Codonopsis lanceolata Extract Restores Smooth Muscle Vasorelaxation in Rat Carotid Arteries Even under High Extracellular K+ Concentrations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Plant Extracts and Chemicals
2.2. Experimental Animals and Myography
2.3. Involvements of Extracellular Ca2+ Influx and Intracellular Ca2+ Release in the Effects of ECL on Vascular Tension
2.4. Involvement of K+ Channels in the Effects of ECL on Vascular Tension
2.5. Statistical Analysis
3. Results
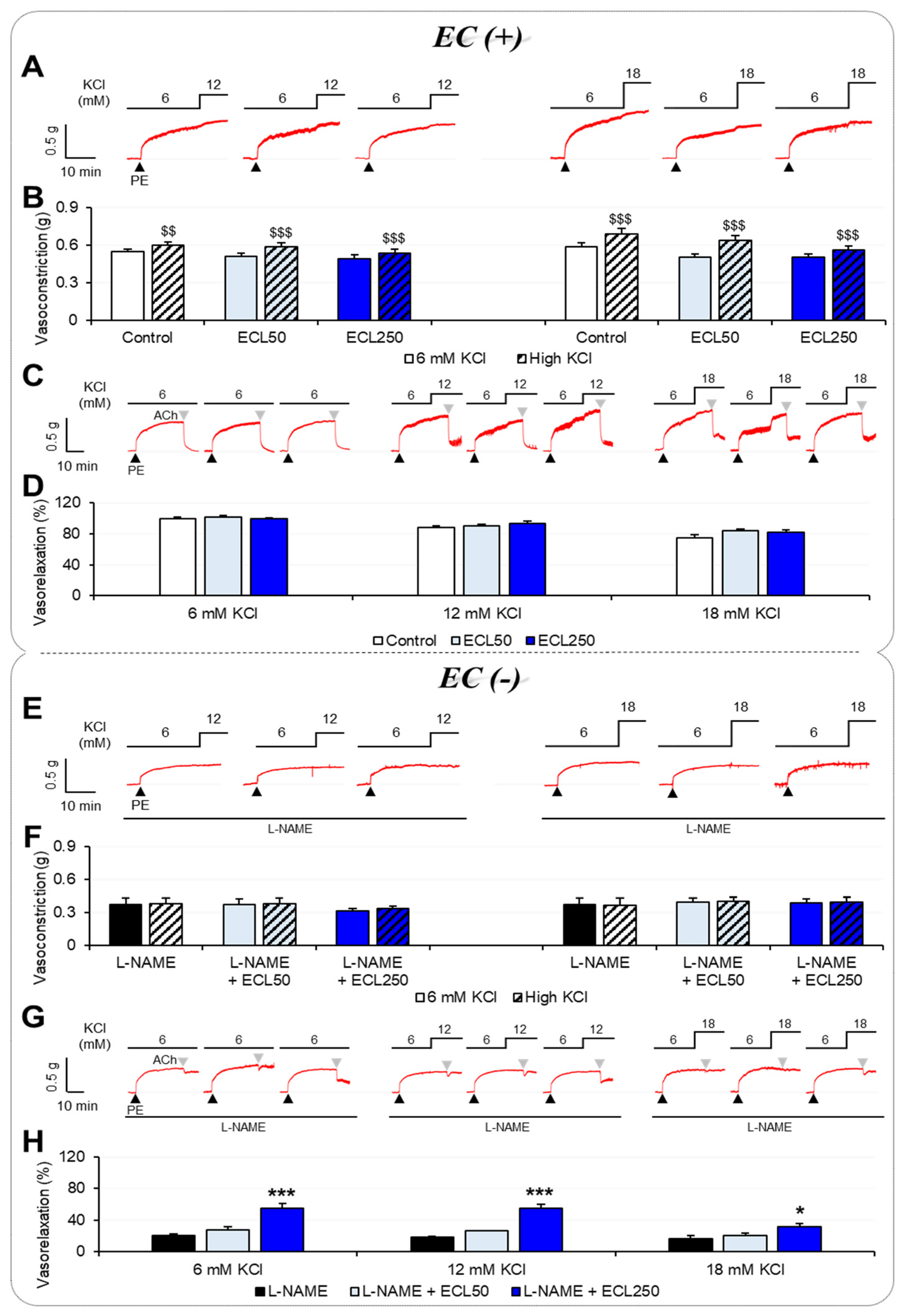
3.1. Effects of ECL on Vascular Tension According to [K+]o
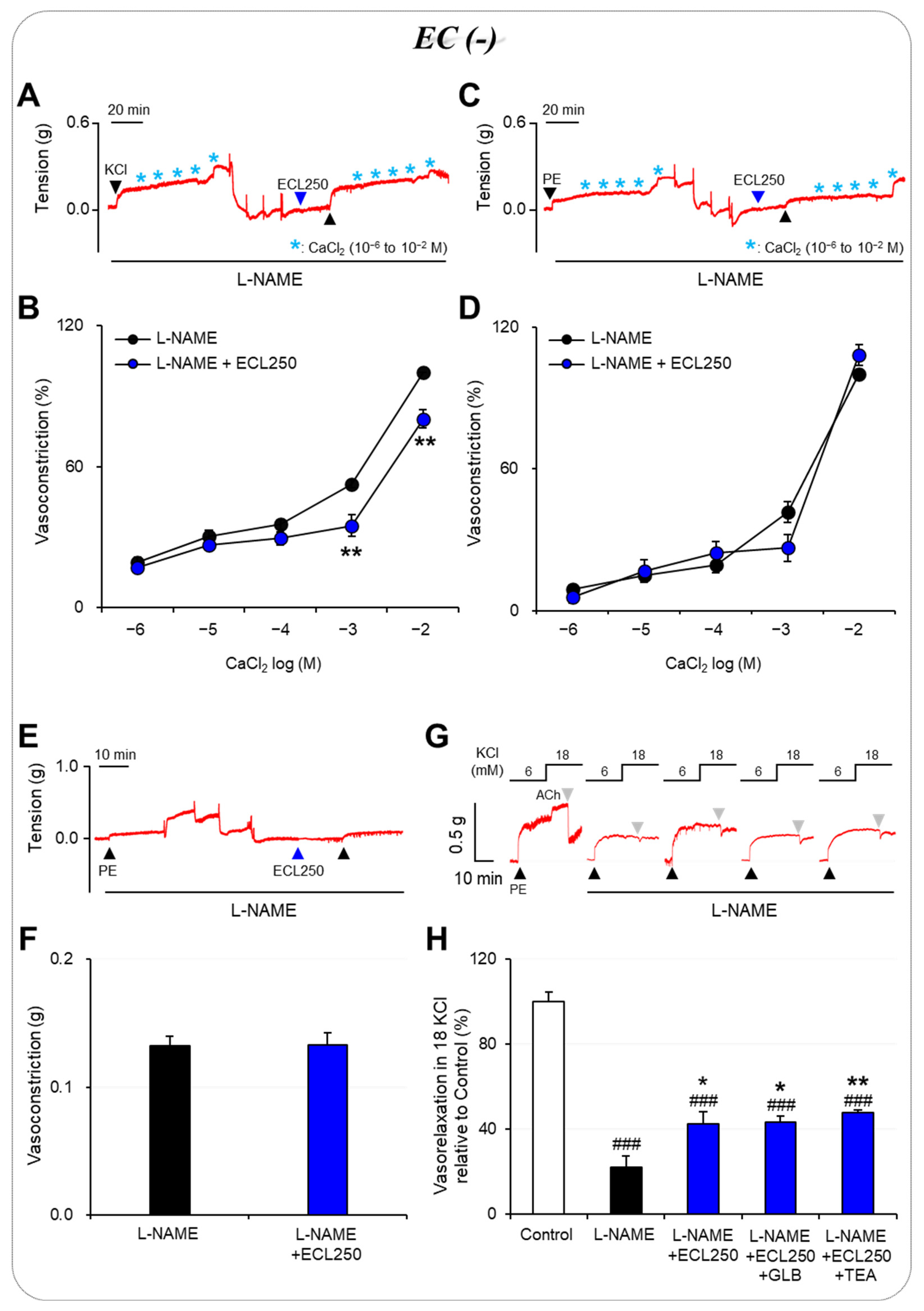
3.2. Involvement of Ca2+ Influx or Release in the Effect of ECL
3.3. Involvement of K+ Channels in the Effect of ECL
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garland, C.J.; Dora, K.A. Endothelium-Dependent Hyperpolarization: The Evolution of Myoendothelial Microdomains. J. Cardiovasc. Pharmacol. 2021, 78, S3–S12. [Google Scholar] [CrossRef]
- Akata, T. General anesthetics and vascular smooth muscle: Direct actions of general anesthetics on cellular mechanisms regulating vascular tone. Anesthesiology 2007, 106, 365–391. [Google Scholar] [CrossRef]
- Daghbouche-Rubio, N.; López-López, J.R.; Pérez-García, M.T.; Cidad, P. Vascular smooth muscle ion channels in essential hypertension. Front. Physiol. 2022, 13, 1016175. [Google Scholar] [CrossRef]
- Ekmekcioglu, C.; Elmadfa, I.; Meyer, A.L.; Moeslinger, T. The role of dietary potassium in hypertension and diabetes. J. Physiol. Biochem. 2016, 72, 93–106. [Google Scholar] [CrossRef]
- Palmer, B.F.; Clegg, D.J. Physiology and pathophysiology of potassium homeostasis. Adv. Physiol. Educ. 2016, 40, 480–490. [Google Scholar] [CrossRef]
