Abstract
Evidence for the effects of dietary diversity changes and cognitive frailty (CF) in the older adults is not clear. This study aimed to investigate the relationship between dietary diversity changes and CF in older adults Chinese. A total of 14,382 participants (mean age: 82.3 years) were enrolled. Dietary diversity scores (DDSs) were collected and calculated using a food frequency questionnaire. DDS changes between baseline and first follow-up were categorized into nine patterns. The associations between DDS changes and the incidence of CF were estimated using Cox proportional hazards models. During an 80,860 person-year follow-up, 3023 CF cases were identified. Groups with a decrease in DDS had increased CF risk compared with the high-to-high DDS group, with adjusted hazard ratios (HRs; 95% confidence intervals (Cis)) of 1.30 (1.06, 1.59), 2.04 (1.51, 2.74), and 1.81 (1.47, 2.22) for high-to-medium, high-to-low, and medium-to-low groups, respectively. Lower overall DDS groups were associated with greater CF risks, with HRs (95% CIs) of 1.49 (1.19, 1.86) for the low-to-medium group and 1.96 (1.53, 2.52) for the low-to-low group. Compared with the high-to-high group, significant associations with CF were found in other DDS change groups; HRs ranged from 1.38 to 3.12 for the plant-based DDS group and from 1.24 to 1.32 for the animal-based DDS group. Additionally, extreme and moderate declines in overall DDS increased CF risk compared with stable DDS, with HRs (95% CIs) of 1.67 (1.50, 1.86) and 1.13 (1.03, 1.24), respectively. In conclusion, among older adults, a declining or persistently low DDS and a moderately or extremely declining DDS were linked to higher incident CF. Plant-based DDS changes correlated more strongly with CF than animal-based DDS changes.
1. Introduction
The rising prevalence of age-related diseases among the older adults has garnered increasing attention due to the aging of the global population. Cognitive frailty (CF) is a clinical symptom combining cognitive impairment and physical frailty [1], reflecting a state of nonspecific vulnerability. Previous studies have investigated whether CF may increase the risk of adverse outcomes [2,3] and mortality [4,5] in comparison with physical frailty or cognitive impairment alone [6,7,8]. Importantly, CF can be reversible if effective interventions are employed [9]. Therefore, it is imperative to identify modifiable risk factors and implement preventive and health promotion strategies in the early or reversible stages.
In recent years, the modifiable risk factor of overall diet quality has received plenty of attention, with consideration given to the effects of various nutrients and other bioactive constituents [10,11,12]. The dietary diversity score (DDS) is recognized as a valid and clinically pertinent measure that reflects nutrient sufficiency and diet quality among the numerous methods used to evaluate overall diet. Some studies have found a correlation or interaction between DDS and nutritional status [13,14], suggesting that a higher DDS may be indicative of nutrient sufficiency and improved health in older adults. Furthermore, the DDS has been shown to be related to the risk of numerous diseases [15,16]. Cross-sectional research has suggested that CF deterioration was associated with a lower DDS [17], but its design limited any inference of a causal relationship. Moreover, the above study only measured baseline DDS; ignoring measurement bias could alter the results with the evolution of diet during follow-up. Dietary diversity changes have been confirmed to be linked to cognitive impairment [18] and mortality [19] in the older adults in previous longitudinal studies. However, the association between changes from baseline DDS to follow-up DDS and CF has yet to be fully explored.
To fill this knowledge gap, we explored DDS change patterns and CF on the basis of a prospective cohort study of the Chinese Longitudinal Healthy Longevity Survey (CLHLS).
2. Materials and Methods
2.1. Study Setting and Participants
This study included participants in the CLHLS. The CLHLS commenced in 1998 and enrolled new participants during every follow-up to maintain the sample size. It covers nearly 85% of the population across 23 provinces in China. Data were obtained through a structured questionnaire through face-to-face interviews; the details regarding the study design were reported earlier [20].
