A Program of Life-Style Modification Improved the Body Weight and Micronutrient Status in Obese Patients after Bariatric Surgery
Abstract
:1. Introduction
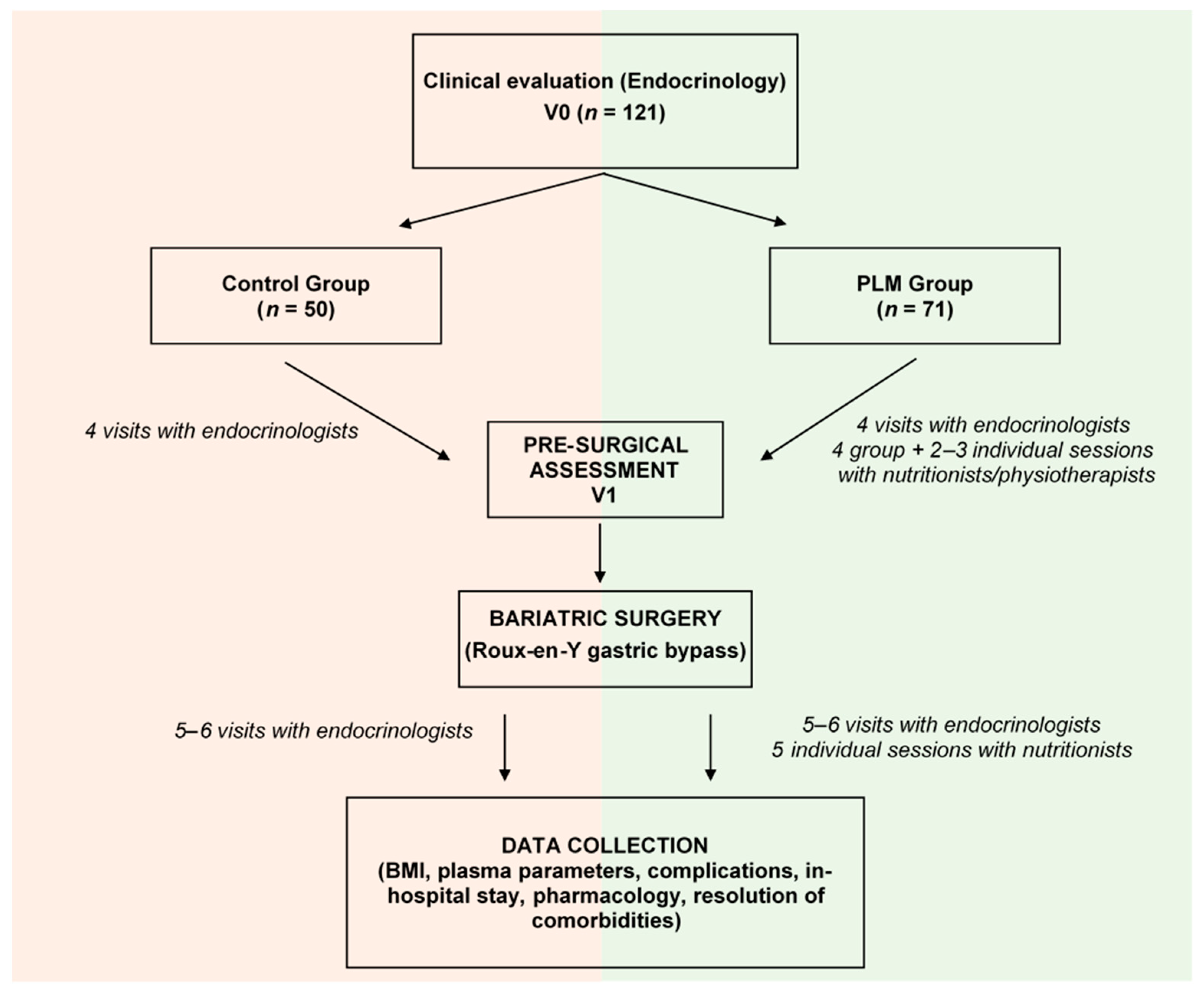
2. Methodology
2.1. Bariatric Surgery for Severe Obesity
2.1.1. Selection of Patients
2.1.2. The Roux-en-Y Gastric Bypass
2.2. Anthropometric and Biochemical Parameters
2.3. The Program for Lifestyle Modification
3. Data Analysis
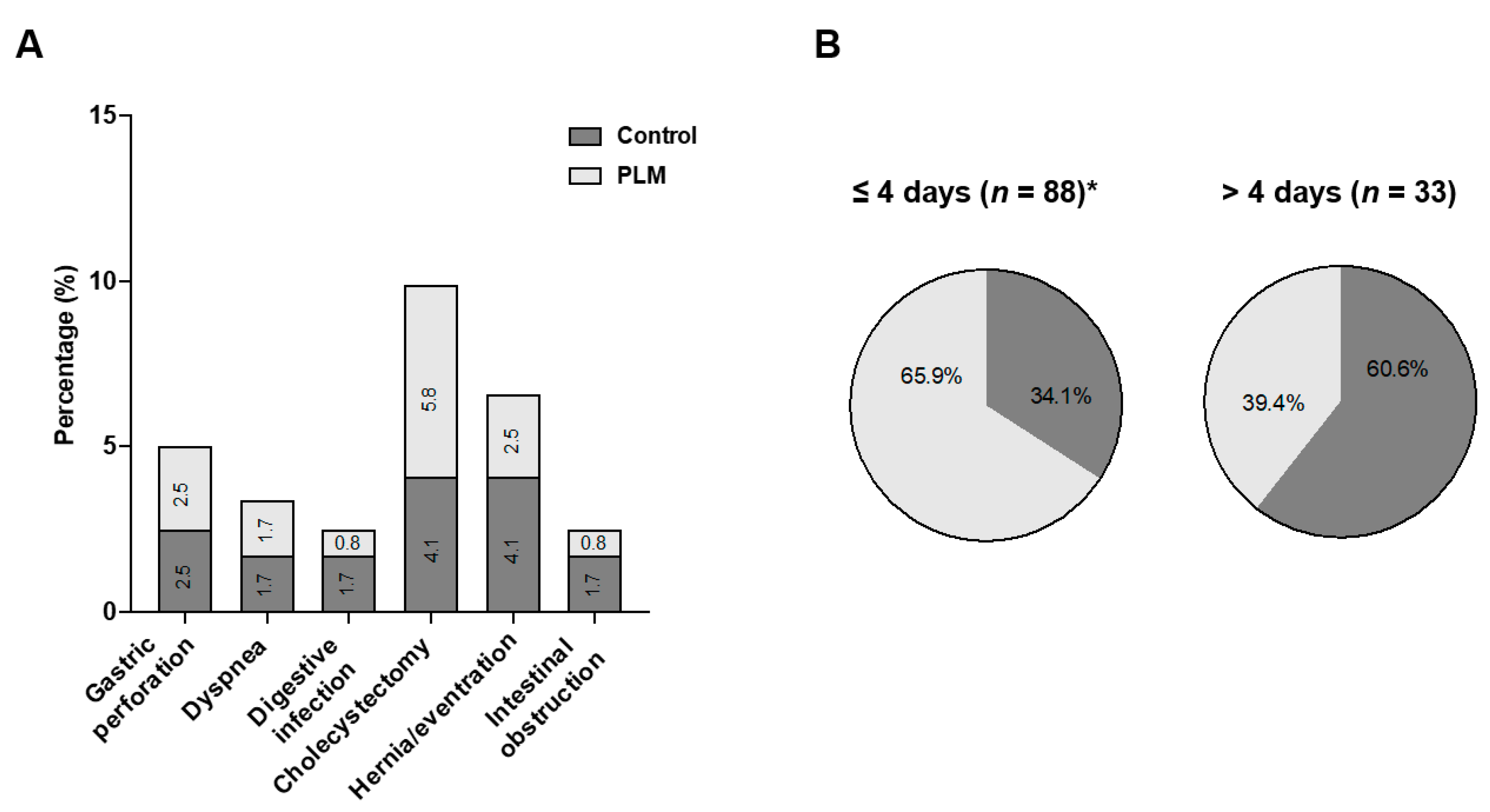
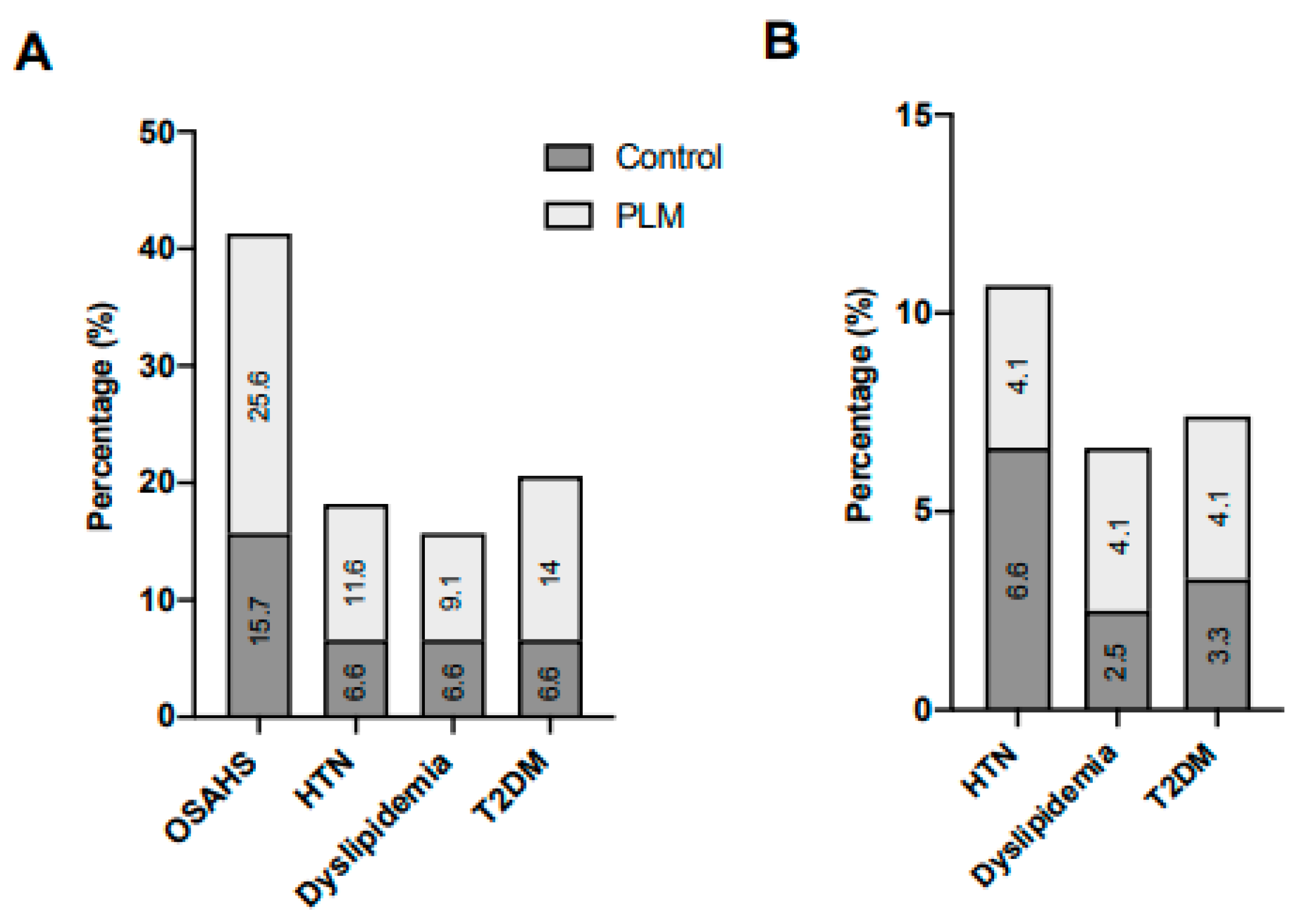
4. Results
4.1. Characterization of the Morbid Obese Population
4.2. Lifestyle Modification at the Pre-Surgical Stage
4.3. Lifestyle Modification at the Post-Surgical Phase
4.3.1. Reduction in the Body Mass Index after the RYGB and PLM
4.3.2. Mineral Metabolism after the RYGB and PLM
5. Discussion
6. Limitation of the Study
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Finkelstein, E.A.; Khavjou, O.A.; Thompson, H.; Trogdon, J.G.; Pan, L.; Sherry, B.; Dietz, W. Obesity and severe obesity forecasts through 2030. Am. J. Prev. Med. 2012, 42, 563–570. [Google Scholar] [CrossRef]
- de Gonzalez, A.B.; Hartge, P.; Cerhan, J.R.; Flint, A.J.; Hannan, L.; MacInnis, R.J.; Moore, S.C.; Tobias, G.S.; Anton-Culver, H.; Freeman, L.B.; et al. Body-Mass Index and Mortality among 1.46 Million White Adults. N. Engl. J. Med. 2010, 363, 2211–2219. [Google Scholar] [CrossRef] [PubMed]
- Kitahara, C.M.; Flint, A.J.; Berrington de Gonzalez, A.; Bernstein, L.; Brotzman, M.; MacInnis, R.J.; Moore, S.C.; Robien, K.; Rosenberg, P.S.; Singh, P.N.; et al. Association between class III obesity (BMI of 40–59 kg/m2) and mortality: A pooled analysis of 20 prospective studies. PLoS Med. 2014, 11, e1001673. [Google Scholar] [CrossRef] [PubMed]
- Meneses, D.; Olveira, A.; Corripio, R.; Méndez, M.D.C.; Romero, M.; Calvo-Viñuelas, I.; Herranz, L.; Vicent, D.; de-Cos-Blanco, A.I. Prevalence and predictors of non-alcoholic steatohepatitis in patients with morbid obesity. Endocrinol. Diabetes Nutr. 2022, 69, 178–188. [Google Scholar] [CrossRef]
- Berardi, G.; Vitiello, A.; Abu-Abeid, A.; Schiavone, V.; Franzese, A.; Velotti, N.; Musella, M. Micronutrients Deficiencies in Candidates of Bariatric Surgery: Results from a Single Institution over a 1-Year Period. Obes. Surg. 2023, 33, 212–218. [Google Scholar] [CrossRef] [PubMed]
- An, P.; Wan, S.; Luo, Y.; Luo, J.; Zhang, X.; Zhou, S.; Xu, T.; He, J.; Mechanick, J.I.; Wu, W.-C.; et al. Micronutrient Supplementation to Reduce Cardiovascular Risk. J. Am. Coll. Cardiol. 2022, 80, 2269–2285. [Google Scholar] [CrossRef]
