Use of Peak Glucose Level and Peak Glycemic Gap in Mortality Risk Stratification in Critically Ill Patients with Sepsis and Prior Diabetes Mellitus of Different Body Mass Indexes
Abstract
1. Background
2. Methods
2.1. Subjects and Study Design
2.2. Definitions and Criteria
2.3. Data Collection
2.4. Statistical Methods
3. Results
3.1. Baseline Characteristics of Patients with Sepsis, Stratified by HbA1c
3.2. Baseline Characteristics of Patients with Sepsis, Stratified by Peak Blood Glucose
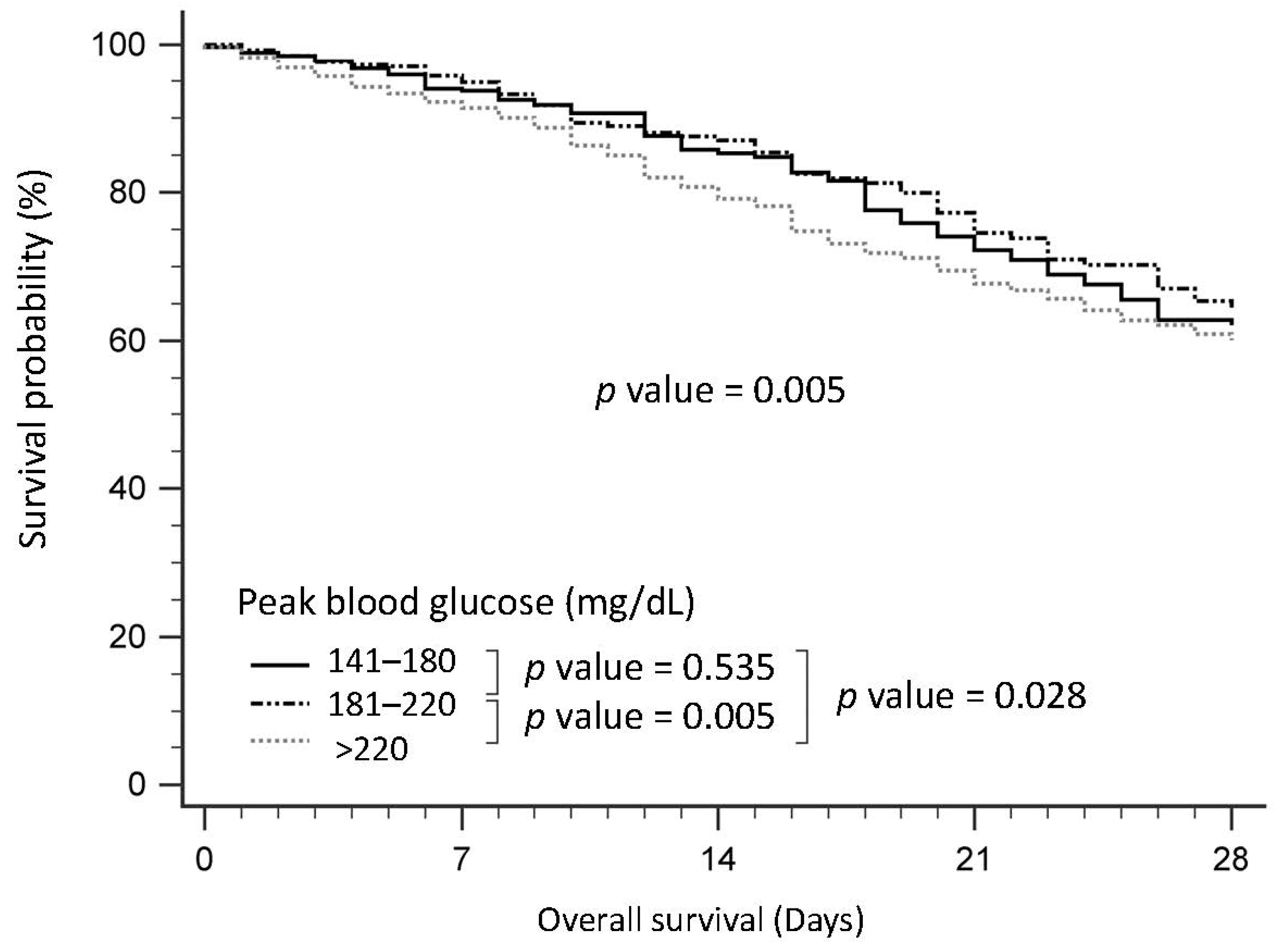
3.3. Survival of Patients with Sepsis, Stratified by Peak Blood Glucose
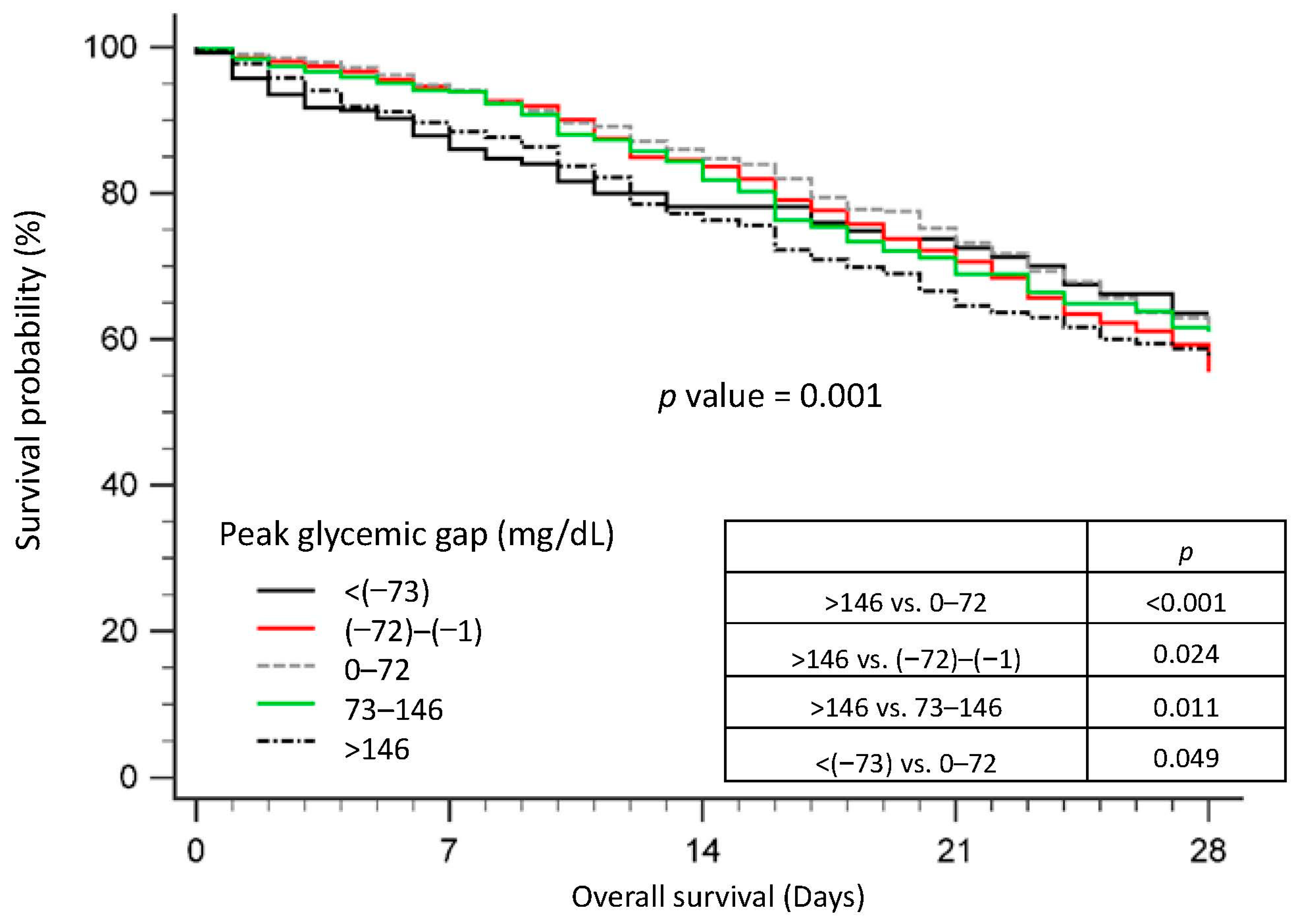
3.4. Survival of Patients with Sepsis, Stratified by the Peak Glycemic Gap
3.5. Prediction of Mortality in Patients with Sepsis Using Modified SOFA-pg and Modified SOFA-pgg Scores
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Plummer, M.P.; Deane, A.M. Dysglycemia and Glucose Control During Sepsis. Clin. Chest Med. 2016, 37, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonca, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Ingelfinger, J.R.; Jarcho, J.A. Increase in the Incidence of Diabetes and Its Implications. N. Engl. J. Med. 2017, 376, 1473–1474. [Google Scholar] [CrossRef]
- Stegenga, M.E.; Vincent, J.L.; Vail, G.M.; Xie, J.; Haney, D.J.; Williams, M.D.; Bernard, G.R.; van der Poll, T. Diabetes does not alter mortality or hemostatic and inflammatory responses in patients with severe sepsis. Crit. Care Med. 2010, 38, 539–545. [Google Scholar] [CrossRef]
- van Vught, L.A.; Holman, R.; de Jonge, E.; de Keizer, N.F.; van der Poll, T. Diabetes Is Not Associated With Increased 90-Day Mortality Risk in Critically Ill Patients With Sepsis. Crit. Care Med. 2017, 45, e1026–e1035. [Google Scholar] [CrossRef]
- Esper, A.M.; Moss, M.; Martin, G.S. The effect of diabetes mellitus on organ dysfunction with sepsis: An epidemiological study. Crit. Care 2009, 13, R18. [Google Scholar] [CrossRef]
- Schuetz, P.; Castro, P.; Shapiro, N.I. Diabetes and sepsis: Preclinical findings and clinical relevance. Diabetes Care 2011, 34, 771–778. [Google Scholar] [CrossRef]
- Hsieh, M.S.; Hu, S.Y.; How, C.K.; Seak, C.J.; Hsieh, V.C.; Lin, J.W.; Chen, P.-C. Hospital outcomes and cumulative burden from complications in type 2 diabetic sepsis patients: A cohort study using administrative and hospital-based databases. Ther. Adv. Endocrinol. Metab. 2019, 10, 2042018819875406. [Google Scholar] [CrossRef]
- Trevelin, S.C.; Carlos, D.; Beretta, M.; da Silva, J.S.; Cunha, F.Q. Diabetes Mellitus and Sepsis: A Challenging Association. Shock 2017, 47, 276–287. [Google Scholar] [CrossRef]