- Sarnowski, A.; Gama, R.M.; Dawson, A.; Mason, H.; Banerjee, D. Hyperkalemia in Chronic Kidney Disease: Links, Risks and Management. Int. J. Nephrol. Renovasc. Dis. 2022, 15, 215–228. [Google Scholar] [CrossRef]
- Emmett, M. Review of Clinical Disorders Causing Metabolic Acidosis. Adv. Chronic Kidney Dis. 2022, 29, 355–363. [Google Scholar] [CrossRef]
- Navarrete, N. Hyperkalemia in electrical burns: A retrospective study in Colombia. Burns 2018, 44, 941–946. [Google Scholar] [CrossRef]
- Nwokocha, C.; Palacios, J.; Ojukwu, V.E.; Nna, V.U.; Owu, D.U.; Nwokocha, M.; McGrowder, D.; Orie, N.N. Oxidant-induced disruption of vascular K+ channel function: Implications for diabetic vasculopathy. Arch. Physiol. Biochem. 2022, 1–12. [Google Scholar] [CrossRef]
- Knox, M.; Vinet, R.; Fuentes, L.; Morales, B.; Martínez, J.L. A Review of Endothelium-Dependent and -Independent Vasodilation Induced by Phytochemicals in Isolated Rat Aorta. Animals 2019, 9, 623. [Google Scholar] [CrossRef]
- Dogan, M.F.; Yildiz, O.; Arslan, S.O.; Ulusoy, K.G. Potassium channels in vascular smooth muscle: A pathophysiological and pharmacological perspective. Fundam. Clin. Pharmacol. 2019, 33, 504–523. [Google Scholar] [CrossRef]
- Hossen, M.J.; Kim, M.Y.; Kim, J.H.; Cho, J.Y. Codonopsis lanceolata: A Review of Its Therapeutic Potentials. Phytother. Res. 2016, 30, 347–356. [Google Scholar] [CrossRef]
- Ushijima, M.; Komoto, N.; Sugizono, Y.; Mizuno, I.; Sumihiro, M.; Ichikawa, M.; Hayama, M.; Kawahara, N.; Nakane, T.; Shirota, O.; et al. Triterpene glycosides from the roots of Codonopsis lanceolata. Chem. Pharm. Bull. 2008, 56, 308–314. [Google Scholar] [CrossRef]
- Hyam, S.R.; Jang, S.E.; Jeong, J.J.; Joh, E.H.; Han, M.J.; Kim, D.H. Echinocystic acid, a metabolite of lancemaside A, inhibits TNBS-induced colitis in mice. Int. Immunopharmacol. 2013, 15, 433–441. [Google Scholar] [CrossRef]