From the CLHLS, we analyzed three successive 9-year cohorts of longitudinal data (the 2002, 2005, and 2008 cohorts); each consisting of four waves of data (participants recruited in the 2002 cohort were followed up in the 2005, 2008, and 2011 waves; those newly recruited in the 2005 cohort were followed up in the 2008, 2011, and 2014 waves; and those newly recruited in the 2008 cohort were followed up in the 2011, 2014, and 2017 waves. Of the initial 48,656 individuals who participated in our study, 26,533 participants were excluded: 460 with an age of <65 years, 15 with incomplete dietary data at baseline, 24,425 with incomplete dietary data at the first follow-up, 1596 with CF at baseline, and 15 who were lost to the follow-up CF assessment. Among the remaining 22,123 participants, we excluded those duplicated in three cohorts (n = 7741), resulting in a final sample size of 14,382 (Figure S1).
2.2. Definitions of DDS Change Patterns
The valid and reliable simplified food-frequency questionnaire was used to collect the frequency of consumption of the following 9 food items [21]: fresh vegetables, fruit, tea, garlic, beans, preserved vegetables, meat, fish, and eggs. We calculated the DDS according to the method proposed by Kant [22] and considered both the number and frequency of food categories. In general, we constructed an overall DDS (a score of 0 to 18), a plant-based DDS (a score of 0 to 12), and an animal-based DDS (a score of 0 to 6). Subsequently, we divided participants into 3 groups by the trisection of the total scores (high, medium, and low) and combined both baseline and the first follow-up visit DDS to form 9 relative DDS change patterns. We also computed the absolute change scores (the numerical value of the first follow-up DDS minus the baseline DDS) and categorized participants into 5 absolute DDS change patterns [19]. More details are provided in the supplementary methods [19,23,24,25,26,27].
2.3. Assessment of CF
Cognition impairment was defined by combining education with the scores of the Chinese version of the Mini-Mental State Examination. Physical frailty was evaluated using the modified Fried criteria. The present study defined CF as the co-occurrence of cognitive impairment and physical frailty [1] (details can be found in the supplementary methods). The follow-up period was from enrolment until the end of follow-up or incident CF, whichever occurred first.
2.4. Assessment of Covariates
We included explanatory variable groups based on previous studies [18,19]. Potential confounders included demographics, lifestyle, and health status. Demographics were age (continuous), sex, living areas, marital status, occupation, years of education, income source, and sufficient income. Lifestyle included living arrangement, body mass index (BMI; continuous), regular exercise, smoking status, and drinking status. Health status included 4 chronic diseases: hypertension, diabetes, stroke, and heart disease.
2.5. Statistical Analysis
The missing data rates for all variables were less than 1.31% (Table S1). Multiple imputations were used to impute missing covariates in order to reduce inferential bias and maximize statistical power [28]. The Cox proportional hazards regression model was used to calculate the hazard ratios (HRs) and 95% confidence intervals (CIs) for DDS change patterns and CF risk.
We tested the assumption of the proportional risk and found that it was satisfied. Two sets of models were conducted. The base model controlled only for age and sex; the fully adjusted model further controlled for living area, marital status, occupation, years of education, income source, sufficient income, living arrangement, BMI, regular exercise, smoking status, drinking status, hypertension, diabetes, stroke, and heart disease.
Subgroup analyses were conducted according to age, sex, living areas, smoking status, drinking status, and regular exercise to examine the association between DDS change patterns and CF. To evaluate the robustness of the findings, multiple sensitivity analyses were performed, including further adjustment for the number of teeth and the usage of dentures, additional adjustment for the year of recruitment, the exclusion of participants with dementia at baseline, and the exclusion of participants with missing covariates. Analyses were all performed using R 4.2.2. with a two-sided p-value of <0.05 indicating statistical significance.
3. Results
3.1. Baseline Characteristics
A total of 14,382 participants (53.7% women; mean age: 82.3 years) were included in the present study. According to relative DDS change patterns, the low-to-high group had the fewest participants (117, 0.8%), while the medium-to-medium group had the most participants (6939, 48.2%). Participants in the high-to-high pattern group were significantly younger and were more likely to be male, to live in urban areas, to be married, no not be a farmer, to have longer years of education, to have a pension and sufficient income, to live with a family member, to have a higher BMI, to regularly exercise, to be a current or former smoker, to be a current or former drinker, and to have hypertension, diabetes, and heart disease (Table 1).

Table 1.
Baseline characteristics of older adults according to DDS change patterns.
