Prevalence of Undernutrition in Community-Dwelling Older Adults in The Netherlands: Application of the SNAQ65+ Screening Tool and GLIM Consensus Criteria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design
2.2. Study Samples
2.3. Assessment of Undernutrition
2.3.1. SNAQ65+ Screening
2.3.2. GLIM Diagnosis
2.4. SNAQ65+ and GLIM Criteria Used to Assess Undernutrition
2.4.1. Involuntary Weight Loss
2.4.2. Low BMI
2.4.3. Low MUAC
2.4.4. Decreased Muscle Mass
2.4.5. Reduced Food Intake
2.4.6. Poor Appetite
2.4.7. Mobility Limitations
2.4.8. Inflammation
2.5. Other Descriptive Variables
2.6. Statistical Analysis
3. Results
3.1. Sample Characteristics
3.2. Prevalence of Undernutrition Based on SNAQ65+ Screening
3.3. Prevalence of Undernutrition Based on GLIM Diagnosis
3.4. Prevalence of Undernutrition by Sample Subgroups
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations. The World Population Prospects 2022: Summary of Results; United Nations: New York, NY, USA, 2022; pp. 9–11. [Google Scholar]
- de Jong, A.; te Riele, S.; Huisman, C.; van Duin, C.; Husby, T.; Stoeldraijer, L. Regionale Bevolkings—En Huishoudensprognose 2022–2050: Steden en Randgemeenten Groeien Verder; CBS: Den Haag, The Netherlands, 2022; pp. 24–28. [Google Scholar]
- Almohaisen, N.; Gittins, M.; Todd, C.; Sremanakova, J.; Sowerbutts, A.M.; Aldossari, A.; Almutairi, A.; Jones, D.; Burden, S. Prevalence of Undernutrition, Frailty and Sarcopenia in Community-Dwelling People Aged 50 Years and Above: Systematic Review and Meta-Analysis. Nutrients 2022, 14, 1537. [Google Scholar] [CrossRef]
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correira, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef]
- Volkert, D.; Kiesswetter, E.; Cederholm, T.; Donini, L.M.; Eglseer, D.; Norman, K.; Schneider, S.M.; Ströbele-Benschop, N.; Trobahn, G.; Wirth, R.; et al. Development of a Model on Determinants of Malnutrition in Persons: A MaNuEl Project. Gerontol. Geriatr. Med. 2019, 5, 2333721419858438. [Google Scholar] [CrossRef]
- Dent, E.; Wright, O.R.L.; Woo, J.; Hoogendijk, E. Malnutrition in older adults. Lancet 2023, 401, 951–966. [Google Scholar] [CrossRef] [PubMed]
- Kupisz-Urbanska, M.; Marcinowska-Suchowierska, E. Malnutrition in Older Adults-Effect on Falls and Fractures: A Narrative Review. Nutrients 2022, 14, 3123. [Google Scholar] [CrossRef] [PubMed]
- Lesourd, B.M. Nutrition: A major factor influencing immunity in the elderly. J. Nutr. Health Aging 2004, 8, 28–37. [Google Scholar] [PubMed]
- Rasheed, S.; Woods, R.T. Malnutrition and quality of life in older people: A systematic review and meta-analysis. Ageing Res. Rev. 2013, 12, 561–566. [Google Scholar] [CrossRef]
- de Miranda, J.M.A.; Viana, D.M.O.; Dos Santos, A.A.L.; Costa, A.F.A.; Dantas, B.; de Miranda, F.A.N.; Mendes, F.R.P.; Torres, L.S.S. Quality of Life in Community-Dwelling Older People with Functional and Nutritional Impairment and Depressive Symptoms: A Comparative Cross-Sectional Study in Brazil and Portugal. Geriatrics 2022, 7, 96. [Google Scholar] [CrossRef]