- Joh, E.H.; Lee, I.A.; Han, S.J.; Chae, S.; Kim, D.H. Lancemaside A ameliorates colitis by inhibiting NF-kappaB activation in TNBS-induced colitis mice. Int. J. Colorectal. Dis. 2010, 25, 545–551. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Zhang, L.; Yuan, Y.; Yu, D. Codonopsis lanceolata polysaccharide CLPS alleviates high fat/high sucrose diet-induced insulin resistance via anti-oxidative stress. Int. J. Biol. Macromol. 2020, 145, 944–949. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, H.; Kim, J.; Seol, G.H.; Lee, K.W. Lancemaside A, a major triterpene saponin of Codonopsis lanceolata enhances regulation of nitric oxide synthesis via eNOS activation. BMC Complement. Altern. Med. 2019, 19, 110. [Google Scholar] [CrossRef]
- Shin, Y.K.; Han, A.Y.; Hsieh, Y.S.; Kwon, S.; Kim, J.; Lee, K.W.; Seol, G.H. Lancemaside A from Codonopsis lanceolata prevents hypertension by inhibiting NADPH oxidase 2-mediated MAPK signalling and improving NO bioavailability in rats. J. Pharm. Pharmacol. 2019, 71, 1458–1468. [Google Scholar] [CrossRef]
- Han, A.Y.; Lee, Y.S.; Kwon, S.; Lee, H.S.; Lee, K.W.; Seol, G.H. Codonopsis lanceolata extract prevents hypertension in rats. Phytomedicine 2018, 39, 119–124. [Google Scholar] [CrossRef]
- Shin, Y.K.; Hsieh, Y.S.; Han, A.Y.; Lee, K.W.; Seol, G.H. Beneficial effects of Codonopsis lanceolata extract on systolic blood pressure levels in prehypertensive adults: A double-blind, randomized controlled trial. Phytother. Res. 2020, 34, 340–348. [Google Scholar] [CrossRef]
- Kim, M.K.; Han, A.Y.; Shin, Y.K.; Lee, K.W.; Seol, G.H. Codonopsis lanceolata Contributes to Ca2+ Homeostasis by Mediating SOCE and PLC/IP3 Pathways in Vascular Endothelial and Smooth Muscle Cells. Planta Medica 2020, 86, 1345–1352. [Google Scholar] [CrossRef]