3.2. Relative DDS Change Patterns and CF
During the 80,860 person-years of follow-up (with a median follow-up time of 5.5 years), 3023 incident CF cases were recorded. The incidence of CF was lowest in the high-to-high group (23.9 per 1000 person-years), while it was greatest in the high-to-low group (71.4 per 1000 person-years). Compared with participants in the high-to-high group, a significantly increased risk of CF was observed in participants in the groups with a decrease in DDS. Specifically, regarding overall DDS, the adjusted HRs (95%CI) of developing CF were 1.30 (1.06, 1.59) for the high-to-medium group, 2.04 (1.51, 2.74) for the high-to-low group, and 1.81 (1.47, 2.22) for the medium-to-low group. Lower overall DDS patterns also increased CF risk with HRs of 1.49 (1.19, 1.86) for the low-to-medium group and 1.96 (1.53, 2.52) for the low-to-low group (Figure 1A).
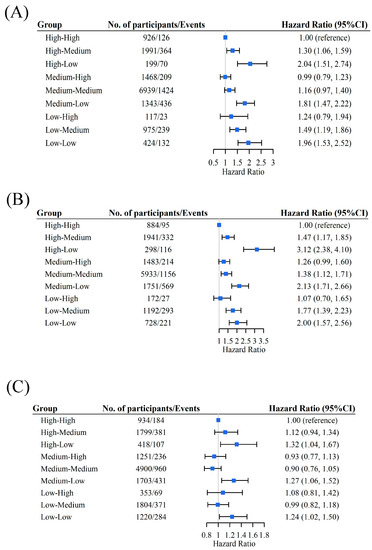
Figure 1.
Association between change patterns of overall DDS (A), plant-based DDS (B), and animal-based DDS (C) and CF. Adjusted for age, sex, living area, marital status, occupation, years of education, income source, sufficient income, living arrangement, BMI, regular exercise, smoking status, drinking status, hypertension, diabetes, stroke, and heart disease. The blue boxes in the figure represent the hazard ratios, and the horizontal lines indicate the corresponding 95% confidence intervals.
Similar relationships were observed between plant-based and animal-based DDSs and CF. Compared with the high-to-high group, significant HRs related to plant-based DDS ranged from 1.38 to 3.12 for the different plant food groups, and significant HRs related to animal-based DDS ranged from 1.24 to 1.32 for the different animal food groups. In particular, compared with the high-to-high group, the HR estimates for the plant-based DDS were 1.47 (1.17, 1.85) in the high-to-medium group, 3.12 (2.38, 4.10) in the high-to-low group, 1.38 (1.12, 1.71) in the medium-to-medium group, 2.13 (1.71, 2.66) in the medium-to-low group, 1.77 (1.39, 2.23) in the low-to-medium group, and 2.00 (1.57, 2.56) in the low-to-low group (Figure 1B). For the animal-based DDS, the HR estimates were 1.32 (1.04, 1.67) in the high-to-low group, 1.27 (1.06, 1.52) in the medium-to-low group, and 1.24 (1.02, 1.50) in the low-to-low group (Figure 1C). Table 2 demonstrates the relationship between numerous food items and CF. These results were consistent with the main findings.

Table 2.
The association between DDS change patterns and CF in nine foods.
Subgroup analyses (Table 3) showed a significant interaction between DDS change and sex on CF. Specifically, males were more susceptible to the effects of DDS reduction on CF risk (p for interaction = 0.020). Additionally, a significant interaction was observed between DDS change and CF stratified by smoking status (p for interaction = 0.026). When stratified by age, living area, drinking, and regular exercise, the associations resembled our major findings. When we further adjusted for the number of teeth, usage of dentures, and the year of recruitment; excluded participants with dementia at baseline; or excluded participants with missing covariates, the sensitivity analyses indicated no substantial changes (Table S3).

Table 3.
The associations between DDS change patterns and CF in subgroups.
3.3. Absolute DDS Change Patterns and CF
We regarded a DDS that remained stable as a reference and found that a declining DDS was associated with a higher risk of CF. Specifically, the HRs (95% CI) were 1.67 (1.50, 1.86) for the extreme decline group and 1.13 (1.03, 1.24) for the moderate decline group, whereas the moderate and extreme improvement groups did not have a significantly increased risk of CF (p > 0.05) (Figure 2). Similar relationships were observed between plant-based or animal-based absolute DDS change patterns and CF (Table S4) and in the subgroup analyses (Table S5).