- Wong, C.K.; Ho, A.W.; Tong, P.C.; Yeung, C.Y.; Chan, J.C.; Kong, A.P.; Lam, C.W. Aberrant expression of soluble co-stimulatory molecules and adhesion molecules in type 2 diabetic patients with nephropathy. J. Clin. Immunol. 2008, 28, 36–43. [Google Scholar] [CrossRef]
- Ali, N.A.; O’Brien, J.M., Jr.; Dungan, K.; Phillips, G.; Marsh, C.B.; Lemeshow, S.; Connors, A.F.; Preiser, J.-C. Glucose variability and mortality in patients with sepsis. Crit. Care Med. 2008, 36, 2316–2321. [Google Scholar] [CrossRef] [PubMed]
- Cherkas, A.; Holota, S.; Mdzinarashvili, T.; Gabbianelli, R.; Zarkovic, N. Glucose as a major antioxidant: When, what for and why it fails? Antioxidants 2020, 9, 140. [Google Scholar] [CrossRef] [PubMed]
- Hung, K.-Y.; Tsai, Y.-H.; Lin, C.-Y.; Chang, Y.-C.; Wang, Y.-H.; Lin, M.-C.; Fang, W.-F. Application of Peak Glucose Range and Diabetes Status in Mortality Risk Stratification in Critically Ill Patients with Sepsis. Diagnostics 2021, 11, 1798. [Google Scholar] [CrossRef] [PubMed]
- Cely, C.M.; Arora, P.; Quartin, A.A.; Kett, D.H.; Schein, R.M. Relationship of baseline glucose homeostasis to hyperglycemia during medical critical illness. Chest 2004, 126, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.-I.; Wang, J.-C.; Chang, W.-C.; Hsu, C.-W.; Chu, C.-M.; Tsai, S.-H. Usefulness of glycemic gap to predict ICU mortality in critically ill patients with diabetes. Medicine 2015, 94, e1525. [Google Scholar] [CrossRef]
- Bellaver, P.; Schaeffer, A.F.; Dullius, D.P.; Viana, M.V.; Leitão, C.B.; Rech, T.H. Association of multiple glycemic parameters at intensive care unit admission with mortality and clinical outcomes in critically ill patients. Sci. Rep. 2019, 9, 18498. [Google Scholar] [CrossRef]
- Chen, P.-C.; Tsai, S.-H.; Wang, J.-C.; Tzeng, Y.-S.; Wang, Y.-C.; Chu, C.-M.; Chu, S.-J.; Liao, W.-I. An elevated glycemic gap predicts adverse outcomes in diabetic patients with necrotizing fasciitis. PLoS ONE 2019, 14, e0223126. [Google Scholar] [CrossRef]
- Fong, K.M.; Au, S.Y.; Ng, G.W.Y. Glycemic control in critically ill patients with or without diabetes. BMC Anesthesiol. 2022, 22, 227. [Google Scholar] [CrossRef]
- Liao, W.-I.; Sheu, W.H.-H.; Chang, W.-C.; Hsu, C.-W.; Chen, Y.-L.; Tsai, S.-H. An elevated gap between admission and A1C-derived average glucose levels is associated with adverse outcomes in diabetic patients with pyogenic liver abscess. PLoS ONE 2013, 8, e64476. [Google Scholar] [CrossRef]
- Kosiborod, M.; Rathore, S.S.; Inzucchi, S.E.; Masoudi, F.A.; Wang, Y.; Havranek, E.P.; Krumholz, H.M. Admission glucose and mortality in elderly patients hospitalized with acute myocardial infarction: Implications for patients with and without recognized diabetes. Circulation 2005, 111, 3078–3086. [Google Scholar] [CrossRef]
