Causal Link between Gut Microbiota, Neurophysiological States, and Bone Diseases: A Comprehensive Mendelian Randomization Study
Abstract
:1. Introduction
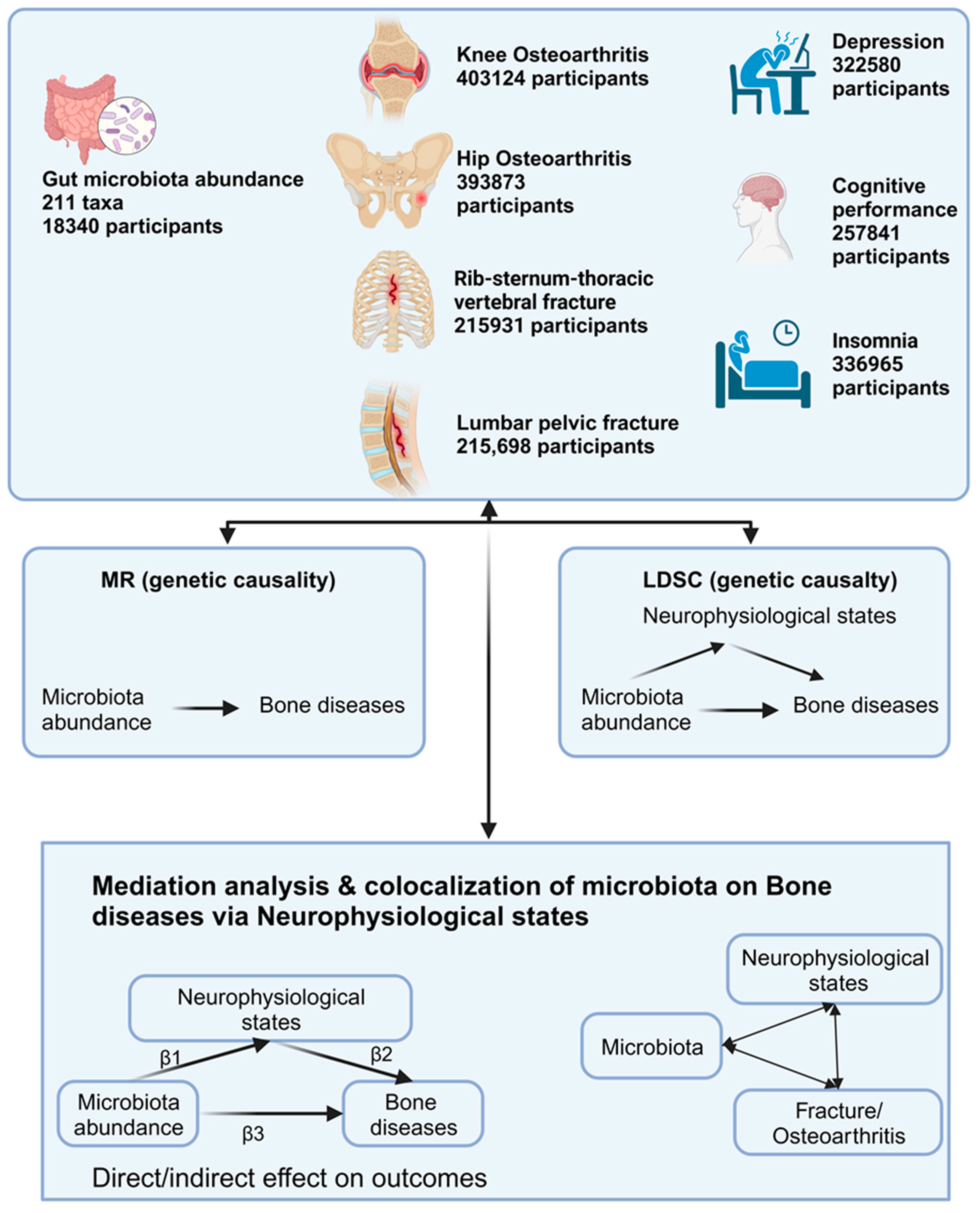
2. Materials and Methods
2.1. Instrument Variables Selection
2.2. Genetic Causality and Correlation of Microbiota and Bone Diseases
2.3. Mediation Analysis and Colocalization
3. Results
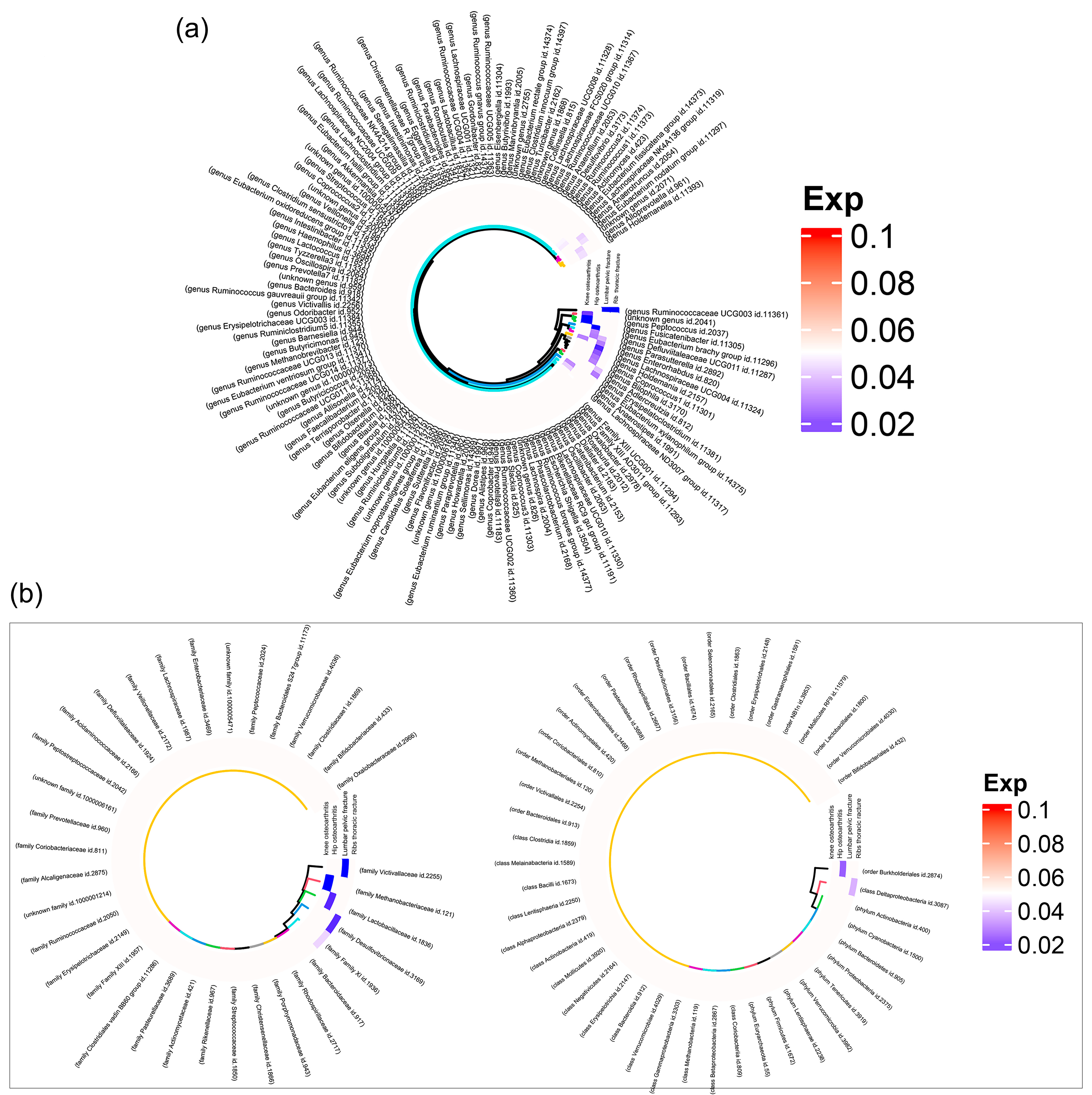
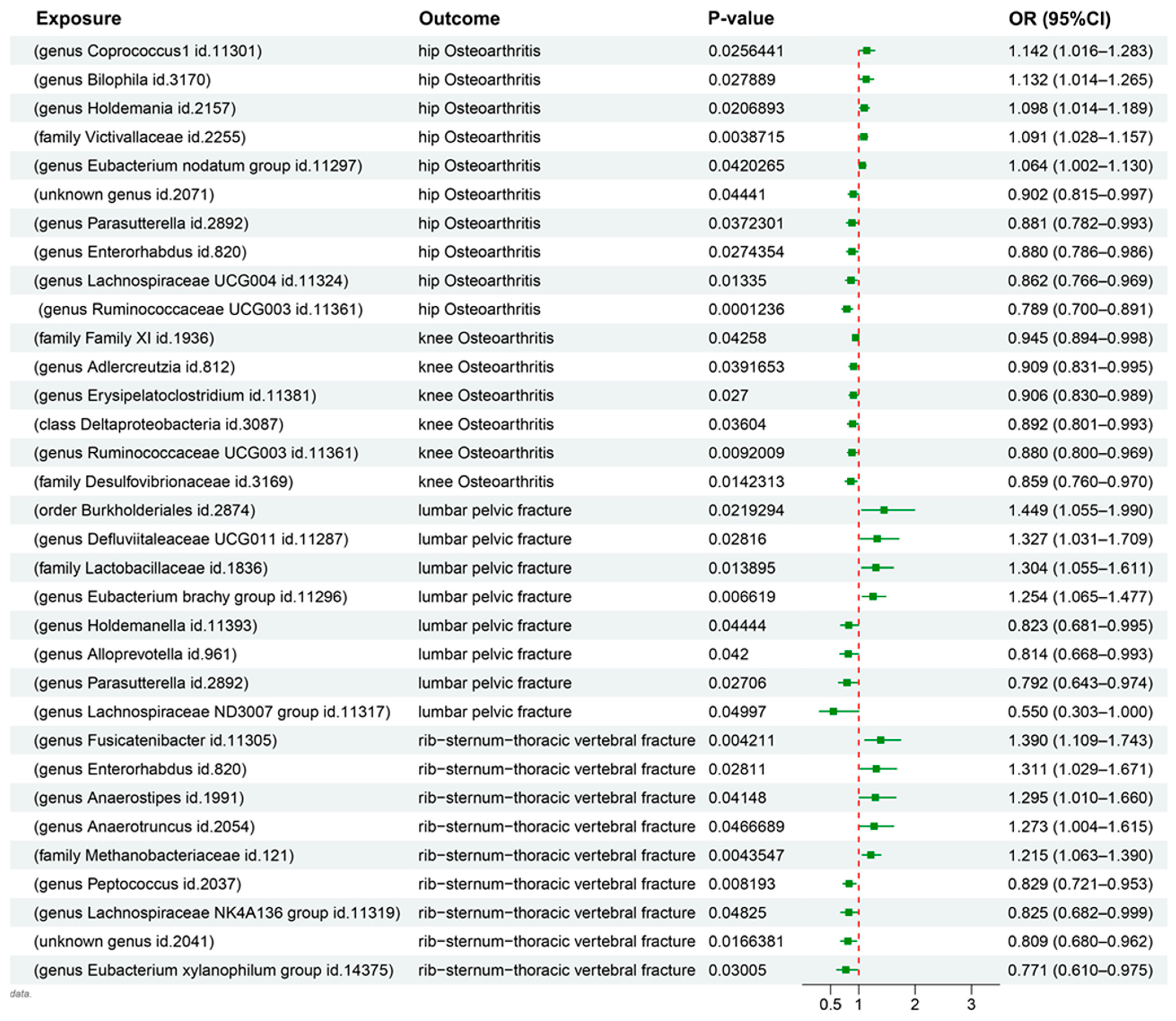
3.1. MR Results
3.2. LDSC Results
3.3. Mediation MR Results
3.4. Gene Colocalization Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, J.; Huang, X.; Dou, L.; Yan, M.; Shen, T.; Tang, W.; Li, J. Aging and aging-related diseases: From molecular mechanisms to interventions and treatments. Signal Transduct. Target. Ther. 2022, 7, 391. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, P.; Rejnmark, L.; Mosekilde, L. Osteoarthritis and Risk of Fractures. Calcif. Tissue Int. 2009, 84, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Yu, H.; Huang, X.; Shen, J.; Xiao, G.; Chen, L.; Wang, H.; Xing, L.; Chen, D. Current understanding of osteoarthritis pathogenesis and relevant new approaches. Bone Res. 2022, 10, 60. [Google Scholar] [CrossRef] [PubMed]
- Martel-Pelletier, J.; Barr, A.J.; Cicuttini, F.M.; Conaghan, P.G.; Cooper, C.; Goldring, M.B.; Goldring, S.R.; Jones, G.; Teichtahl, A.J.; Pelletier, J.-P. Osteoarthritis. Nat. Rev. Dis. Primers 2016, 2, 16072. [Google Scholar] [CrossRef] [PubMed]
- Bhanushali, A.; Kovoor, J.G.; Stretton, B.; Kieu, J.T.; Bright, R.A.; Hewitt, J.N.; Ovenden, C.D.; Gupta, A.K.; Afzal, M.Z.; Edwards, S.; et al. Outcomes of early versus delayed weight-bearing with intramedullary nailing of tibial shaft fractures: A systematic review and meta-analysis. Eur. J. Trauma. Emerg. Surg. 2022, 48, 3521–3527. [Google Scholar] [CrossRef]
- Rubenstein, L.Z. Falls in older people: Epidemiology, risk factors and strategies for prevention. Age Ageing 2006, 35, ii37–ii41. [Google Scholar] [CrossRef]