- González-Parra, E.; Aceña, Á.; Lorenzo, Ó.; Tarín, N.; González-Casaus, M.L.; Cristóbal, C.; Huelmos, A.; Mahíllo-Fernández, I.; Pello, A.M.; Carda, R.; et al. Important abnormalities of bone mineral metabolism are present in patients with coronary artery disease with a mild decrease of the estimated glomerular filtration rate. J. Bone Miner. Metab. 2016, 34, 587–598. [Google Scholar] [CrossRef]
- Robertson, A.G.N.; Wiggins, T.; Robertson, F.P.; Huppler, L.; Doleman, B.; Harrison, E.M.; Hollyman, M.; Welbourn, R. Perioperative mortality in bariatric surgery: Meta-analysis. Br. J. Surg. 2021, 108, 892–897. [Google Scholar] [CrossRef]
- Osland, E.J.; Yunus, R.M.; Khan, S.; Memon, M.A. Five-year Comorbidity Outcomes in Laparoscopic Vertical Sleeve Gastrectomy (LVSG) and Laparoscopic Roux-en-Y Gastric Bypass (LRYGB): A Systematic Review and Meta-analysis of Randomized Controlled Trials. Surg. Laparosc. Endosc. Percutan. Tech. 2023, 33, 241–248. [Google Scholar] [CrossRef]
- Sherf Dagan, S.; Goldenshluger, A.; Globus, I.; Schweiger, C.; Kessler, Y.; Kowen Sandbank, G.; Ben-Porat, T.; Sinai, T. Nutritional Recommendations for Adult Bariatric Surgery Patients: Clinical Practice. Adv. Nutr. 2017, 8, 382–394. [Google Scholar] [CrossRef]
- Cooper, T.C.; Simmons, E.B.; Webb, K.; Burns, J.L.; Kushner, R.F. Trends in Weight Regain Following Roux-en-Y Gastric Bypass (RYGB) Bariatric Surgery. Obes. Surg. 2015, 25, 1474–1481. [Google Scholar] [CrossRef] [PubMed]
- Ieong, K.; Ardila-Gatas, J.; Yang, J.; Zhang, X.; Tsui, S.T.; Spaniolas, K.; Pryor, A.D. Bone mineral density changes after bariatric surgery. Surg. Endosc. 2021, 35, 4763–4770. [Google Scholar] [CrossRef]
- Greco, C.; Passerini, F.; Coluccia, S.; Teglio, M.; Bondi, M.; Mecheri, F.; Trapani, V.; Volpe, A.; Toschi, P.; Madeo, B.; et al. Long-term trajectories of bone metabolism parameters and bone mineral density (BMD) in obese patients treated with metabolic surgery: A real-world, retrospective study. J. Endocrinol. Investig. 2023. [Google Scholar] [CrossRef] [PubMed]
- Gils Contreras, A.; Bonada Sanjaume, A.; Becerra-Tomás, N.; Salas-Salvadó, J. Adherence to Mediterranean Diet or Physical Activity After Bariatric Surgery and Its Effects on Weight Loss, Quality of Life, and Food Tolerance. Obes. Surg. 2020, 30, 687–696. [Google Scholar] [CrossRef]
- Schiavo, L.; Pilone, V.; Rossetti, G.; Iannelli, A. The Role of the Nutritionist in a Multidisciplinary Bariatric Surgery Team. Obes. Surg. 2019, 29, 1028–1030. [Google Scholar] [CrossRef]