- Tsai, Y.-H.; Lin, C.-Y.; Chen, Y.-M.; Chang, Y.-P.; Hung, K.-Y.; Chang, Y.-C.; Chen, H.C.; Huang, K.-T.; Chen, Y.C.; Wang, Y.H.; et al. Impact of Body Mass Index on the Survival of Patients with Sepsis with Different Modified NUTRIC Scores. Nutrients 2021, 13, 1873. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.C.; Chan, Y.Y.; Kao Yang, Y.H.; Lin, S.J.; Hung, M.J.; Chien, R.N.; Lai, C.; Lai, E.C. The Chang Gung Research Database—A multi-institutional electronic medical records database for real-world epidemiological studies in Taiwan. Pharmacoepidemiol. Drug Saf. 2019, 28, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.N.; Chen, Y.T.; Chu, H.; Shih, C.J.; Ou, S.M.; Hsu, Y.T.; Chen, R.-C.; Quraishi, S.A.; Aisiku, I.P.; Seethala, R.R.; et al. Association of pre-hospital theophylline use and mortality in chronic obstructive pulmonary disease patients with sepsis. Respir. Med. 2017, 125, 33–38. [Google Scholar] [CrossRef]
- Nathan, D.M.; Kuenen, J.; Borg, R.; Zheng, H.; Schoenfeld, D.; Heine, R.J.; A1c-Derived Average Glucose (ADAG) Study Group. Translating the A1C assay into estimated average glucose values. Diabetes Care 2008, 31, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- Seymour, C.W.; Liu, V.X.; Iwashyna, T.J.; Brunkhorst, F.M.; Rea, T.D.; Scherag, A.; Rubenfeld, G.; Kahn, J.M.; Shankar-Hari, M.; Singer, M.; et al. Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 762–774. [Google Scholar] [CrossRef]
- Schuler, A.; Wulf, D.A.; Lu, Y.; Iwashyna, T.J.; Escobar, G.J.; Shah, N.H.; Liu, V.X. The Impact of Acute Organ Dysfunction on Long-Term Survival in Sepsis. Crit. Care Med. 2018, 46, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Raith, E.P.; Udy, A.A.; Bailey, M.; McGloughlin, S.; MacIsaac, C.; Bellomo, R.; Pilcher, D.V.; Australian and New Zealand Intensive Care Society (ANZICS) Centre for Outcomes and Resource Evaluation (CORE). Prognostic Accuracy of the SOFA Score, SIRS Criteria, and qSOFA Score for In-Hospital Mortality Among Adults with Suspected Infection Admitted to the Intensive Care Unit. JAMA 2017, 317, 290–300. [Google Scholar] [CrossRef]
- Chao, W.-C.; Tseng, C.-H.; Wu, C.-L.; Shih, S.-J.; Yi, C.-Y.; Chan, M.-C. Higher glycemic variability within the first day of ICU admission is associated with increased 30-day mortality in ICU patients with sepsis. Ann. Intensive Care 2020, 10, 17. [Google Scholar] [CrossRef]
- Wang, J.; Yan, R.; Wen, J.; Kong, X.; Li, H.; Zhou, P.; Zhu, H.; Su, X.; Ma, J. Association of lower body mass index with increased glycemic variability in patients with newly diagnosed type 2 diabetes: A cross-sectional study in China. Oncotarget 2017, 8, 73133. [Google Scholar] [CrossRef]