- Rodríguez-Sánchez, B.; Sulo, S.; Carnicero, J.A.; Rueda, R.; Rodríguez-Mañas, L. Malnutrition Prevalence and Burden on Healthcare Resource Use Among Spanish Community-Living Older Adults: Results of a Longitudinal Analysis. Clin. Outcomes Res. 2020, 12, 355–367. [Google Scholar] [CrossRef]
- Söderström, L.; Rosenblad, A.; Thors Adolfsson, E.; Bergkvist, L. Malnutrition is associated with increased mortality in older adults regardless of the cause of death. Br. J. Nutr. 2017, 117, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Caçador, C.; Teixeira-Lemos, E.; Oliveira, J.; Pinheiro, J.; Mascarenhas-Melo, F.; Ramos, F. The Relationship between Nutritional Status and Functional Capacity: A Contribution Study in Institutionalised Portuguese Older Adults. Int. J. Environ. Res. Public Health 2021, 18, 3789. [Google Scholar] [CrossRef]
- Ge, L.; Yap, C.W.; Heng, B.H. Association of Nutritional Status with physical Function and Disability in Community-Dwelling Older Adults: A Longitudinal Data Analysis. J. Nutr. Gerontol. Geriatr. 2020, 39, 131–142. [Google Scholar] [CrossRef]
- Schilp, J.; Kruizenga, H.M.; Wijnhoven, H.A.H.; Leistra, E.; Evers, A.M.; van Binsbergen, J.J.; Deeg, D.J.H.; Visser, M. High prevalence of undernutrition in Dutch community-dwelling older individuals. Nutrition 2012, 28, 1151–1156. [Google Scholar] [CrossRef]
- CBS (Centraal Bureau Voor de Statistiek). Woonsituatie van Personen 70 Jaar of Ouder, 2018–2022. Available online: https://www.cbs.nl/nl-nl/maatwerk/2022/38/woonsituatie-van-personen-van-70-jaar-of-ouder-2018-2022 (accessed on 30 July 2023).
- NZa (Nederlandse Zorgautoriteit). Zorg Voor Ouderen 2018. Available online: https://puc.overheid.nl/nza/doc/PUC_234967_22/1/ (accessed on 16 August 2023).
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 207–217. [Google Scholar] [CrossRef]
- Hoogendijk, E.O.; Deeg, D.J.H.; De Breij, S.; Klokgieters, S.S.; Kok, A.L.; Stringa, N.; Timmermans, E.J.; van Schoor, N.M.; van Zutphen, E.M.; van der Horst, M.H.L.; et al. The longitudinal aging study Amsterdam: Cohort update 2019 and additional data collections. Eur. J. Epidemiol. 2020, 35, 61–74. [Google Scholar] [CrossRef]
- Hoogendijk, E.O.; Deeg, D.J.H.; Poppelaars, J.; van der Horst, M.H.L.; van Groenou, M.I.B.; Comijs, H.C.; Pasman, H.R.W.; van Schoor, N.M.; Suanet, B.; Thomése, F.; et al. The longitudinal aging study Amsterdam: Cohort update 2016 and major findings. Eur. J. Epidemiol. 2016, 31, 927–945. [Google Scholar] [CrossRef]
- Huisman, M.; Poppelaars, J.; van der Horst, M.H.L.; Beekman, A.T.F.; Brug, J.; van Tilburg, T.G.; Deeg, D.J.H. Cohort profile: The longitudinal aging study Amsterdam. Int. J. Epidemiol. 2011, 40, 868–876. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Public Health and the Environment (RIVM). Voedselconsumptiepeiling 2019–2021 (Food Consumption Survey 2019–2021). Available online: https://www.wateetnederland.nl/ (accessed on 24 June 2023).