- Eisenberg, D.; Shikora, S.A.; Aarts, E.; Aminian, A.; Angrisani, L.; Cohen, R.V.; de Luca, M.; Faria, S.L.; Goodpaster, K.P.S.; Haddad, A.; et al. 2022 American Society of Metabolic and Bariatric Surgery (ASMBS) and International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO) Indications for Metabolic and Bariatric Surgery. Obes. Surg. 2023, 33, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Bauer, K.; Lau, T.; Schwille-Kiuntke, J.; Schild, S.; Hauner, H.; Stengel, A.; Zipfel, S.; Mack, I. Conventional weight loss interventions across the different BMI obesity classes: A systematic review and quantitative comparative analysis. Eur. Eat. Disord. Rev. J. Eat. Disord. Assoc. 2020, 28, 492–512. [Google Scholar] [CrossRef] [PubMed]
- Parrott, J.; Frank, L.; Rabena, R.; Craggs-Dino, L.; Isom, K.A.; Greiman, L. American Society for Metabolic and Bariatric Surgery Integrated Health Nutritional Guidelines for the Surgical Weight Loss Patient 2016 Update: Micronutrients. Surg. Obes. Relat. Dis. Off. J. Am. Soc. Bariatr. Surg. 2017, 13, 727–741. [Google Scholar] [CrossRef]
- Csendes, A.; Burdiles, P.; Papapietro, K.; Diaz, J.C.; Maluenda, F.; Burgos, A.; Rojas, J. Results of gastric bypass plus resection of the distal excluded gastric segment in patients with morbid obesity. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 2005, 9, 121–131. [Google Scholar] [CrossRef]
- Thorell, A.; MacCormick, A.D.; Awad, S.; Reynolds, N.; Roulin, D.; Demartines, N.; Vignaud, M.; Alvarez, A.; Singh, P.M.; Lobo, D.N. Guidelines for Perioperative Care in Bariatric Surgery: Enhanced Recovery After Surgery (ERAS) Society Recommendations. World J. Surg. 2016, 40, 2065–2083. [Google Scholar] [CrossRef]
- Sabench Pereferrer, F.; Molina López, A.; Vives Espelta, M.; Raga Carceller, E.; Blanco Blasco, S.; Buils Vilalta, F.; París Sans, M.; Piñana Campón, M.L.; Hernández González, M.; Sánchez Marín, A.; et al. Weight Loss Analysis According to Different Formulas after Sleeve Gastrectomy With or Without Antral Preservation: A Randomised Study. Obes. Surg. 2017, 27, 1254–1260. [Google Scholar] [CrossRef] [PubMed]
- Castro Vázquez, J.; Saravia Barahona, F.; Loureiro González, C.; Leturio Fernández, S.; García Fernández, M.; Moro Delgado, A.; Barrenetxea Asua, J.; Ortiz Lacorzana, J.; Díez del Val, I. Sleeve gastrectomy as a surgical technique in bariatric surgery: Results of safety and effectiveness. Cir. Esp. Engl. Ed. 2022, 100, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Llamas, F.; López-Contreras, M.J.; Blanco, M.J.; López-Azorín, F.; Zamora, S.; Moreiras, O. Seemingly paradoxical seasonal influences on vitamin D status in nursing-home elderly people from a Mediterranean area. Nutrition 2008, 24, 414–420. [Google Scholar] [CrossRef]