- Nienow, M.K.; Foley, S.P.; Nowak, K.L.; Braunschweig, C.A.; Peterson, S.J. Relationship between blood glucose variability and muscle composition in ICU patients receiving nutrition support: A pilot study. Clin. Nutr. ESPEN 2021, 46, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Hung, K.-Y.; Chen, T.-H.; Lee, Y.-F.; Fang, W.-F. Using Body Composition Analysis for Improved Nutritional Intervention in Septic Patients: A Prospective Interventional Study. Nutrients 2023, 15, 3814. [Google Scholar] [CrossRef] [PubMed]
- Clain, J.; Ramar, K.; Surani, S.R. Glucose control in critical care. World J. Diabetes 2015, 6, 1082. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, J.; Bircher, N.; Krinsley, J.; Agus, M.; Braithwaite, S.S.; Deutschman, C.; Freire, A.X.; Geehan, D.; Kohl, B.; Nasraway, S.A.; et al. Guidelines for the use of an insulin infusion for the management of hyperglycemia in critically ill patients. Crit. Care Med. 2012, 40, 3251–3276. [Google Scholar] [CrossRef]
- Investigators, N.-S.S. Intensive versus conventional glucose control in critically ill patients. N. Engl. J. Med. 2009, 360, 1283–1297. [Google Scholar]
- Luethi, N.; Cioccari, L.; Crisman, M.; Bellomo, R.; Eastwood, G.M.; Mårtensson, J. Prevalence of ketosis, ketonuria, and ketoacidosis during liberal glycemic control in critically ill patients with diabetes: An observational study. Crit. Care 2016, 20, 297. [Google Scholar] [CrossRef][Green Version]
- Kar, P.; Plummer, M.P.; Bellomo, R.; Jenkins, A.J.; Januszewski, A.S.; Chapman, M.J.; Jones, K.L.; Horowitz, M.; Deane, A.M. Liberal glycemic control in critically ill patients with type 2 diabetes: An exploratory study. Crit. Care Med. 2016, 44, 1695–1703. [Google Scholar] [CrossRef]
- Yao, Y.; Zhao, Y.-H.; Zheng, W.-H.; Huang, H.-B. Subcutaneous continuous glucose monitoring in critically ill patients during insulin therapy: A meta-analysis. Am. J. Transl. Res. 2022, 14, 4757. [Google Scholar]
- Marik, P.E.; Raghavan, M. Stress-hyperglycemia, insulin and immunomodulation in sepsis. Intensive Care Med. 2004, 30, 748–756. [Google Scholar] [CrossRef]
- Plummer, M.P.; Bellomo, R.; Cousins, C.E.; Annink, C.E.; Sundararajan, K.; Reddi, B.A.; Raj, J.P.; Chapman, M.J.; Horowitz, M.; Deane, A.M. Dysglycaemia in the critically ill and the interaction of chronic and acute glycaemia with mortality. Intensive Care Med. 2014, 40, 973–980. [Google Scholar] [CrossRef]
- Jiang, Y.-D.; Chang, C.-H.; Tai, T.-Y.; Chen, J.-F.; Chuang, L.-M. Incidence and prevalence rates of diabetes mellitus in Taiwan: Analysis of the 2000–2009 Nationwide Health Insurance database. J. Formos. Med. Assoc. 2012, 111, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Reaven, P.D.; Emanuele, N.V.; Wiitala, W.L.; Bahn, G.D.; Reda, D.J.; McCarren, M.; Duckworth, W.C.; Hayward, R.A. Intensive Glucose Control in Patients with Type 2 Diabetes—15-Year Follow-up. N. Engl. J. Med. 2019, 380, 2215–2224. [Google Scholar] [CrossRef]