- Fang, E.F.; Xie, C.; Schenkel, J.A.; Wu, C.; Long, Q.; Cui, H.; Aman, Y.; Frank, J.; Liao, J.; Zou, H.; et al. A research agenda for ageing in China in the 21st century (2nd edition): Focusing on basic and translational research, long-term care, policy and social networks. Ageing Res. Rev. 2020, 64, 101174. [Google Scholar] [CrossRef]
- Fairley, J.L.; Seneviwickrama, M.; Yeh, S.; Anthony, S.; Chou, L.; Cicuttini, F.M.; Sullivan, K.; Briggs, A.M.; Wluka, A.E. Person-centred care in osteoarthritis and inflammatory arthritis: A scoping review of people’s needs outside of healthcare. BMC Musculoskelet. Disord. 2021, 22, 341. [Google Scholar] [CrossRef]
- Vincent, H.K.; Horodyski, M.; Vincent, K.R.; Brisbane, S.T.; Sadasivan, K.K. Psychological Distress After Orthopedic Trauma: Prevalence in Patients and Implications for Rehabilitation. PM&R 2015, 7, 978–989. [Google Scholar] [CrossRef]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.; Finlay, B.B. Gut microbiota in health and disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Cong, Y. Gut microbiota-derived metabolites in the regulation of host immune responses and immune-related inflammatory diseases. Cell. Mol. Immunol. 2021, 18, 866–877. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Yu, X.-J.; Yu, L.-L.; Tian, F.-W.; Zhao, J.-X.; Zhang, H.; Chen, W.; Zhai, Q.-X. The influence of gut microbiome on bone health and related dietary strategies against bone dysfunctions. Food Res. Int. 2021, 144, 110331. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Greenbaum, J.; Shen, H.; Deng, H.W. Association Between Gut Microbiota and Bone Health: Potential Mechanisms and Prospective. J. Clin. Endocrinol. Metab. 2017, 102, 3635–3646. [Google Scholar] [CrossRef]
- Lu, L.; Chen, X.; Liu, Y.; Yu, X. Gut microbiota and bone metabolism. FASEB J. 2021, 35, e21740. [Google Scholar] [CrossRef]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; van Harsselaar, J.; et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef]
- Medawar, E.; Haange, S.-B.; Rolle-Kampczyk, U.; Engelmann, B.; Dietrich, A.; Thieleking, R.; Wiegank, C.; Fries, C.; Horstmann, A.; Villringer, A.; et al. Gut microbiota link dietary fiber intake and short-chain fatty acid metabolism with eating behavior. Transl. Psychiatry 2021, 11, 500. [Google Scholar] [CrossRef]