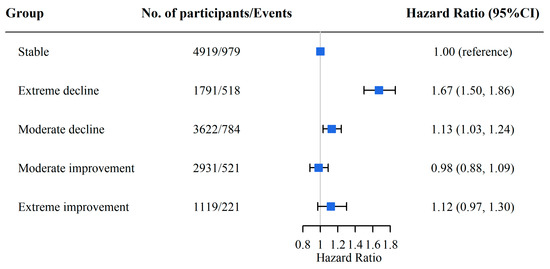
Figure 2.
The association between absolute DDS change groups and CF. Adjusted for age, sex, living area, marital status, occupation, years of education, income source, sufficient income, living arrangement, BMI, regular exercise, smoking status, drinking status, hypertension, diabetes, stroke, and heart disease. The blue boxes in the figure represent the hazard ratios, and the horizontal lines indicate the corresponding 95% confidence intervals.
4. Discussion
In this community-based prospective study involving 14,382 Chinese adults aged 65 years and older, participants with the highest DDS had the lowest incidence rates of CF. We found that a consistently higher DDS was related to a lower CF risk, while DDSs that declined extremely or remained low were related to a higher CF risk. We provided evidence that dynamic changes in DDS, as a flexible intervention strategy, have potential benefits among the older adults. These results highlight the importance of higher dietary diversity for CF prevention.
Dietary diversity has been identified as beneficial for reductions in cognitive impairment [29] and mortality risk [30]. However, the relationship between DDS changes and the incident CF remains unknown. In this study, a higher DDS was related to decreased CF risk, consistent with previous investigations of various dietary quality indices, such as the Mediterranean diet, the Healthy Eating Index 2015, the Mediterranean–DASH Intervention for Neurodegenerative Delay, and the Mediterranean Dietary Approach to Systolic Hypertension diet [31,32,33]. Given the challenges of assessing overall dietary quality with the measurement of the abovementioned indices in a clinical setting, the DDS emerges as a more practical approach without quantitative measurements. Our findings emphasize the significance of maintaining a high DDS, which may have valuable public health implications for older adults.
Global dietary guidelines recommend a varied diet [34,35,36], although specifics may vary by the study population and country. A monotonous diet devoid of a variety of carbohydrate foods, fruits, vegetables, and animal foods may not provide all the necessary nutrients [37]. Low dietary diversity scores may increase the likelihood of nutritional deficiencies, particularly in older adults [38]. Both declining and persistently low plant-based and animal-based DDSs were significantly associated with an increased risk of CF, whereas the association was stronger for plant-based DDS changes. In addition, consuming more fresh vegetables, fruit, tea, garlic, and meat was associated with lower incident CF. Previous studies have indicated that an increase in the consumption of these nutrients can reduce the incidence of cognitive impairment and physical frailty [39,40,41,42]. Several plausible mechanisms could explain this relationship. For instance, fresh vegetables, fruit, tea, and garlic are regarded as good sources of antioxidants to reduce inflammation and oxidative stress, which mitigate the loss of muscle and bone mass and the degeneration of central nervous system function [43]. Meat contains high-quality protein, which is beneficial for reversing or preventing age-related muscle tissue wasting and cognitive dysfunction [44].
Our findings indicate that maintaining a stable, high DDS can significantly reduce CF risk. This is partially consistent with the results of the Rotterdam study [45], which found that adherence to a healthy dietary pattern resulted in less physical frailty over time. It is noteworthy that the interpretation of DDS for specific foods was consistent with the overall DDS, incorporating a simultaneous analysis of all nine dietary categories. For example, in our study, when fresh vegetable consumption dropped from “often-to-often” to “often-to-occasionally”, the incidence of CF increased by 27.5 per 1000 person-years, indicating that even a slight reduction in the frequency at which older individuals consume vegetables can have negative consequences.