- Finfer, S.; Liu, B.; Chittock, D.R.; Norton, R.; Myburgh, J.A.; McArthur, C.; The NICE-SUGAR Study Investigators. Hypoglycemia and risk of death in critically ill patients. N. Engl. J. Med. 2012, 367, 1108–1118. [Google Scholar] [PubMed]
- Lanspa, M.J.; Krinsley, J.S.; Hersh, A.M.; Wilson, E.L.; Holmen, J.R.; Orme, J.F.; Morris, A.H.; Hirshberg, E.L. Percentage of Time in Range 70 to 139 mg/dL Is Associated With Reduced Mortality Among Critically Ill Patients Receiving IV Insulin Infusion. Chest 2019, 156, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Cismondi, F.; Fialho, A.S.; Vieira, S.M.; Reti, S.R.; Sousa, J.M.; Finkelstein, S.N. Missing data in medical databases: Impute, delete or classify? Artif. Intell. Med. 2013, 58, 63–72. [Google Scholar] [CrossRef]
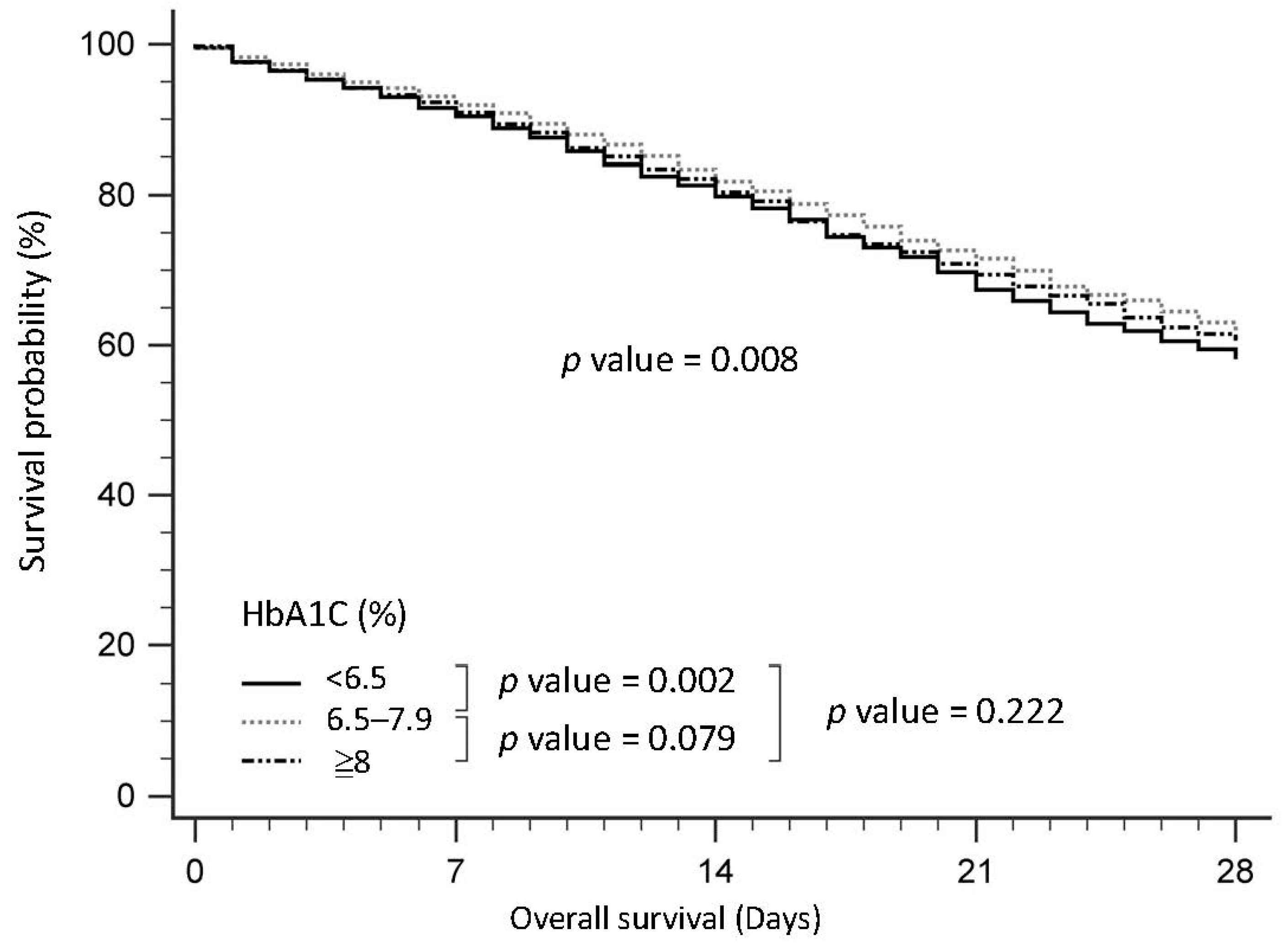
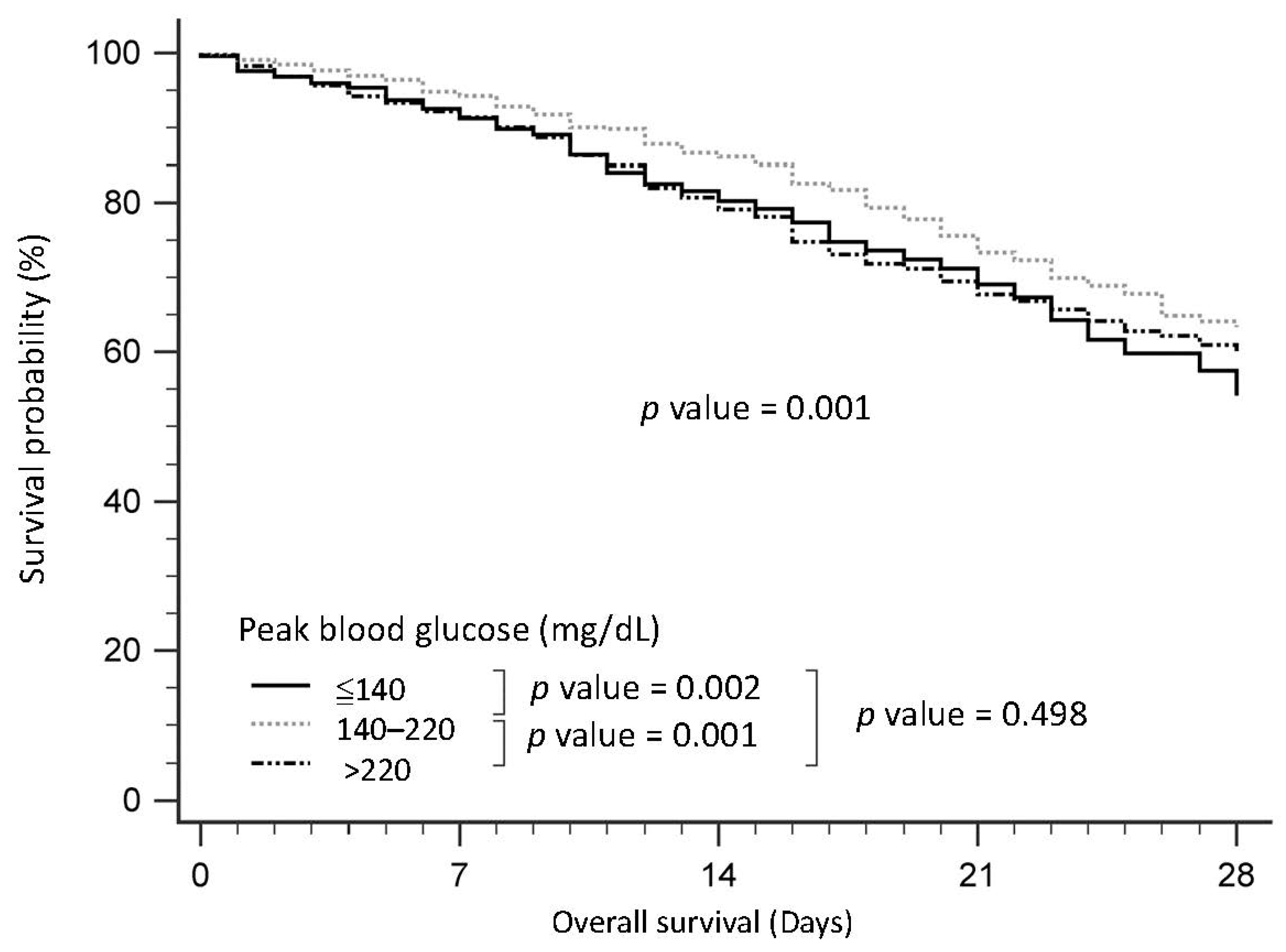

| HbA1c < 6.5% | 6.5≤ HbA1c < 8% | HbA1c ≥ 8% | p | Glucose ≤ 140 (mg/dL) | 140 < Glucose ≤ 220 (mg/dL) | Glucose > 220 (mg/dL) | p | |
|---|---|---|---|---|---|---|---|---|
| n = 4847 | n = 6050 | n = 4987 | n = 998 | n = 1740 | n = 2647 | |||
| Age, years | 67.2 (58.3–75.3) | 65.5 (56.9–73.6) | 60.9 (52.3–69.4) | <0.001 | 63.8 (54.5–73.3) | 63.8 (55.5–73.2) | 61.7 (53.0–70.5) | <0.001 |
| BMI, kg/m2 | 23.3 (20.5–26.4) | 24.4 (21.7–27.4) | 24.6 (22.1–27.6) | <0.001 | 24.2 (21.6–27.4) | 24.7 (22.0–27.8) | 24.3 (21.8–27.2) | 0.006 |
| Gender (F), % | 2088 (43.1%) | 2490 (41.2%) | 1999 (40.1%) | 0.009 | 328 (32.9%) | 567 (32.6%) | 1068 (40.3%) | <0.001 |
| CCI score | 4.0 (2.0–6.0) | 3.0 (2.0–5.0) | 3.0 (2.0–5.0) | <0.001 | 3.0 (2.0–5.0) | 3.0 (2.0–5.0) | 3.0 (2.0–5.0) | 0.023 |
| DCSI | 4.0 (2.0–6.0) | 4.0 (2.0–6.0) | 4.0 (2.0–6.0) | 0.201 | 3.0 (2.0–5.0) | 3.0 (2.0–6.0) | 4.0 (2.0–6.0) | <0.001 |
| Pneumonia | 1990 (41.1%) | 2338 (38.6%) | 1685 (33.8%) | <0.001 | 289 (29.0%) | 510 (29.3%) | 833 (32.5%) | 0.185 |
| UTI | 989 (20.4%) | 1160 (19.2%) | 868 (17.4%) | 0.001 | 162 (16.2%) | 230 (13.2%) | 427 (16.1%) | 0.019 |
| Vasopressor | 1156 (23.8%) | 1369 (22.6%) | 1086 (21.8%) | 0.048 | 215 (21.5%) | 266 (15.3%) | 585 (22.1%) | <0.001 |
| Ventilator | 2706 (55.8%) | 3288 (54.3%) | 2489 (49.9%) | <0.001 | 420 (42.1%) | 701 (40.3%) | 1401 (52.9%) | <0.001 |
| HD | 1224 (25.3%) | 2339 (20.3%) | 856 (17.2%) | <0.001 | 191 (19.1%) | 264 (15.2%) | 467 (17.6%) | 0.018 |
| 1st Day Glucose, mg/dL | 159.5 (120.0–212.8) | 203.0 (154.0–271.0) | 299.5 (213.0–416.0) | <0.001 | 113.0 (92.0–127.0) | 175.0 (157.0–197.0) | 314.0 (256.0–418.0) | <0.001 |
| Peak Glucose, mg/dL | 163.0 (123.0–216.8) | 207.0 (158.0–280.0) | 307.0 (220.0–432.0) | <0.001 | 103.0 (93.0–128.0) | 178.0 (160.0–198.0) | 320.0 (262.0–432.0) | <0.001 |
| Day1 | ||||||||