- Ros, E. The PREDIMED study. Endocrinol. Diabetes Nutr. 2017, 64, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Tabesh, M.R.; Maleklou, F.; Ejtehadi, F.; Alizadeh, Z. Nutrition, Physical Activity, and Prescription of Supplements in Pre- and Post-bariatric Surgery Patients: A Practical Guideline. Obes. Surg. 2019, 29, 3385–3400. [Google Scholar] [CrossRef] [PubMed]
- Cunha, G.M.; Guzman, G.; Correa De Mello, L.L.; Trein, B.; Spina, L.; Bussade, I.; Marques Prata, J.; Sajoux, I.; Countinho, W. Efficacy of a 2-Month Very Low-Calorie Ketogenic Diet (VLCKD) Compared to a Standard Low-Calorie Diet in Reducing Visceral and Liver Fat Accumulation in Patients with Obesity. Front. Endocrinol. 2020, 11, 607. [Google Scholar] [CrossRef] [PubMed]
- Chakhtoura, M.; Nakhoul, N.; Akl, E.; Mantzoros, C.; El Hajj Fuleihan, G. Guidelines on Vitamin D Replacement in Bariatric Surgery: Identification and Systematic Appraisal. Metabolism 2016, 65, 586–597. [Google Scholar] [CrossRef]
- Mechanick, J.I.; Apovian, C.; Brethauer, S.; Timothy Garvey, W.; Joffe, A.M.; Kim, J.; Kushner, R.F.; Lindquist, R.; Pessah-Pollack, R.; Seger, J.; et al. Clinical Practice Guidelines for the Perioperative Nutrition, Metabolic, and Nonsurgical Support of Patients Undergoing Bariatric Procedures—2019 Update: Cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, The Obesity Society, American Society for Metabolic and Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists. Obesity (Silver Spring) 2020, 28, O1–O58. [Google Scholar] [CrossRef]
- Di Lorenzo, N.; Antoniou, S.A.; Batterham, R.L.; Busetto, L.; Godoroja, D.; Iossa, A.; Carrano, F.M.; Agresta, F.; Alarçon, I.; Azran, C.; et al. Clinical practice guidelines of the European Association for Endoscopic Surgery (EAES) on bariatric surgery: Update 2020 endorsed by IFSO-EC, EASO and ESPCOP. Surg. Endosc. 2020, 34, 2332–2358. [Google Scholar] [CrossRef]
- Fried, M.; Yumuk, V.; Oppert, J.M.; Scopinaro, N.; Torres, A.; Weiner, R.; Yashkov, Y.; Frühbeck, G.; International Federation for Surgery of Obesity and Metabolic Disorders-European Chapter (IFSO-EC); European Association for the Study of Obesity (EASO); et al. Interdisciplinary European guidelines on metabolic and bariatric surgery. Obes. Surg. 2014, 24, 42–55. [Google Scholar] [CrossRef]