- Seely, K.D.; Kotelko, C.A.; Douglas, H.; Bealer, B.; Brooks, A.E. The Human Gut Microbiota: A Key Mediator of Osteoporosis and Osteogenesis. Int. J. Mol. Sci. 2021, 22, 9452. [Google Scholar] [CrossRef]
- Kesika, P.; Suganthy, N.; Sivamaruthi, B.S.; Chaiyasut, C. Role of gut-brain axis, gut microbial composition, and probiotic intervention in Alzheimer’s disease. Life Sci. 2021, 264, 118627. [Google Scholar] [CrossRef]
- Morais, L.H.; Schreiber, H.L.; Mazmanian, S.K. The gut microbiota–brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef]
- Ma, Q.; Xing, C.; Long, W.; Wang, H.Y.; Liu, Q.; Wang, R.-F. Impact of microbiota on central nervous system and neurological diseases: The gut-brain axis. J. Neuroinflammation 2019, 16, 53. [Google Scholar] [CrossRef] [PubMed]
- Roos, P.M. Osteoporosis in neurodegeneration. J. Trace Elem. Med. Biol. 2014, 28, 418–421. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, Z.; Alexander, P.G.; Ocasio-Nieves, B.D.; Yocum, L.; Lin, H.; Tuan, R.S. Pathogenesis of Osteoarthritis: Risk Factors, Regulatory Pathways in Chondrocytes, and Experimental Models. Biology 2020, 9, 194. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Zhou, S.; Li, S.; Kuang, L.; Chen, H.; Luo, X.; Ouyang, J.; He, M.; Du, X.; Chen, L. Exosomes: Roles and therapeutic potential in osteoarthritis. Bone Res. 2020, 8, 25. [Google Scholar] [CrossRef]
- Ramsey, N.F.; Jansma, J.M.; Jager, G.; Van Raalten, T.; Kahn, R.S. Neurophysiological factors in human information processing capacity. Brain 2004, 127, 517–525. [Google Scholar] [CrossRef]
- Xie, N.; Wang, Z.; Shu, Q.; Liang, X.; Wang, J.; Wu, K.; Nie, Y.; Shi, Y.; Fan, D.; Wu, J. Association between Gut Microbiota and Digestive System Cancers: A Bidirectional Two-Sample Mendelian Randomization Study. Nutrients 2023, 15, 2937. [Google Scholar] [CrossRef]
- Wang, F.; Li, N.; Ni, S.; Min, Y.; Wei, K.; Sun, H.; Fu, Y.; Liu, Y.; Lv, D. The Effects of Specific Gut Microbiota and Metabolites on IgA Nephropathy-Based on Mendelian Randomization and Clinical Validation. Nutrients 2023, 15, 2407. [Google Scholar] [CrossRef]