| 1st Day APACHE II | 17.0 (13.0–22.0) | 16.0 (12.0–21.0) | 15.0 (10.0–20.0) | <0.001 | 15.0 (10.0–20.0) | 14.0 (10.0–19.0) | 16.0 (11.0–21.0) | <0.001 |
| 1st Day Temperature, °C | 36.5 (36.0–37.1) | 36.6 (36.1–37.2) | 36.6 (36.1–37.2) | <0.001 | 36.6 (36.0–37.1) | 36.6 (36.0–37.1) | 36.6 (36.0–37.2) | 0.974 |
| 1st Day SBP, mmHg | 135.0 (114.0–156.0) | 136.0 (115.0–155.0) | 134.0 (114.0–154.0) | 0.095 | 137.0 (113.0–157.0) | 138.0 (118.0–157.0) | 132.0 (113.0–154.0) | <0.001 |
| 1st Day DBP, mmHg | 72.0 (60.0–84.0) | 72.0 (61.0–84.0) | 73.0 (62.0–86.0) | 0.005 | 73.0 (62.0–85.0) | 74.0 (63.0–86.0) | 73.0 (60.0–86.0) | 0.03 |
| 1st Day Pulse Pressure, mmHg | 60.0 (46.0–79.0) | 61.0 (46.0–77.0) | 59.0 (44.0–75.0) | <0.001 | 60.0 (46.0–78.0) | 61.0 (46.0–79.0) | 58.0 (44.0–74.0) | <0.001 |
| 1st Day WBC, 1000/μL | 10.4 (7.5–14.2) | 10.8 (8.0–14.7) | 11.1 (8.3–14.9) | <0.001 | 9.6 (7.2–13.1) | 10.2 (7.8–13.1) | 11.9 (8.7–15.8) | <0.001 |
| 1st Day qSOFA | 1.0 (1.0–2.0) | 1.0 (0.0–2.0) | 1.0 (0.0–2.0) | 0.013 | 1.0 (0.0–1.0) | 1.0 (0.0–1.0) | 1.0 (0.0–2.0) | <0.001 |
| 1st Day SOFA | 5.0 (3.0–7.0) | 4.0 (2.0–6.0) | 4.0 (2.0–7.0) | 0.010 | 5.0 (3.0–7.0) | 4.0 (2.0–6.0) | 5.0 (3.0–7.0) | <0.001 |
| Day3 | n = 4678 | n = 5891 | n = 4816 | n = 967 | n = 1714 | n = 2565 | ||
| 3rd Day APACHE II | 15.0 (11.0–20.0) | 14.0 (10.0–19.0) | 13.0 (9.0–17.0) | <0.001 | 13.0 (9.0–18.0) | 12.0 (9.0–17.0) | 13.0 (9.0–18.0) | <0.001 |
| 3rd Day Temperature, °C | 36.5 (36.0–37.1) | 36.6 (36.1–37.1) | 36.6 (36.1–37.1) | <0.001 | 36.5 (36.0–37.0) | 36.5 (36.0–37.0) | 36.6 (36.1–37.2) | 0.02 |
| 3rd Day SBP, mmHg | 128.0 (110.0–147.0) | 128.0 (111.0–147.0) | 126.0 (109.8–145.0) | <0.001 | 126.0 (109.0–144.0) | 126.0 (110.0–146.0) | 124.0 (109.0–143.0) | 0.035 |
| 3rd Day DBP, mmHg | 64.0 (55.0–75.0) | 65.0 (55.0–75.0) | 66.0 (56.0–77.0) | <0.001 | 65.0 (56.0–76.0) | 65.0 (56.0–76.0) | 64.5 (55.0–75.0) | 0.122 |
| 3rd Day Pulse Pressure, mmHg | 62.0 (49.0–78.0) | 62.0 (49.0–78.0) | 60.0 (46.0–75.0) | <0.001 | 59.0 (47.0–76.0) | 60.0 (46.0–76.0) | 60.0 (46.0–75.0) | 0.472 |
| 3rd Day WBC, 1000/μL | 5.9 (5.6–6.2) | 7.1 (6.7–7.4) | 9.3 (8.5–10.6) | <0.001 | 6.4 (5.8–7.2) | 6.8 (6.1–7.7) | 7.9 (6.9–9.7) | <0.001 |
| 3rd Day qSOFA | 1.0 (1.0–2.0) | 1.0 (1.0–2.0) | 1.0 (1.0–2.0) | 0.204 | 1.0 (1.0–1.0) | 1.0 (0.0–1.0) | 1.0 (1.0–2.0) | <0.001 |
| 3rd Day SOFA | 6.0 (4.0–8.0) | 5.0 (3.0–8.0) | 5.0 (3.0–8.0) | <0.001 | 7.0 (4.0–9.0) | 5.0 (4.0–8.0) | 6.0 (3.0–8.0) | 0.023 |
| Mortality | ||||||||
| 7 days | 373 (7.7%) | 385 (6.4%) | 353 (7.1%) | 0.025 | 64 (6.4%) | 66 (3.8%) | 175 (6.6%) | <0.001 |
| 28 days | 917 (18.9%) | 958 (15.8%) | 783 (15.7%) | <0.001 | 165 (16.5%) | 179 (10.3%) | 398 (15.0%) | <0.001 |