Another interesting finding was that participants with an extreme decline or moderate decline in DDS had a higher CF risk than those with stable DDSs. Our investigation regarding the characteristics of participants with an extreme decline or moderate decline in DDS reveals that the higher risk may be associated with inferior familial care (such as not being married due to being single, divorced, or bereaved), and a higher prevalence of chronic diseases (such as hypertension) (Table S2). Surprisingly, those in the high-to-high group were mainly smokers and drinkers (Table 1); this finding exhibited contrary characteristics to our initial expectations and is presumed to be associated with living in urban areas or receiving better family care.
The strengths of this study include the prospective design, large sample size, and the evaluation of dynamic changes in diet quality. However, several limitations should be considered. First, the research did not record quantitative food consumption data; therefore, energy intake was not accounted for. Second, self-reported data to calculate DDS may have introduced recall bias. Third, given the observational nature of the study, residual confounding and reverse causality may exist. Finally, caution should be exercised when generalizing these findings to other populations or countries, as the study was conducted among Chinese older adults.
5. Conclusions
A declining or persistently low DDS and a moderately or extremely declining DDS were associated with a significantly higher incidence of CF risk among Chinese adults aged 65 years and older. Notably, the relationship between plant-based DDS changes and CF was stronger than that between animal-based DDS changes and CF. This is the first study, to our knowledge, to provide evidence of the association between changes in DDS and CF among the Chinese older adults population. Our results emphasize the importance of dynamic changes in dietary diversity for the prevention of CF and improved health among older adults, providing a practical message to this population.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu15173784/s1, Figure S1. Flowchart of participant enrollment. Table S1. The numbers (percentages) of participants with missing covariates. Table S2. Baseline characteristics of older adults according to absolute DDS change patterns. Table S3. Sensitivity analyses of the association between DDS change patterns and CF. Table S4. The association between absolute DDS change groups and CF in plant-based and animal-based DDS. Table S5. The association between absolute DDS change groups and CF in subgroups.
Author Contributions
C.M. and W.-F.Z. designed the research and developed the analytical plan. C.M., W.-F.Z. and W.-Q.S. performed the statistical analyses and had primary responsibility for writing the manuscript. C.M., X.-M.S., V.B.K. and X.G. directed the study. W.-F.Z., W.-Q.S., X.-M.W., Z.-H.L., D.S., D.L., P.-D.Z., Q.-Q.S., F.L. and Y.N. contributed to the acquisition or interpretation of the data. J.-X.X., Z.-T.C., C.L., S.-T.L., X.-G.L., X.-R.L. and Y.-B.L. contributed to data cleaning. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme (2019), the Construction of the High-level University of Guangdong (G623330580 and G621331128), the Guangdong Graduate Innovation Program (D222330012), the Innovation and Entrepreneurship Training Program for College Students (202112121025), the Scientific Enlightenment Plan of Southern Medical University (2021), and the National Institutes of Health (NIH P30-AG028716). The funders played no role in the study design or implementation; manuscript preparation, review, or approval; or the decision to submit the manuscript for publication.
Institutional Review Board Statement