- Seol, G.H.; Ahn, S.C.; Kim, J.A.; Nilius, B.; Suh, S.H. Inhibition of endothelium-dependent vasorelaxation by extracellular K+: A novel controlling signal for vascular contractility. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H329–H339. [Google Scholar] [CrossRef]
- Aekthammarat, D.; Tangsucharit, P.; Pannangpetch, P.; Sriwantana, T.; Sibmooh, N. Moringa oleifera leaf extract enhances endothelial nitric oxide production leading to relaxation of resistance artery and lowering of arterial blood pressure. Biomed Pharmacother. 2020, 130, 110605. [Google Scholar] [CrossRef]
- Yang, S.; Xu, Z.; Lin, C.; Li, H.; Sun, J.; Chen, J.; Wang, C. Schisantherin A causes endothelium-dependent and -independent vasorelaxation in isolated rat thoracic aorta. Life Sci. 2020, 245, 117357. [Google Scholar] [CrossRef]
- Tykocki, N.R.; Boerman, E.M.; Jackson, W.F. Smooth Muscle Ion Channels and Regulation of Vascular Tone in Resistance Arteries and Arterioles. Compr. Physiol. 2017, 7, 485–581. [Google Scholar] [CrossRef]
- Afsar, S.; Hemsinli, D.; Ozyazgan, S.; Akkan, A.G.; Arslan, C. The Effects of Potassium Channels in Human Internal Mammary Artery. Pharmacology 2016, 97, 72–77. [Google Scholar] [CrossRef]
- Pathan, A.R.; Rusch, N.J. Two-pore domain K+ channels: Evidence for TWIK-2 in blood pressure regulation. Hypertension 2011, 58, 539–541. [Google Scholar] [CrossRef]
- Hu, G.Y.; Peng, C.; Xie, X.F.; Xiong, L.; Zhang, S.Y.; Cao, X.Y. Patchouli alcohol isolated from Pogostemon cablin mediates endothelium-independent vasorelaxation by blockade of Ca2+ channels in rat isolated thoracic aorta. J. Ethnopharmacol. 2018, 220, 188–196. [Google Scholar] [CrossRef]
- Panizo, N.; Rubio-Navarro, A.; Amaro-Villalobos, J.M.; Egido, J.; Moreno, J.A. Molecular Mechanisms and Novel Therapeutic Approaches to Rhabdomyolysis-Induced Acute Kidney Injury. Kidney Blood Press. Res. 2015, 40, 520–532. [Google Scholar] [CrossRef]