- Jamal, M.K.; DeMaria, E.J.; Johnson, J.M.; Carmody, B.J.; Wolfe, L.G.; Kellum, J.M.; Meador, J.G. Insurance-mandated preoperative dietary counseling does not improve outcome and increases dropout rates in patients considering gastric bypass surgery for morbid obesity. Surg. Obes. Relat. Dis. Off. J. Am. Soc. Bariatr. Surg. 2006, 2, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Franz, M.J.; VanWormer, J.J.; Crain, A.L.; Boucher, J.L.; Histon, T.; Caplan, W.; Bowman, J.D.; Pronk, N.P. Weight-loss outcomes: A systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J. Am. Diet. Assoc. 2007, 107, 1755–1767. [Google Scholar] [CrossRef] [PubMed]
- Benotti, P.N.; Still, C.D.; Wood, G.C.; Akmal, Y.; King, H.; El Arousy, H.; Dancea, H.; Gerhard, G.S.; Petrick, A.; Strodel, W. Preoperative weight loss before bariatric surgery. Arch Surg. 2009, 144, 1150–1155. [Google Scholar] [CrossRef] [PubMed]
- Tewksbury, C.; Crowley, N.; Parrott, J.M.; Andromalos, L.; Isom, K.A.; Smith, E.; Allison, K.C. Weight Loss Prior to Bariatric Surgery and 30-Day Mortality, Readmission, Reoperation, and Intervention: An MBSAQIP Analysis of 349,016 Cases. Obes. Surg. 2019, 29, 3622–3628. [Google Scholar] [CrossRef] [PubMed]
- Giordano, S.; Victorzon, M. The impact of preoperative weight loss before laparoscopic gastric bypass. Obes. Surg. 2014, 24, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Verde, L.; Sulu, C.; Katsiki, N.; Hassapidou, M.; Frias-Toral, E.; Cucalón, G.; Pazderska, A.; Yumuk, V.D.; Colao, A.; et al. Mediterranean Diet and Obesity-related Disorders: What is the Evidence? Curr. Obes. Rep. 2022, 11, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.S.; Dhaliwal, S.S.; Hills, A.P.; Pal, S. The effect of 12 weeks of aerobic, resistance or combination exercise training on cardiovascular risk factors in the overweight and obese in a randomized trial. BMC Public Health 2012, 12, 704. [Google Scholar] [CrossRef]
- Johns, D.J.; Hartmann-Boyce, J.; Jebb, S.A.; Aveyard, P. Diet or Exercise Interventions vs Combined Behavioral Weight Management Programs: A Systematic Review and Meta-Analysis of Direct Comparisons. J. Acad. Nutr. Diet. 2014, 114, 1557–1568. [Google Scholar] [CrossRef]
- Barrea, L.; Muscogiuri, G.; Laudisio, D.; Pugliese, G.; de Alteriis, G.; Colao, A.; Savastano, S. Influence of the Mediterranean Diet on 25-Hydroxyvitamin D Levels in Adults. Nutrients 2020, 12, 1439. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, Z.-B. Exercise: A Possibly Effective Way to Improve Vitamin D Nutritional Status. Nutrients 2022, 14, 2652. [Google Scholar] [CrossRef]