- Barbosa-Silva, T.G.; Bielemann, R.M.; Gonzalez, M.C.; Menezes, A.M.B. Prevalence of sarcopenia among community-dwelling elderly of a medium-sized South American city: Results of the COMO VAI? Study. J. Cachex-Sarcopenia Muscle 2016, 7, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Menant, J.; Steele, J.; Menz, H.; Munro, B.; Lord, S. Effects of Footwear Features on Balance and Stepping in Older People. Gerontology 2008, 54, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Frank, E.; Dunlop, A.L. What does a patient’s outfit weight? Fam. Med. 2000, 32, 595–596. [Google Scholar]
- Wijnhoven, H.A.H.; Schilp, J.; de van der Schueren, M.A.E.v.B.; de Vet, H.C.W.; Kruizenga, H.M.; Deeg, D.J.H.; Ferrucci, L.; Visser, M. Development and validation of criteria for determining undernutrition in community-dwelling older men and women: The Short Nutritional Assessment Questionnaire 65+. Clin. Nutr. 2012, 31, 351–358. [Google Scholar] [CrossRef]
- Radloff, L.S. The CES-D scale: A self-reported depression scale for research in the general population. Appl. Psychol. Meas. 1977, 1, 385–401. [Google Scholar] [CrossRef]
- Demirdag, F.; Kolbasi, E.N.; Pehlivan, O. Prevalence of Malnutrition According to the Global Leadership Initiative on Malnutrition Criteria in Community—Dwelling Older Adults in Turkey. Medeni. Med. J. 2022, 37, 234–323. [Google Scholar] [CrossRef]
- Kokura, Y.; Momosaki, R. Prevalence of Malnutrition Assessed by the GLIM Criteria and Association with Activities of Daily Living in Older Residents in an Integrated Facility for Medical and Long-term Care. Nutrients 2022, 14, 3656. [Google Scholar] [CrossRef]
- Kriegsman, D.M.; Penninx, B.W.; van Eijk, J.T.; Boeke, A.J.; Deeg, D.J.H. Self-reports and general practitioner information on the presence of chronic diseases in community dwelling elderly. A study on the accuracy of patients’ self-reports and on determinants of inaccuracy. J. Clin. Epidemiol. 1996, 49, 1407–1417. [Google Scholar] [CrossRef]
- CBS (Centraal Bureau voor de Statistiek). Lengte en Gewicht van Personen, Ondergewicht en Overgewicht; Vanaf 1981. Available online: https://opendata.cbs.nl/statline#/CBS/nl/dataset/81565NED/table?ts=1685547230797 (accessed on 31 May 2023).