- Sanchez-Lopez, E.; Coras, R.; Torres, A.; Lane, N.E.; Guma, M. Synovial inflammation in osteoarthritis progression. Nat. Rev. Rheumatol. 2022, 18, 258–275. [Google Scholar] [CrossRef]
- Glyn-Jones, S.; Palmer, A.J.; Agricola, R.; Price, A.J.; Vincent, T.L.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef]
- Mahmoudian, A.; Lohmander, L.S.; Mobasheri, A.; Englund, M.; Luyten, F.P. Early-stage symptomatic osteoarthritis of the knee—Time for action. Nat. Rev. Rheumatol. 2021, 17, 621–632. [Google Scholar] [CrossRef]
- Barrio, C.; Arias-Sánchez, S.; Martín-Monzón, I. The gut microbiota-brain axis, psychobiotics and its influence on brain and behaviour: A systematic review. Psychoneuroendocrinology 2022, 137, 105640. [Google Scholar] [CrossRef]
- Pettit, R.W.; Amos, C.I. Linkage Disequilibrium Score Statistic Regression for Identifying Novel Trait Associations. Curr. Epidemiol. Rep. 2022, 9, 190–199. [Google Scholar] [CrossRef]
- Zeng, P.; Shao, Z.; Zhou, X. Statistical methods for mediation analysis in the era of high-throughput genomics: Current successes and future challenges. Comput. Struct. Biotechnol. J. 2021, 19, 3209–3224. [Google Scholar] [CrossRef] [PubMed]
- Claus, S.P.; Ellero, S.L.; Berger, B.; Krause, L.; Bruttin, A.; Molina, J.; Paris, A.; Want, E.J.; de Waziers, I.; Cloarec, O.; et al. Colonization-induced host-gut microbial metabolic interaction. mBio 2011, 2, e00271-10. [Google Scholar] [CrossRef] [PubMed]
- Skrivankova, V.W.; Richmond, R.C.; Woolf, B.A.R.; Yarmolinsky, J.; Davies, N.M.; Swanson, S.A.; VanderWeele, T.J.; Higgins, J.P.T.; Timpson, N.J.; Dimou, N.; et al. Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization: The STROBE-MR Statement. JAMA 2021, 326, 1614–1621. [Google Scholar] [CrossRef]
- Liu, X.; Tang, S.; Zhong, H.; Tong, X.; Jie, Z.; Ding, Q.; Wang, D.; Guo, R.; Xiao, L.; Xu, X.; et al. A genome-wide association study for gut metagenome in Chinese adults illuminates complex diseases. Cell Discov. 2021, 7, 9. [Google Scholar] [CrossRef]