The study was conducted with the ethical approval of the Biomedical Ethics Committee of Peking University (IRB00001052-13074).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Available from the Peking University on request (https://opendata.pku.edu.cn; accessed on 3 April 2020).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kelaiditi, E.; Cesari, M.; Canevelli, M.; Abellan van Kan, G.; Ousset, P.J.; Gillette-Guyonnet, S.; Ritz, P.; Duveau, F.; Soto, M.E.; Provencher, V.; et al. Cognitive frailty: Rational and definition from an (I.A.N.A./I.A.G.G.) international consensus group. J. Nutr. Health Aging 2013, 17, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Nyunt, M.S.Z.; Gao, Q.; Feng, L.; Lee, T.S.; Tsoi, T.; Chong, M.S.; Lim, W.S.; Collinson, S.; Yap, P.; et al. Physical Frailty, Cognitive Impairment, and the Risk of Neurocognitive Disorder in the Singapore Longitudinal Ageing Studies. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Zin Nyunt, M.S.; Gao, Q.; Feng, L.; Yap, K.B.; Ng, T.P. Cognitive Frailty and Adverse Health Outcomes: Findings From the Singapore Longitudinal Ageing Studies (SLAS). J. Am. Med. Dir. Assoc. 2017, 18, 252–258. [Google Scholar] [CrossRef]
- Esteban-Cornejo, I.; Cabanas-Sánchez, V.; Higueras-Fresnillo, S.; Ortega, F.B.; Kramer, A.F.; Rodriguez-Artalejo, F.; Martinez-Gomez, D. Cognitive Frailty and Mortality in a National Cohort of Older Adults: The Role of Physical Activity. Mayo Clin. Proc. 2019, 94, 1180–1189. [Google Scholar] [CrossRef]
- Liu, L.K.; Chen, C.H.; Lee, W.J.; Wu, Y.H.; Hwang, A.C.; Lin, M.H.; Shimada, H.; Peng, L.N.; Loh, C.H.; Arai, H.; et al. Cognitive Frailty and Its Association with All-Cause Mortality Among Community-Dwelling Older Adults in Taiwan: Results from I-Lan Longitudinal Aging Study. Rejuvenation Res. 2018, 21, 510–517. [Google Scholar] [CrossRef]
- Solfrizzi, V.; Scafato, E.; Lozupone, M.; Seripa, D.; Giannini, M.; Sardone, R.; Bonfiglio, C.; Abbrescia, D.I.; Galluzzo, L.; Gandin, C.; et al. Additive Role of a Potentially Reversible Cognitive Frailty Model and Inflammatory State on the Risk of Disability: The Italian Longitudinal Study on Aging. Am. J. Geriatr. Psychiatry 2017, 25, 1236–1248. [Google Scholar] [CrossRef] [PubMed]
- Robertson, D.A.; Savva, G.M.; Kenny, R.A. Frailty and cognitive impairment--a review of the evidence and causal mechanisms. Ageing Res. Rev. 2013, 12, 840–851. [Google Scholar] [CrossRef]
- Matusik, P.; Tomaszewski, K.; Chmielowska, K.; Nowak, J.; Nowak, W.; Parnicka, A.; Dubiel, M.; Gąsowski, J.; Grodzicki, T. Severe frailty and cognitive impairment are related to higher mortality in 12-month follow-up of nursing home residents. Arch. Gerontol. Geriatr. 2012, 55, 22–24. [Google Scholar] [CrossRef]
- Ruan, Q.; Yu, Z.; Chen, M.; Bao, Z.; Li, J.; He, W. Cognitive frailty, a novel target for the prevention of elderly dependency. Ageing Res Rev. 2015, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Di Giosia, P.; Stamerra, C.A.; Giorgini, P.; Jamialahamdi, T.; Butler, A.E.; Sahebkar, A. The role of nutrition in inflammaging. Ageing Res. Rev. 2022, 77, 101596. [Google Scholar] [CrossRef]
- Shlisky, J.; Bloom, D.E.; Beaudreault, A.R.; Tucker, K.L.; Keller, H.H.; Freund-Levi, Y.; Fielding, R.A.; Cheng, F.W.; Jensen, G.L.; Wu, D.; et al. Nutritional Considerations for Healthy Aging and Reduction in Age-Related Chronic Disease. Adv. Nutr. 2017, 8, 17–26. [Google Scholar] [CrossRef]
- Kurotani, K.; Honjo, K.; Nakaya, T.; Ikeda, A.; Mizoue, T.; Sawada, N.; Tsugane, S.; Japan Public Health Center-based Prospective Study Group. Diet Quality Affects the Association between Census-Based Neighborhood Deprivation and All-Cause Mortality in Japanese Men and Women: The Japan Public Health Center-Based Prospective Study. Nutrients 2019, 11, 2194. [Google Scholar] [CrossRef]