- Caprio, M.; Infante, M.; Calanchini, M.; Mammi, C.; Fabbri, A. Vitamin D: Not just the bone. Evidence for beneficial pleiotropic extraskeletal effects. Eat. Weight Disord. EWD 2017, 22, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Vimaleswaran, K.S.; Berry, D.J.; Lu, C.; Tikkanen, E.; Pilz, S.; Hiraki, L.T.; Cooper, J.D.; Dastani, Z.; Li, R.; Houston, D.K.; et al. Causal Relationship between Obesity and Vitamin D Status: Bi-Directional Mendelian Randomization Analysis of Multiple Cohorts. PLoS Med. 2013, 10, e1001383. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.T.; Kenney, W.L. The vitamin D-folate hypothesis in human vascular health. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2019, 317, R491–R501. [Google Scholar] [CrossRef]
- de Azevedo Muner Ferreira, B.; Fonseca, D.C.; Sala, P.; Alves, J.T.M.; Prudêncio, A.P.A.; Machado, N.M.; Marques, M.; Barcelos, S.; Ishida, R.K.; Guarda, I.F.M.S.; et al. Roux-en-Y gastric bypass affects the expression of genes related to the intestinal folate metabolism pathway in obese women. Nutrition 2023, 112, 112054. [Google Scholar] [CrossRef] [PubMed]
- Lespessailles, E.; Toumi, H. Vitamin D alteration associated with obesity and bariatric surgery. Exp. Biol. Med. 2017, 242, 1086–1094. [Google Scholar] [CrossRef]
- Vranić, L.; Mikolašević, I.; Milić, S. Vitamin D Deficiency: Consequence or Cause of Obesity? Medicina 2019, 55, 541. [Google Scholar] [CrossRef]

| Total (n = 121) | Control (n = 50) | PLM (n = 71) | p Value | |
|---|---|---|---|---|
| Glucose (mg/dL) | 94 (111) | 95 (34) | 97.5 (20) | 0.533 |
| Hemoglobin A1C (%) | 5.2 (4.8) | 5.95 (1.75) | 6.01 (0.77) | 0.147 |
| Total cholesterol (mg/dL) | 189.93 ± 36.94 | 191.36 ± 38.66 | 188.78 ± 35.96 | 0.714 |
| LDL-cholesterol (mg/dL) | 116.67 ± 32.97 | 114.2 ± 32.96 | 118.3 ± 33.12 | 0.529 |
| HDL-cholesterol (mg/dL) | 45.96 ± 11.2 | 46.18 ± 10.74 | 45.82 ± 11.51 | 0.901 |
| Triglycerides (mg/dL) | 154 (128) | 141 (108) | 113 (57) | 0.261 |
| Sodium (mmol/L) | 140.63 ± 2.35 | 140.8 ± 2.79 | 140.51 ± 2.01 | 0.512 |
| Potassium (mmol/L) | 4.4 (0.4) | 4.35 (0.45) | 4.4 (0.5) | 0.073 |
| Phosphorus (mg/dL) | 3.54 ± 0.6 | 3.72 ± 0.8 | 3.47 ± 0.49 | 0.104 |
| Calcium (mg/dL) | 9.13 ± 0.3 | 9.13 ± 0.35 | 9.13 ± 0.28 | 0.974 |
| Iron (ng/dL) | 75.21 ± 27.77 | 76.55 ± 29.28 | 74.29 ± 26.99 | 0.738 |
| Vitamin D (ng/mL) | 21.21 ± 11.25 | 25.24 ± 13.68 | 19.31 ± 9.21 | 0.015 |
| Vitamin B12 (ng/mL) | 365.56 ± 146.8 | 363.44 ± 148.47 | 400.87 ± 138.03 | 0.299 |