| 90 days | 1217 (26.3%) | 1375 (22.7%) | 1059 (21.2%) | <0.001 | 215 (21.5%) | 246 (14.1%) | 524 (19.8%) | <0.001 |
| Hospital Mortality | 1293 (26.7%) | 1405 (23.2%) | 1074 (21.5%) | <0.001 | 221 (22.1%) | 250 (14.4%) | 533 (20.1%) | <0.001 |
| Peak Glucose Level (Pg), mg/dL | 140 < Pg ≤ 180 (n = 998) | 180 <Pg ≤ 220 (n = 1470) | Pg > 220 (n = 2647) | p |
|---|---|---|---|---|
| Age, years | 64.0 (55.6–73.4) | 63.6 (55.4–72.8) | 61.7 (53.0–70.5) | <0.001 |
| BMI, kg/m2 | 24.6 (22.0–27.4) | 24.9 (22.1–28.4) | 24.3 (21.8–27.2) | 0.002 |
| Gender(F), % | 287 (30.9%) | 280 (34.5%) | 1068 (40.3%) | <0.001 |
| CCI_score | 3.0 (2.0–5.0) | 3.0 (2.0–5.0) | 3.0 (2.0–5.0) | 0.022 |
| DCSI | 3.0 (2.0–6.0) | 3.0 (2.0–6.0) | 4.0 (2.0–6.0) | 0.014 |
| Pneumonia | 273 (29.4%) | 237 (29.2%) | 833 (31.5%) | 0.315 |
| UTI | 112 (12.1%) | 118 (14.5%) | 427 (16.1%) | 0.011 |
| Vasopressor | 138 (14.9%) | 128 (15.8%) | 586 (22.1%) | <0.001 |
| Ventilator | 351 (37.8%) | 350 (43.2%) | 1401 (52.9%) | <0.001 |
| HD | 143 (15.4%) | 121 (14.9%) | 467 (17.6%) | 0.096 |
| 1st Day Glucose, mg/dL | 160.0 (149.0–170.0) | 199.0 (187.6–209.0) | 314.0 (256.0–418.0) | <0.001 |
| Peak Glucose, mg/dL | 161.0 (151.0–170.0) | 200.0 (190.0–210.0) | 320.0 (262.0–432.0) | <0.001 |
| Day 1 | ||||
| 1st Day APACHE II | 14.0 (10.0–19.0) | 14.0 (10.0–19.0) | 16.0 (11.0–21.0) | <0.001 |
| 1st Day Temperature,°C | 36.6 (36.0–37.1) | 36.5 (36.0–37.1) | 36.6 (36.0–37.2) | 0.972 |
| 1st Day SBP, mmHg | 139.0 (119.0–158.0) | 138.0 (116.0–157.0) | 132.0 (113.0–154.0) | <0.001 |
| 1st Day DBP, mmHg | 75.0 (63.0–87.0) | 74.0 (63.0–85.0) | 73.0 (60.0–86.0) | 0.016 |
| 1st Day Pulse pressure, mmHg | 61.0 (48.0–78.0) | 60.0 (44.8–79.0) | 58.0 (44.0–74.0) | <0.001 |
| 1st Day WBC, 1000/μL | 9.9 (7.6–12.6) | 10.5 (8.2–13.6) | 11.9 (8.7–15.8) | <0.001 |
| 1st Day qSOFA | 1.0 (0.0–1.0) | 1.0 (0.0–1.0) | 1.0 (0.0–2.0) | <0.001 |
| 1st Day SOFA | 4.0 (2.0–6.0) | 4.0 (2.0–6.0) | 5.0 (3.0–7.0) | <0.001 |
| Day 3 | n = 915 | n = 799 | n = 2565 | |
| 3rd Day APACHE II | 12.0 (9.0–17.0) | 13.0 (9.0–17.0) | 13.0 (9.0–18.0) | <0.001 |
| 3rd Day Temperature,°C | 36.6 (36.0–37.0) | 36.5 (36.0–37.0) | 36.6 (36.1–37.2) | 0.332 |
| 3rd Day SBP, mmHg | 129.0 (110.0–148.0) | 124.0 (110.0–144.0) | 124.0 (109.0–143.0) | 0.006 |
| 3rd Day DBP, mmHg | 65.0 (56.0–78.0) | 65.0 (55.0–74.0) | 64.5 (55.0–75.0) | 0.015 |
| 3rd Day Pulse pressure, mmHg | 61.0 (46.0–76.0) | 60.0 (46.0–75.0) | 60.0 (46.0–75.0) | 0.473 |
| 3rd Day WBC, 1000/μL | 6.6 (6.0–7.4) | 7.0 (6.2–7.9) | 7.9 (6.9–9.7) | <0.001 |
| 3rd Day qSOFA | 1.0 (0.0–1.0) | 1.0 (1.0–2.0) | 1.0 (1.0–2.0) | <0.001 |
| 3rd Day SOFA | 6.0 (4.0–9.0) | 5.0 (4.0–7.0) | 6.0 (3.0–8.0) | 0.236 |
| Mortality | ||||
| 7 days | 38 (4.1%) | 28 (3.5%) | 175 (6.6%) | <0.001 |