- Otsuka, R.; Tange, C.; Nishita, Y.; Kato, Y.; Tomida, M.; Imai, T.; Ando, F.; Shimokata, H. Dietary Diversity and All-Cause and Cause-Specific Mortality in Japanese Community-Dwelling Older Adults. Nutrients 2020, 12, 1052. [Google Scholar] [CrossRef]
- Maila, G.; Audain, K.; Marinda, P.A. Association between dietary diversity, health and nutritional status of older persons in rural Zambia. S. Afr. J. Clin. Nutr. 2021, 34, 34–39. [Google Scholar] [CrossRef]
- Rezazadegan, M.; Mirjalili, F.; Jalilpiran, Y.; Aziz, M.; Jayedi, A.; Setayesh, L.; Yekaninejad, M.S.; Casazza, K.; Mirzaei, K. The Association Between Dietary Diversity Score and Odds of Diabetic Nephropathy: A Case-Control Study. Front. Nutr. 2022, 9, 767415. [Google Scholar] [CrossRef]
- Torres-Collado, L.; García-de la Hera, M.; Cano-Ibañez, N.; Bueno-Cavanillas, A.; Vioque, J. Association between Dietary Diversity and All-Cause Mortality: A Multivariable Model in a Mediterranean Population with 18 Years of Follow-Up. Nutrients 2022, 14, 1583. [Google Scholar] [CrossRef]
- Huang, W.C.; Huang, Y.C.; Lee, M.S.; Chang, H.Y.; Doong, J.Y. Frailty Severity and Cognitive Impairment Associated with Dietary Diversity in Older Adults in Taiwan. Nutrients 2021, 13, 418. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, W.T.; Wang, J.H.; Shen, D.; Zhang, P.D.; Li, Z.H.; Chen, P.L.; Zhang, X.R.; Huang, Q.M.; Zhong, W.F.; et al. Association between Dietary Diversity Changes and Cognitive Impairment among Older People: Findings from a Nationwide Cohort Study. Nutrients 2022, 14, 1251. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, X.R.; Li, Z.H.; Zhang, Y.J.; Lv, Y.B.; Wang, Z.H.; Shen, D.; Chen, P.L.; Zhong, W.F.; Huang, Q.M.; et al. Association of dietary diversity changes and mortality among older people: A prospective cohort study. Clin. Nutr. 2021, 40, 2620–2629. [Google Scholar] [CrossRef]
- Yi, Z.D., Jr.; Vlosky, D.A.; Gu, D. Healthy Longevity in China: Demographic, Socioeconomic, and Psychological Dimensions; Springer: Berlin/Heidelberg, Germany, 2008; Volume 20. [Google Scholar]
- Zhao, W.; Hasegawa, K.; Chen, J. The use of food-frequency questionnaires for various purposes in China. Public Health Nutr. 2002, 5, 829–833. [Google Scholar] [CrossRef]
- Kant, A.K.; Schatzkin, A.; Harris, T.B.; Ziegler, R.G.; Block, G. Dietary diversity and subsequent mortality in the First National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Am. J. Clin. Nutr. 1993, 57, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Kraus, V.B.; Gao, X.; Yin, Z.; Zhou, J.; Mao, C.; Duan, J.; Zeng, Y.; Brasher, M.S.; Shi, W.; et al. Higher dietary diversity scores and protein-rich food consumption were associated with lower risk of all-cause mortality in the oldest old. Clin. Nutr. 2020, 39, 2246–2254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Gu, D.; Hayward, M.D. Early life influences on cognitive impairment among oldest old Chinese. J. Gerontol. B Psychol. Sci. Soc. Sci. 2008, 63, S25–S33. [Google Scholar] [CrossRef]
- Zhang, M.; Katzman, R.; Salmon, D.; Jin, H.; Cai, G.; Wang, Z.; Qu, G.; Grant, I.; Yu, E.; Levy, P.; et al. The prevalence of dementia and Alzheimer’s disease in Shanghai, China: Impact of age, gender, and education. Ann. Neurol. 1990, 27, 428–437. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Wang, H.Y.; Lv, X.; Du, J.; Kong, G.; Zhang, L. Age- and Gender-Specific Prevalence of Frailty and Its Outcomes in the Longevous Population: The Chinese Longitudinal Healthy Longevity Study. Front. Med. (Lausanne) 2021, 8, 719806. [Google Scholar] [CrossRef]
- Romaniuk, H.; Patton, G.C.; Carlin, J.B. Multiple imputation in a longitudinal cohort study: A case study of sensitivity to imputation methods. Am. J. Epidemiol. 2014, 180, 920–932. [Google Scholar] [CrossRef]