- Lima, J.; Dias, A.J.B.; Burgel, C.F.; Bernandes, S.; Gonzalez, M.C.; Silva, F.M. Complementarity of nutritional screening tools to GLIM criteria on malnutrition diagnosis in hospitalised patients: A secondary analysis of a longitudinal study. Clin. Nutr. 2022, 41, 2325–2332. [Google Scholar] [CrossRef] [PubMed]
- Ozer, N.T.; Akin, S.; Gunes Sahin, G.; Sahin, S. Prevalence of malnutrition diagnosed by the Global Leadership Initiative on Malnutrition and Mini Nutritional Assessment in older adult outpatients and comparison between the Global Leadership Initiative on Malnutrition and Mini Nutritional Assessment energy-protein intake: A cross-sectional study. J. Parenter. Enter. Nutr. 2022, 46, 367–377. [Google Scholar] [CrossRef]
- Powell-Tuck, J.; Hennessy, E.M. A comparison of mid upper arm circumference, body mass index and weight loss as indices of undernutrition in acutely hospitalized patients. Clin. Nutr. 2003, 22, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Wijnhoven, H.A.H.; de van der Schueren, M.A.E.v.B.; Heymans, M.W.; de Vet, H.C.W.; Kruizenga, H.M.; Twisk, J.W.; Visser, M. Low mid-upper arm circumference, calf circumference, and body mass index and moratlity in older persons. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- Monteagudo, C.; Dijkstra, S.C.; Visser, M. Self-perception of body weight status in older Dutch adults. J. Nutr. Health Aging 2015, 19, 612–618. [Google Scholar] [CrossRef]
| UNDERNUTRITION SCREENING SAMPLE (SNAQ65+) | UNDERNUTRITION DIAGNOSIS SAMPLE (GLIM) | |||||||
|---|---|---|---|---|---|---|---|---|
| LASA | DNFCS | LASA and DNFCS Combined | LASA SUBGROUP | |||||
| n | (N = 1138) | n | (N = 607) | n | (N = 1745) | n | (N = 700) | |
| Sex female, n(%) | 1138 | 586 (51.5) | 607 | 296 (48.8) | 1745 | 882 (50.5) | 700 | 339 (48.4) |
| Age (years), mean ± SD | 1138 | 75.4 ± 6.9 | 607 | 70.7 ± 3.9 | 1745 | 73.7 ± 6.4 | 700 | 74.9 ± 6.6 |
| Age group, n(%) | 1138 | 607 | 1745 | 700 | ||||
| 65–69 years | 303 (26.6) | 276 (45.5) | 579 (33.2) | 202 (28.9) | ||||
| 70–74 years | 303 (26.6) | 213 (35.1) | 516 (29.6) | 193 (27.6) | ||||
| 75–79 years | 240 (22.1) | 118 (19.4) | 358 (20.5) | 144 (20.6) | ||||