| 28 days | 97 (10.4%) | 82 (10.1%) | 398 (15.0%) | <0.001 |
| 90 days | 133 (14.3%) | 113 (13.9%) | 524 (19.8%) | <0.001 |
| Hospital mortality | 133 (14.3%) | 117 (14.4%) | 533 (20.1%) | <0.001 |
| SOFA Day 3 | mSOFA-pg (Peak Glucose) | mSOFA-pgg (Peak Glucose Gap) | |
|---|---|---|---|
| 28-day mortality OR (p); r2 | 1.277 (<0.001); 0.166 | 1.288 (<0.000); 0.209 | 1.286 (<0.001); 0.215 |
| 90-day mortality | 1.307 (<0.001); 0.199 | 1.314 (<0.001); 0.245 | 1.301 (<0.001); 0.241 |
| mNUTRIC score < 6 | |||
| 28-day mortality OR (p); r2 | 1.231 (<0.001); 0.129 | 1.215 (<0.000); 0.129 | 1.204 (<0.001); 1.130 |
| 90-day mortality | 1.233 (<0.001); 0.138 | 1.213 (<0.000);0.135 | 1.218 (<0.001); 0.152 |
| mNUTRIC score ≥ 6 | |||
| 28-day mortality OR (p); r2 | 1.745 (<0.001); 0.407 | 1.689 (<0.000); 0.428 | 1.576 (<0.001); 1.375 |
| 90-day mortality | 1.524 (0.001); 0.303 | 1.494 (0.001); 0.328 | 1.359 (0.002); 0.236 |
| BMI < 18.5 | |||
| 28-day mortality OR (p); r2 | 1.304 (<0.001); 0.205 | 1.413 (0.004); 0.377 | 1.364 (0.007); 0.330 |
| 90-day mortality | 1.376 (<0.001); 0.255 | 1.356 (0.006); 0.325 | 1.287 (0.014); 0.253 |
| 18.5 ≤ BMI < 25 | |||
| 28-day mortality OR (p); r2 | 1.266 (<0.001); 0.148 | 1.242 (<0.001); 0.163 | 1.220 (<0.001); 0.149 |
| 90-day mortality | 1.294 (<0.001); 0.180 | 1.288 (<0.001); 0.221 | 1.265 (<0.001); 0.207 |
| BMI ≥ 25 | |||
| 28-day mortality OR (p); r2 | 1.310 (<0.001); 0201 | 1.347 (<0.001); 0.258 | 1.398 (<0.001); 0.310 |
| 90-day mortality | 1.343 (<0.001); 0.237 | 1.365 (<0.001); 0.283 | 1.378 (<0.001); 0.305 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, Y.-H.; Hung, K.-Y.; Fang, W.-F. Use of Peak Glucose Level and Peak Glycemic Gap in Mortality Risk Stratification in Critically Ill Patients with Sepsis and Prior Diabetes Mellitus of Different Body Mass Indexes. Nutrients 2023, 15, 3973. https://doi.org/10.3390/nu15183973
Tsai Y-H, Hung K-Y, Fang W-F. Use of Peak Glucose Level and Peak Glycemic Gap in Mortality Risk Stratification in Critically Ill Patients with Sepsis and Prior Diabetes Mellitus of Different Body Mass Indexes. Nutrients. 2023; 15(18):3973. https://doi.org/10.3390/nu15183973
Chicago/Turabian StyleTsai, Yi-Hsuan, Kai-Yin Hung, and Wen-Feng Fang. 2023. "Use of Peak Glucose Level and Peak Glycemic Gap in Mortality Risk Stratification in Critically Ill Patients with Sepsis and Prior Diabetes Mellitus of Different Body Mass Indexes" Nutrients 15, no. 18: 3973. https://doi.org/10.3390/nu15183973
APA StyleTsai, Y.-H., Hung, K.-Y., & Fang, W.-F. (2023). Use of Peak Glucose Level and Peak Glycemic Gap in Mortality Risk Stratification in Critically Ill Patients with Sepsis and Prior Diabetes Mellitus of Different Body Mass Indexes. Nutrients, 15(18), 3973. https://doi.org/10.3390/nu15183973