- Lee, J.J.; Wedow, R.; Okbay, A.; Kong, E.; Maghzian, O.; Zacher, M.; Nguyen-Viet, T.A.; Bowers, P.; Sidorenko, J.; Karlsson Linnér, R.; et al. Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nat. Genet. 2018, 50, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Howard, D.M.; Adams, M.J.; Shirali, M.; Clarke, T.K.; Marioni, R.E.; Davies, G.; Coleman, J.R.I.; Alloza, C.; Shen, X.; Barbu, M.C.; et al. Genome-wide association study of depression phenotypes in UK Biobank identifies variants in excitatory synaptic pathways. Nat. Commun. 2018, 9, 1470. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Torous, J.; Kossowsky, J.; Chen, C.-Y.; Huang, H.; Wright, A. Genome-wide association analysis of insomnia using data from Partners Biobank. Sci. Rep. 2020, 10, 6928. [Google Scholar] [CrossRef]
- Burgess, S.; Davey Smith, G.; Davies, N.M.; Dudbridge, F.; Gill, D.; Glymour, M.M.; Hartwig, F.P.; Kutalik, Z.; Holmes, M.V.; Minelli, C.; et al. Guidelines for performing Mendelian randomization investigations: Update for summer 2023. Wellcome Open Res. 2019, 4, 186. [Google Scholar] [CrossRef]
- Hou, T.; Dai, H.; Wang, Q.; Hou, Y.; Zhang, X.; Lin, H.; Wang, S.; Li, M.; Zhao, Z.; Lu, J.; et al. Dissecting the causal effect between gut microbiota, DHA, and urate metabolism: A large-scale bidirectional Mendelian randomization. Front. Immunol. 2023, 14, 1148591. [Google Scholar] [CrossRef] [PubMed]
- Foley, C.N.; Staley, J.R.; Breen, P.G.; Sun, B.B.; Kirk, P.D.W.; Burgess, S.; Howson, J.M.M. A fast and efficient colocalization algorithm for identifying shared genetic risk factors across multiple traits. Nat. Commun. 2021, 12, 764. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Chen, X.; Gong, P.; Li, G.; Yao, W.; Yang, W. The Gut-Organ-Axis Concept: Advances the Application of Gut-on-Chip Technology. Int. J. Mol. Sci. 2023, 24, 4089. [Google Scholar] [CrossRef]