| 80–84 years | 173 (15.2) | NA | 173 (9.9) | 96 (13.7) | ||||
| ≥85 years | 119 (10.5) | NA | 119 (6.8) | 65 (9.3) | ||||
| BMI (kg/m2), mean ±SD | 1131 | 27.1 ± 4.7 | 607 | 26.9 ± 4.9 | 1738 | 27.0 ± 4.8 | 700 | 26.7 ± 7 |
| BMI, n (%) | 1131 | 607 | 1738 | 700 | ||||
| Underweight | 94 (8.3) | 42 (6.9) | 136 (7.8) | 56 (8.0) | ||||
| Normal and overweight | 796 (70.4) | 443 (73.0) | 1239 (71.3) | 510 (72.9) | ||||
| Obese | 241 (21.3) | 122 (20.1) | 363 (20.9) | 134 (19.1) | ||||
| Region of The Netherlands, n (%) | 1138 | 607 | 1745 | 700 | ||||
| West | 509 (44.7) | 282 (46.5) | 791 (45.3) | 329 (47.0) | ||||
| Northeast | 376 (33.0) | 174 (28.7) | 550 (31.5) | 213 (30.4) | ||||
| South | 253 (22.2) | 151 (24.9) | 404 (23.2) | 158 (22.6) | ||||
| Education level, n (%) | 1138 | 601 | 1739 | 700 | ||||
| Low | 139 (12.2) | 27 (4.5) | 166 (9.5) | 75 (10.7) | ||||
| Medium | 387 (34.0) | 197 (32.8) | 584 (33.6) | 223 (31.9) | ||||
| High | 612 (53.8) | 377 (62.7) | 989 (56.9) | 402 (57.4) | ||||
| Living situation, n (%) | 1125 | 607 | 1732 | 695 | ||||
| Alone | 358 (31.8) | 166 (27.3) | 524 (30.3) | 213 (30.6) | ||||
| With others | 767 (68.2) | 441 (72.7) | 1208 (69.7) | 482 (69.4) | ||||
| Receiving formal home care, n (%) | 1138 | 700 | ||||||
| Yes | 165 (14.5) | NA | NA | 81 (11.6) | ||||
| No | 973 (85.5) | 619 (88.4) | ||||||
| Self-rated health, n (%) | 1138 | 700 | ||||||
| Good | 743 (65.3) | NA | NA | 478 (68.3) | ||||
| Poor | 395 (34.7) | 222 (31.7) | ||||||
| Individual SNAQ | ||||||||
| ≥4 kg involuntary weight loss, n (%) | 1138 | 64 (5.6) | 607 | 25 (4.1) | 1745 | 89 (5.1) | 700 | 30 (4.3) |
| MUAC < 25 cm, n (%) * | 1138 | 33 (2.9) | 607 | 34 (5.6) | 1745 | 67 (3.8) | 700 | 11 (1.6) |
| Appetite last week, n (%) | 1138 | 607 | 1745 | 700 | ||||
| Good | 1009 (88.7) | 589 (97.0) | 1598 (91.6) | 652 (93.1) | ||||
| Poor | 129 (11.3) | 18 (3.0) | 147 (8.4) | 48 (6.9) | ||||
| Mobility limitations, n (%) | 1137 | 607 | 1744 | 700 | ||||
| No | 703 (61.8) | 582 (95.9) | 1285 (73.7) | 469 (67.0) | ||||
| Yes | 434 (38.2) | 25 (4.1) | 459 (26.3) | 231 (33.0) | ||||
| Screening result SNAQ65+ | ||||||||
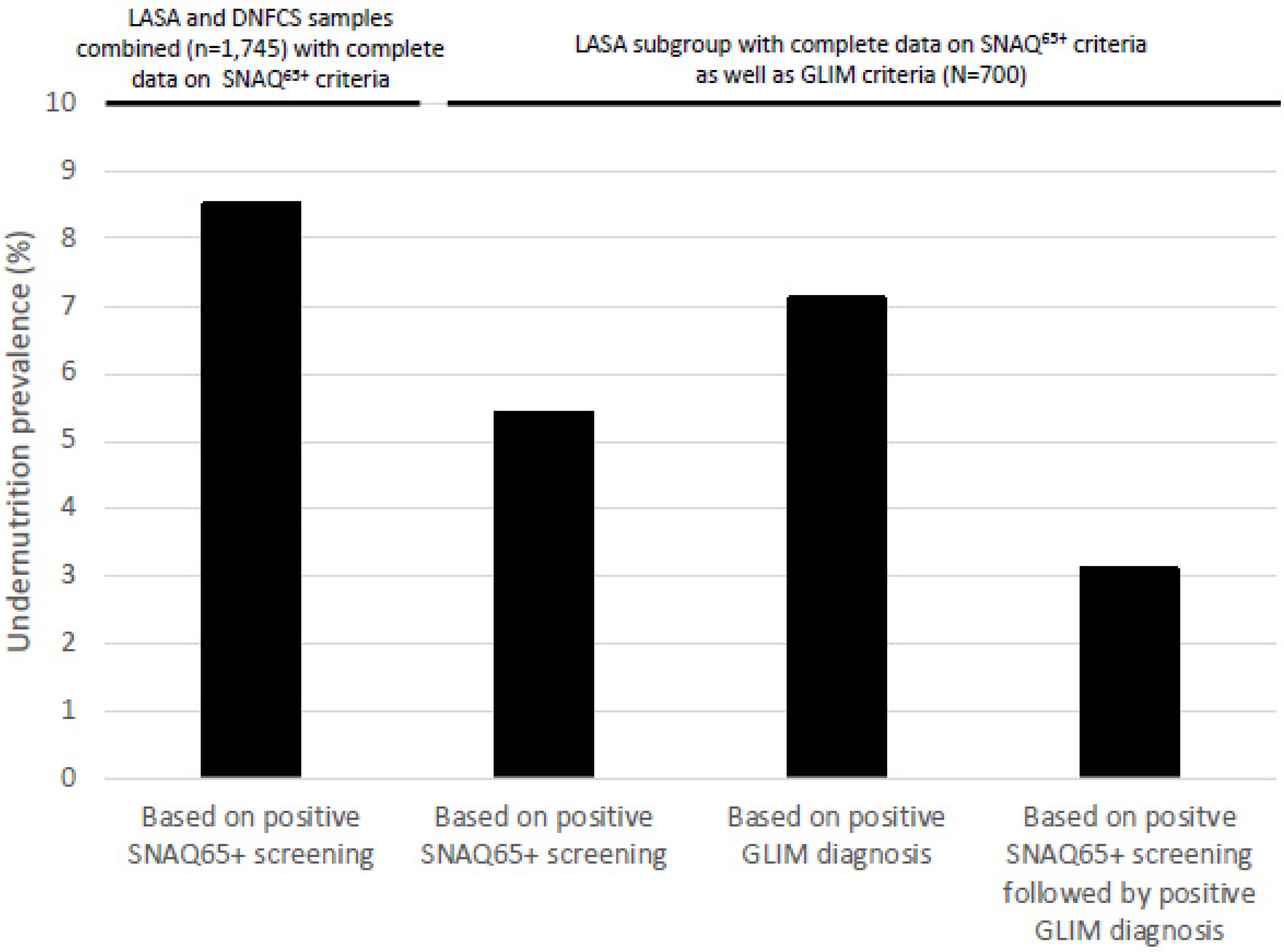