- Zheng, J.; Zhou, R.; Li, F.; Chen, L.; Wu, K.; Huang, J.; Liu, H.; Huang, Z.; Xu, L.; Yuan, Z.; et al. Association between dietary diversity and cognitive impairment among the oldest-old: Findings from a nationwide cohort study. Clin. Nutr. 2021, 40, 1452–1462. [Google Scholar] [CrossRef]
- Kobayashi, M.; Sasazuki, S.; Shimazu, T.; Sawada, N.; Yamaji, T.; Iwasaki, M.; Mizoue, T.; Tsugane, S. Association of dietary diversity with total mortality and major causes of mortality in the Japanese population: JPHC study. Eur. J. Clin. Nutr. 2020, 74, 54–66. [Google Scholar] [CrossRef]
- Jayanama, K.; Theou, O.; Godin, J.; Cahill, L.; Shivappa, N.; Hébert, J.R.; Wirth, M.D.; Park, Y.M.; Fung, T.T.; Rockwood, K. Relationship between diet quality scores and the risk of frailty and mortality in adults across a wide age spectrum. BMC Med. 2021, 19, 64. [Google Scholar] [CrossRef]
- Alaghehband, F.R.; Erkkilä, A.T.; Rikkonen, T.; Sirola, J.; Kröger, H.; Isanejad, M. Association of Baltic Sea and Mediterranean diets with frailty phenotype in older women, Kuopio OSTPRE-FPS study. Eur. J. Nutr. 2021, 60, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Sacks, F.M.; Barnes, L.L.; Bennett, D.A.; Aggarwal, N.T. MIND diet slows cognitive decline with aging. Alzheimers Dement. 2015, 11, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Healthy Diet. Updated. 29 April 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/healthy-diet (accessed on 15 September 2022).
- Phillips, J.A. Dietary Guidelines for Americans, 2020–2025. Workplace Health Saf. 2021, 69, 395. [Google Scholar] [CrossRef] [PubMed]
- Society, C.N. Dietary Guidelines for Chinese Residents (2022). Updated 2022-04-29. Available online: http://dg.cnsoc.org/article/04/K7tlcs-UQh67DBC5XY1Jqw.html (accessed on 15 September 2022).
- Azadbakht, L.; Akbari, F.; Esmaillzadeh, A. Diet quality among Iranian adolescents needs improvement. Public Health Nutr. 2015, 18, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Khorsha, F.; Mirzababaei, A.; Togha, M.; Mirzaei, K. Association of dietary diversity score (DDS) and migraine headache severity among women. Neurol. Sci. 2021, 42, 3403–3410. [Google Scholar] [CrossRef] [PubMed]
- Kojima, G.; Avgerinou, C.; Iliffe, S.; Jivraj, S.; Sekiguchi, K.; Walters, K. Fruit and vegetables Consumption and Frailty: A Systematic Review. J. Nutr. Health Aging 2018, 22, 1010–1017. [Google Scholar] [CrossRef]
- Ng, T.P.; Feng, L.; Niti, M.; Kua, E.H.; Yap, K.B. Tea consumption and cognitive impairment and decline in older Chinese adults. Am. J. Clin. Nutr. 2008, 88, 224–231. [Google Scholar] [CrossRef]
- Rahman, K. Garlic and aging: New insights into an old remedy. Ageing Res. Rev. 2003, 2, 39–56. [Google Scholar] [CrossRef]
- Kouvari, M.; Tyrovolas, S.; Panagiotakos, D.B. Red meat consumption and healthy ageing: A review. Maturitas 2016, 84, 17–24. [Google Scholar] [CrossRef]
- Mulero, J.; Zafrilla, P.; Martinez-Cacha, A. Oxidative stress, frailty and cognitive decline. J. Nutr. Health Aging 2011, 15, 756–760. [Google Scholar] [CrossRef]
- Daly, R.M.; Gianoudis, J.; Prosser, M.; Kidgell, D.; Ellis, K.A.; O’Connell, S.; Nowson, C.A. The effects of a protein enriched diet with lean red meat combined with a multi-modal exercise program on muscle and cognitive health and function in older adults: Study protocol for a randomised controlled trial. Trials 2015, 16, 339. [Google Scholar] [CrossRef] [PubMed]
- de Haas, S.C.; de Jonge, E.A.; Voortman, T.; Graaff, J.S.D.; Franco, O.H.; Ikram, M.A.; Rivadeneira, F.; Kiefte-de Jong, J.C.; Schoufour, J.D. Dietary patterns and changes in frailty status: The Rotterdam study. Eur. J. Nutr. 2018, 57, 2365–2375. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).