- Petersen, P.E.; Ogawa, H. The global burden of periodontal disease: Towards integration with chronic disease prevention and control. Periodontology 2000 2012, 60, 15–39. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Lamont, R.J. Beyond the red complex and into more complexity: The polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Mol. Oral Microbiol. 2012, 27, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Ikram, S.; Hassan, N.; Raffat, M.A.; Mirza, S.; Akram, Z. Systematic review and meta-analysis of double-blind, placebo-controlled, randomized clinical trials using probiotics in chronic periodontitis. J. Investig. Clin. Dent. 2018, 9, e12338. [Google Scholar] [CrossRef]
- Zhou, T.; Xu, W.; Wang, Q.; Jiang, C.; Li, H.; Chao, Y.; Sun, Y. The effect of the “Oral-Gut” axis on periodontitis in inflammatory bowel disease: A review of microbe and immune mechanism associations. Front. Cell. Infect. Microbiol. 2023, 13, 1132420. [Google Scholar] [CrossRef]
- Han, Y.; You, X.; Xing, W.; Zhang, Z.; Zou, W. Paracrine and endocrine actions of bone—The functions of secretory proteins from osteoblasts, osteocytes, and osteoclasts. Bone Res. 2018, 6, 16. [Google Scholar] [CrossRef]
- Hou, Y.F.; Shan, C.; Zhuang, S.Y.; Zhuang, Q.Q.; Ghosh, A.; Zhu, K.C.; Kong, X.K.; Wang, S.M.; Gong, Y.L.; Yang, Y.Y.; et al. Gut microbiota-derived propionate mediates the neuroprotective effect of osteocalcin in a mouse model of Parkinson’s disease. Microbiome 2021, 9, 34. [Google Scholar] [CrossRef]
- Zhou, R.; Guo, Q.; Xiao, Y.; Guo, Q.; Huang, Y.; Li, C.; Luo, X. Endocrine role of bone in the regulation of energy metabolism. Bone Res. 2021, 9, 25. [Google Scholar] [CrossRef]
- La Reau, A.J.; Suen, G. The Ruminococci: Key symbionts of the gut ecosystem. J. Microbiol. 2018, 56, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Rojas, F.U.; López-Sánchez, D.; Meza-Radilla, G.; Méndez-Canarios, A.; Ibarra, J.A.; Estrada-de Los Santos, P. The controversial Burkholderia cepacia complex, a group of plant growth promoting species and plant, animals and human pathogens. Rev. Argent. Microbiol. 2019, 51, 84–92. [Google Scholar] [CrossRef]
- Hasan, N.; Yang, H. Factors affecting the composition of the gut microbiota, and its modulation. PeerJ 2019, 7, e7502. [Google Scholar] [CrossRef]