| Undernutrition, n (%) | 1138 | 92 (8.1) | 607 | 57 (9.4) | 1745 | 149 (8.5) | 700 | 38 (5.4) |
| At-risk, n (%) | 1138 | 72 (6.3) | 607 | 1 (0.2) | 1745 | 73 (4.2) | 700 | 24 (3.4) |
| None, n (%) | 1138 | 974 (85.6) | 607 | 549 (90.4) | 1745 | 1523 (87.3) | 700 | 638 (91.1) |
| ALL | SNAQ65+ Screening Result | |||
|---|---|---|---|---|
| Undernutrition | At Risk | None | ||
| N | 700 | 38 | 24 | 638 |
| Phenotypic criteria, n (%) | ||||
| Unintentional weight loss ≥5% in 6 months | 22 (3.1) | 19 (50.0) | 1 (4.2) | 2 (0.3) |
| Severe | 5 (0.7) | 4 (10.5) | 0 (0.0) | 1 (0.2) |
| Low BMI | ||||
| Severe | 56 (8.0) | 15 (39.5) | 1 (4.2) | 40 (6.3) |
| 13 (1.9) | 8 (21.1) | 0 (0.0) | 5 (0.8) | |
| Reduced muscle mass | 74 (10.6) | 10 (26.3) | 5 (20.8) | 59 (9.2) |
| Severe | 16 (2.3) | 4 (10.5) | 1 (4.2) | 11 (1.7) |
| At least one phenotypic criterion | 126 (18.0) | 33 (86.8) | 5 (20.8) | 88 (13.8) |
| Etiologic criteria, n (%) | ||||
| Reduced intake | 69 (9.9) | 2 (5.3) | 2 (8.3) | 65 (10.2) |
| Inflammation | 227 (32.4) | 20 (52.6) | 13 (54.2) | 194 (30.4) |
| CNSLD | 85 (12.1) | 7 (18.4) | 5 (20.8) | 73 (11.4) |
| Rheumatoid arthritis | 87 (12.4) | 6 (15.8) | 6 (25.0) | 75 (11.8) |
| Non-skin cancer | 92 (13.1) | 9 (23.7) | 4 (16.7) | 79 (12.4) |
| At least one etiologic criterion | 266 (38.0) | 21 (55.3) | 14 (58.3) | 231 (36.2) |
| Diagnosis based on GLIM | ||||
| Undernutrition | 50 (7.1) | 19 (50.0) | 3 (12.5) | 28 (4.4) |
| Moderate undernutrition | 24 (3.4) | 9 (23.7) | 2 (8.3) | 13 (2.0) |
| Severe undernutrition | 26 (3.7) | 10 (26.3) | 1 (4.2) | 15 (2.4) |
| No undernutrition | 650 (92.9) | 19 (50.0) | 21 (87.5) | 610 (95.6) |
| UNDERNUTRITION SCREENING SAMPLE (SNAQ65+) | UNDERNUTRITION DIAGNOSIS SAMPLE (GLIM) | |||
|---|---|---|---|---|
| LASA and DNFCS Combined | p-Value * | LASA Subgroup | p-Value * | |
| N | 1745 | 700 | ||
| Sex, n (%) | 0.001 | 0.16 | ||
| male | 55 (6.4) | 21 (5.8) | ||
| female | 94 (10.7) | 29 (8.6) | ||
| Age, n (%) | <0.001 | 0.46 | ||
| 65–69 years | 28 (4.8) | 11 (5.4) | ||
| 70–74 years | 48 (9.3) | 14 (7.3) | ||
| 75–79 years | 35 (9.8) | 15 (10.4) | ||
| 80–84 years ** | 15 (8.7) | 4 (4.2) | ||
| ≥85 years ** | 23 (19.3) | 6 (9.2) | ||
