar] [CrossRef]
- Rodríguez Almagro, J.; Rodríguez Almagro, D.; Solano Ruiz, C.; Siles González, J.; Hernández Martínez, A. The Experience of Living with a Gluten-Free Diet: An Integrative Review. Gastroenterol. Nurs. 2018, 41, 189–200. [Google Scholar] [CrossRef]
- Usai, P.; Minerba, L.; Marini, B.; Cossu, R.; Spada, S.; Carpiniello, B.; Cuomo, R.; Boy, M.F. Case Control Study on Health-Related Quality of Life in Adult Coeliac Disease. Dig. Liver Dis. 2002, 34, 547–552. [Google Scholar] [CrossRef]
- Hankey, G.L.; Holmes, G.K. Coeliac Disease in the Elderly. Gut 1994, 35, 65–67. [Google Scholar] [CrossRef]
- Haines, M.; Anderson, R.P.; Gibson, P.R. Systematic Review: The Evidence Base for Long-Term Management of Coeliac Disease. Aliment. Pharmacol. Ther. 2008, 28, 1042–1066. [Google Scholar] [CrossRef] [PubMed]
- Long, K.; Rubio-Tapia, A.; Wagie, A.; Melton Iii, L.; Lahr, B.; Van Dyke, C.; Murray, J. The Economics of Coeliac Disease: A Population-Based Study. Aliment. Pharmacol. Ther. 2010, 32, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Mędza, A.; Szlagatys-Sidorkiewicz, A. Nutritional Status and Metabolism in Celiac Disease: Narrative Review. JCM 2023, 12, 5107. [Google Scholar] [CrossRef]
- Simpson, S.; Thompson, T. Nutrition Assessment in Celiac Disease. Gastrointest. Endosc. Clin. 2012, 22, 797–809. [Google Scholar] [CrossRef] [PubMed]
- Dewar, D.H. Celiac Disease: Management of Persistent Symptoms in Patients on a Gluten-Free Diet. WJG 2012, 18, 1348. [Google Scholar] [CrossRef]
- Veeraraghavan, G.; Therrien, A.; Degroote, M.; McKeown, A.; Mitchell, P.D.; Silvester, J.A.; Leffler, D.A.; Leichtner, A.M.; Kelly, C.P.; Weir, D.C. Non-Responsive Celiac Disease in Children on a Gluten Free Diet. World J. Gastroenterol. 2021, 27, 1311. [Google Scholar] [CrossRef]
- Costas-Batlle, C.; Trott, N.; Jeanes, Y.; Seamark, L.; Gardiner, C. A Dietitian-Led Coeliac Service Helps to Identify and Reduce Involuntary Gluten Ingestion with Subsequent Reduction in the Frequency of Repeat Endoscopies. J. Hum. Nutr. Diet. 2023. [Google Scholar] [CrossRef]
- Mulholland, P.; Caddy, G.; Harding, T.; Nichol, A.; Power, N.; Prosser, J. Evaluation of Dietetic Led Coeliac Clinical Annual Review as a Safe and Cost-Effective Solution to Medical Review. J. Hum. Nutr. Diet. 2017, 30 (Suppl. S1), 41–43. [Google Scholar]
- Bebb, J.R.; Lawson, A.; Knight, T.; Long, R.G. Long-Term Follow-up of Coeliac Disease--What Do Coeliac Patients Want? Aliment. Pharmacol. Ther. 2006, 23, 827–831. [Google Scholar] [CrossRef] [PubMed]
- Trott, N.; Raju, S.A.; Rej, A.; Hoffman, O.; Holland, W.; Bebb, J.R.; Seamark, L.; Williams, M.; Costas Batlle, C.; Jeanes, Y.M.; et al. Long-Term Follow-up in Patients with Coeliac Disease in the Pandemic-Era: A View from Sheffield the NHS England National Centre for Adult Coeliac Disease: Follow up Preferences in Adult Coeliac Disease. Gastroenterol. Hepatol. Bed Bench 2023, 16, 158–166. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simón, E.; Molero-Luis, M.; Fueyo-Díaz, R.; Costas-Batlle, C.; Crespo-Escobar, P.; Montoro-Huguet, M.A. The Gluten-Free Diet for Celiac Disease: Critical Insights to Better Understand Clinical Outcomes. Nutrients 2023, 15, 4013. https://doi.org/10.3390/nu15184013
Simón E, Molero-Luis M, Fueyo-Díaz R, Costas-Batlle C, Crespo-Escobar P, Montoro-Huguet MA. The Gluten-Free Diet for Celiac Disease: Critical Insights to Better Understand Clinical Outcomes. Nutrients. 2023; 15(18):4013. https://doi.org/10.3390/nu15184013
Chicago/Turabian StyleSimón, Edurne, Marta Molero-Luis, Ricardo Fueyo-Díaz, Cristian Costas-Batlle, Paula Crespo-Escobar, and Miguel A. Montoro-Huguet. 2023. "The Gluten-Free Diet for Celiac Disease: Critical Insights to Better Understand Clinical Outcomes" Nutrients 15, no. 18: 4013. https://doi.org/10.3390/nu15184013
APA StyleSimón, E., Molero-Luis, M., Fueyo-Díaz, R., Costas-Batlle, C., Crespo-Escobar, P., & Montoro-Huguet, M. A. (2023). The Gluten-Free Diet for Celiac Disease: Critical Insights to Better Understand Clinical Outcomes. Nutrients, 15(18), 4013. https://doi.org/10.3390/nu15184013