- Cabral, B.M.I.; Edding, S.N.; Portocarrero, J.P.; Lerma, E.V. Rhabdomyolysis. Dis. Mon. 2020, 66, 101015. [Google Scholar] [CrossRef]
- Giannoglou, G.D.; Chatzizisis, Y.S.; Misirli, G. The syndrome of rhabdomyolysis: Pathophysiology and diagnosis. Eur. J. Intern. Med. 2007, 18, 90–100. [Google Scholar] [CrossRef]
- Roumeliotis, S.; Mallamaci, F.; Zoccali, C. Endothelial Dysfunction in Chronic Kidney Disease, from Biology to Clinical Outcomes: A 2020 Update. J. Clin. Med. 2020, 9, 2359. [Google Scholar] [CrossRef]
- Khreba, N.; Khedr, D.; Abdel-Baky, A.; Kannishy, G.E.; Samaan, E. Nephron index rather than serum FGF 23 predicts endothelial dysfunction in early but not advanced chronic kidney disease patients. Int. Urol. Nephrol. 2023; in press. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, U.; Shin, Y.K.; Park, J.; Seol, G.H. Codonopsis lanceolata Extract Restores Smooth Muscle Vasorelaxation in Rat Carotid Arteries Even under High Extracellular K+ Concentrations. Nutrients 2023, 15, 3791. https://doi.org/10.3390/nu15173791
Kim U, Shin YK, Park J, Seol GH. Codonopsis lanceolata Extract Restores Smooth Muscle Vasorelaxation in Rat Carotid Arteries Even under High Extracellular K+ Concentrations. Nutrients. 2023; 15(17):3791. https://doi.org/10.3390/nu15173791
Chicago/Turabian StyleKim, Uihwan, You Kyoung Shin, Jubin Park, and Geun Hee Seol. 2023. "Codonopsis lanceolata Extract Restores Smooth Muscle Vasorelaxation in Rat Carotid Arteries Even under High Extracellular K+ Concentrations" Nutrients 15, no. 17: 3791. https://doi.org/10.3390/nu15173791
APA StyleKim, U., Shin, Y. K., Park, J., & Seol, G. H. (2023). Codonopsis lanceolata Extract Restores Smooth Muscle Vasorelaxation in Rat Carotid Arteries Even under High Extracellular K+ Concentrations. Nutrients, 15(17), 3791. https://doi.org/10.3390/nu15173791