| BMI, n (%) | <0.001 | <0.001 | ||
| Underweight | 72 (52.9) | 23 (41.1) | ||
| Normal and overweight | 51 (4.1) | 24 (4.7) | ||
| Obese | 22 (6.1) | 3 (2.2) | ||
| Appetite, n (%) | <0.001 | <0.001 | ||
| Good | 116 (7.3) | 39 (6.0) | ||
| Poor | 33 (22.4) | 11 (22.9) | ||
| Mobility limitations, n (%) | 0.007 | 0.008 | ||
| No | 96 (7.5) | 25 (5.3) | ||
| Yes | 53 (11.5) | 25 (10.8) | ||
| Region, n (%) | 0.39 | 0.29 | ||
| West | 74 (9.4) | 28 (8.5) | ||
| Northeast | 42 (7.6) | 12 (5.6) | ||
| South | 33 (8.2) | 10 (6.3) | ||
| Education, n (%) | 0.24 | 0.07 | ||
| Low | 17 (10.2) | 10 (13.3) | ||
| Medium | 53 (9.1) | 15 (6.7) | ||
| High | 78 (7.9) | 25 (6.2) | ||
| Living situation, n (%) | <0.001 | 0.07 | ||
| Alone | 71 (13.5) | 23 (10.8) | ||
| With others | 76 (6.3) | 25 (5.2) | ||
| Receiving formal home care, n(%) ** | <0.001 | 0.72 | ||
| Yes | 26 (15.8) | 5 (6.2) | ||
| No | 66 (6.8) | 45 (7.3) | ||
| Self-rated health, n (%) ** | <0.001 | 0.05 | ||
| Good | 44 (5.9) | 28 (5.9) | ||
| Poor | 8 (12.2) | 22 (9.9) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zügül, Y.; van Rossum, C.; Visser, M. Prevalence of Undernutrition in Community-Dwelling Older Adults in The Netherlands: Application of the SNAQ65+ Screening Tool and GLIM Consensus Criteria. Nutrients 2023, 15, 3917. https://doi.org/10.3390/nu15183917
Zügül Y, van Rossum C, Visser M. Prevalence of Undernutrition in Community-Dwelling Older Adults in The Netherlands: Application of the SNAQ65+ Screening Tool and GLIM Consensus Criteria. Nutrients. 2023; 15(18):3917. https://doi.org/10.3390/nu15183917
Chicago/Turabian StyleZügül, Yaren, Caroline van Rossum, and Marjolein Visser. 2023. "Prevalence of Undernutrition in Community-Dwelling Older Adults in The Netherlands: Application of the SNAQ65+ Screening Tool and GLIM Consensus Criteria" Nutrients 15, no. 18: 3917. https://doi.org/10.3390/nu15183917
APA StyleZügül, Y., van Rossum, C., & Visser, M. (2023). Prevalence of Undernutrition in Community-Dwelling Older Adults in The Netherlands: Application of the SNAQ65+ Screening Tool and GLIM Consensus Criteria. Nutrients, 15(18), 3917. https://doi.org/10.3390/nu15183917