| PTH (pg/mL) | 51.23 ± 21.82 | 48.39 ± 24.33 | 54.04 ± 21.48 | 0.416 |
| Folic acid (ng/mL) | 6.45 ± 4.22 | 7.38 ± 5.47 | 5.74 ± 2.86 | 0.235 |
| GOT (UI/L) | 19 (20) | 19 (10) | 19.5 (9) | 0.731 |
| GPT (UI/L) | 37 (19) | 26.5 (20) | 24 (13) | 0.494 |
| Creatinine (mg/dL) | 0.94 (0.4) | 0.8 (0.28) | 0.7 (0.2) | 0.019 |
| Uric acid (mg/dL) | 6.45 ± 4.22 | 5.66 ± 1.11 | 5.88 ± 1.36 | 0.45 |
| Albumin (g/dL) | 4.2 (0.1) | 4.19 (0.3) | 4.2 (0.3) | 0.698 |
| Ferritin (ng/mL) | 83 (124) | 97 (167.8) | 75 (87.5) | 0.224 |
| V1 vs. V0 | Control (n = 50) | PLM (n = 71) | p Value |
|---|---|---|---|
| Dif. BMI (kg/m2) | −0.35 (2.25) | −2.76 (2.43) | <0.001 |
| %TWL | 0.78 ± 4.34 | 6.37 ± 4.31 | <0.001 |
| %EWL | 1.93 ± 11.64 | 16.33 ±10.72 | <0.001 |
| Dif. Phosphorus (mg/dL) | −0.35 ± 0.64 | 0.057 ± 0.54 | 0.023 |
| Dif. Vitamin D (ng/mL) | 0.96 (20.76) | 4.3 (11.23) | 0.167 |
| Dif. Calcium (mg/dL) | −0.13 ± 0.43 | 0.047 ± 0.33 | 0.298 |
| Dif. PTH (pg/mL) | 7.57 ± 22.8 | −4.78 ± 23.96 | 0.314 |
| Dif. Folic acid (ng/mL) | 2.45 ± 0.64 | 6.02 ± 11.3 | 0.732 |
| Control (n = 50) | PLM (n = 71) | p Value | |
| V2 vs. V0 | |||
| Dif. BMI (kg/m2) | −11.27 ± 3.21 | −12.72 ± 3.88 | 0.032 |
| %TWL | 25.41 ± 6.38 | 28.07 ± 6.74 | 0.031 |
| %EWL | 68.32 ± 18.88 | 73.21 ± 16.92 | 0.139 |
| Control (n = 50) | PLM (n = 71) | p value | |
| V3 vs. V0 | |||
| Dif. BMI (kg/m2) | −13.87 ± −3.79 | −15.81 ± 4.46 | 0.014 |
| %TWL | 31.3 ± 7.49 | 34.84 ± 7.53 | 0.012 |
| %EWL | 84.05 ± 21.76 | 90.65 ± 17.72 | 0.070 |
| %EBMIL | 94.8 ± 4.38 | 95.68 ± 3.71 | 0.138 |
| %EEBMIL | 90.3 ± 22.95 | 98.17 ± 19.03 | 0.043 |
| Control (n = 50) | PLM (n = 71) | p value | |
| V4 vs. V0 | |||
| Dif. BMI (kg/m2) | −13.53 (6.44) | −16.12 (7.5) | 0.049 |
| %TWL | 32.72 ± 9.09 | 35.79 ± 8.49 | 0.059 |
| %EWL | 65.52 ± 17.80 | 69.85 ± 15.33 | 0.155 |
| %EBMIL | 77.60 ± 22.44 | 82.44 ± 18.38 | 0.196 |
| %EEBMIL | 94.09 ± 25.96 | 100.95 ± 22.07 | 0.120 |
| Coefficient | p Value | 95% CI | R Squared | |
|---|---|---|---|---|
| V1 vs. V0 | ||||
| Dif. BMI (kg/m2) | −2.23 | <0.001 | −2.98 to −1.46 | - |
| %TWL | 5.68 | <0.001 | 4.07 to 7.29 | 0.34 |
| %EWL | 14.6 | <0.001 | 10.48 to 18.72 | 0.35 |
| V2 vs. V0 | ||||
| Dif. BMI (kg/m2) | −1.9 | 0.005 | −3.2 to −0.59 | 0.17 |
| %TWL | 3.2 | 0.008 | 0.875 to 5.67 | 0.15 |
| V3 vs. V0 | ||||
| Dif. BMI (kg/m2) | −2.063 | 0.009 | −3.59 to −0.53 | 0.16 |
| %TWL | 3.41 | 0.015 | 0.67 to 6.16 | 0.16 |
| %EEBMIL | 6.75 | 0.087 | −0.99 to 14.49 | 0.11 |
| V4 vs. V0 | ||||
| Dif. BMI (kg/m2) | −2.21 | 0.044 | −4.35 to −0.63 | - |