- Grieneisen, L.; Dasari, M.; Gould, T.J.; Björk, J.R.; Grenier, J.-C.; Yotova, V.; Jansen, D.; Gottel, N.; Gordon, J.B.; Learn, N.H.; et al. Gut microbiome heritability is nearly universal but environmentally contingent. Science 2021, 373, 181–186. [Google Scholar] [CrossRef]
- Harley, M.E.; Murina, O.; Leitch, A.; Higgs, M.R.; Bicknell, L.S.; Yigit, G.; Blackford, A.N.; Zlatanou, A.; Mackenzie, K.J.; Reddy, K.; et al. TRAIP promotes DNA damage response during genome replication and is mutated in primordial dwarfism. Nat. Genet. 2016, 48, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Guo, Y.; Huang, J.; Deng, Y.; Zang, J.; Huen, M.S.-Y. TRAIP regulates replication fork recovery and progression via PCNA. Cell Discov. 2016, 2, 16016. [Google Scholar] [CrossRef]
- Sanderson, E.; Glymour, M.M.; Holmes, M.V.; Kang, H.; Morrison, J.; Munafò, M.R.; Palmer, T.; Schooling, C.M.; Wallace, C.; Zhao, Q.; et al. Mendelian randomization. Nat. Rev. Methods Primers 2022, 2, 6. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, S.; Chen, Z.; Deng, L.; Chen, Y.; Zhou, W.; Canavese, F.; Li, L. Causal Link between Gut Microbiota, Neurophysiological States, and Bone Diseases: A Comprehensive Mendelian Randomization Study. Nutrients 2023, 15, 3934. https://doi.org/10.3390/nu15183934
Luo S, Chen Z, Deng L, Chen Y, Zhou W, Canavese F, Li L. Causal Link between Gut Microbiota, Neurophysiological States, and Bone Diseases: A Comprehensive Mendelian Randomization Study. Nutrients. 2023; 15(18):3934. https://doi.org/10.3390/nu15183934
Chicago/Turabian StyleLuo, Shaoting, Zhiyang Chen, Linfang Deng, Yufan Chen, Weizheng Zhou, Federico Canavese, and Lianyong Li. 2023. "Causal Link between Gut Microbiota, Neurophysiological States, and Bone Diseases: A Comprehensive Mendelian Randomization Study" Nutrients 15, no. 18: 3934. https://doi.org/10.3390/nu15183934
APA StyleLuo, S., Chen, Z., Deng, L., Chen, Y., Zhou, W., Canavese, F., & Li, L. (2023). Causal Link between Gut Microbiota, Neurophysiological States, and Bone Diseases: A Comprehensive Mendelian Randomization Study. Nutrients, 15(18), 3934. https://doi.org/10.3390/nu15183934