| Control (n = 50) | PLM (n = 71) | p Value | |
| V2 vs. V0 | |||
| Dif. Vitamin D (ng/mL) | 6.30 ± 15.48 | 17.46 ± 13.53 | 0.001 |
| Dif. Phosphorus (mg/dL) | −0.3 (0.75) | 0.1 (0.7) | 0.004 |
| Dif. Folic Acid (ng/mL) | −0.29 (8.22) | 1.52 (5.00) | 0.027 |
| Control (n = 50) | PLM (n = 71) | p value | |
| V3 vs. V0 | |||
| Dif. Vitamin D (ng/mL) | 4.93 ± 16.7 | 15.50 ± 14.62 | 0.004 |
| Dif. Phosphorus (mg/dL) | 0.06 ± 0.91 | 0.48 ± 0.62 | 0.031 |
| Dif. Folic Acid (ng/mL) | −0.85 (6.13) | 2.72 (5.73) | 0.127 |
| Control (n = 50) | PLM (n = 71) | p value | |
| V3 vs. V0 | |||
| Dif. Vitamin D (ng/mL) | 4.93 ± 16.7 | 15.50 ± 14.62 | 0.004 |
| Dif. Phosphorus (mg/dL) | 0.06 ± 0.91 | 0.48 ± 0.62 | 0.031 |
| Dif. Folic Acid (ng/mL) | −0.85 (6.13) | 2.72 (5.73) | 0.127 |
| Coefficient | p Value | 95% CI | R Squared | |
|---|---|---|---|---|
| V2 vs. V0 | ||||
| Dif. Vitamin D (ng/mL) | 8.4 | 0.030 | 0.85 to 15.97 | 0.153 |
| Dif. Phosphorus (mg/dL) | 0.56 | 0.007 | 0.16 to 0.96 | - |
| Dif. Folic Acid (ng/mL) | 2.9 | 0.242 | −2.1 to 7.95 | - |
| V3 vs. V0 | ||||
| Dif. Vitamin D (ng/mL) | 7.12 | 0.088 | −1.1 to 15.43 | 0.124 |
| Dif. Phosphorus (mg/dL) | 0.39 | 0.045 | −0.01 to 0.76 | 0.252 |
| V4 vs. V0 | ||||
| Dif. Vitamin D (ng/mL) | 9.78 | 0.134 | −3.07 to 22.63 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crespo-Yanguas, M.; Lumpuy-Castillo, J.; Espadas, C.; Aragón-Valera, C.; Vázquez, C.; Lorenzo, Ó. A Program of Life-Style Modification Improved the Body Weight and Micronutrient Status in Obese Patients after Bariatric Surgery. Nutrients 2023, 15, 3807. https://doi.org/10.3390/nu15173807
Crespo-Yanguas M, Lumpuy-Castillo J, Espadas C, Aragón-Valera C, Vázquez C, Lorenzo Ó. A Program of Life-Style Modification Improved the Body Weight and Micronutrient Status in Obese Patients after Bariatric Surgery. Nutrients. 2023; 15(17):3807. https://doi.org/10.3390/nu15173807
Chicago/Turabian StyleCrespo-Yanguas, Marta, Jairo Lumpuy-Castillo, Cristina Espadas, Carmen Aragón-Valera, Clotilde Vázquez, and Óscar Lorenzo. 2023. "A Program of Life-Style Modification Improved the Body Weight and Micronutrient Status in Obese Patients after Bariatric Surgery" Nutrients 15, no. 17: 3807. https://doi.org/10.3390/nu15173807
APA StyleCrespo-Yanguas, M., Lumpuy-Castillo, J., Espadas, C., Aragón-Valera, C., Vázquez, C., & Lorenzo, Ó. (2023). A Program of Life-Style Modification Improved the Body Weight and Micronutrient Status in Obese Patients after Bariatric Surgery. Nutrients, 15(17), 3807. https://doi.org/10.3390/nu15173807








