Oral Semaglutide under Human Protocols and Doses Regulates Food Intake, Body Weight, and Glycemia in Diet-Induced Obese Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals
2.3. Study Design
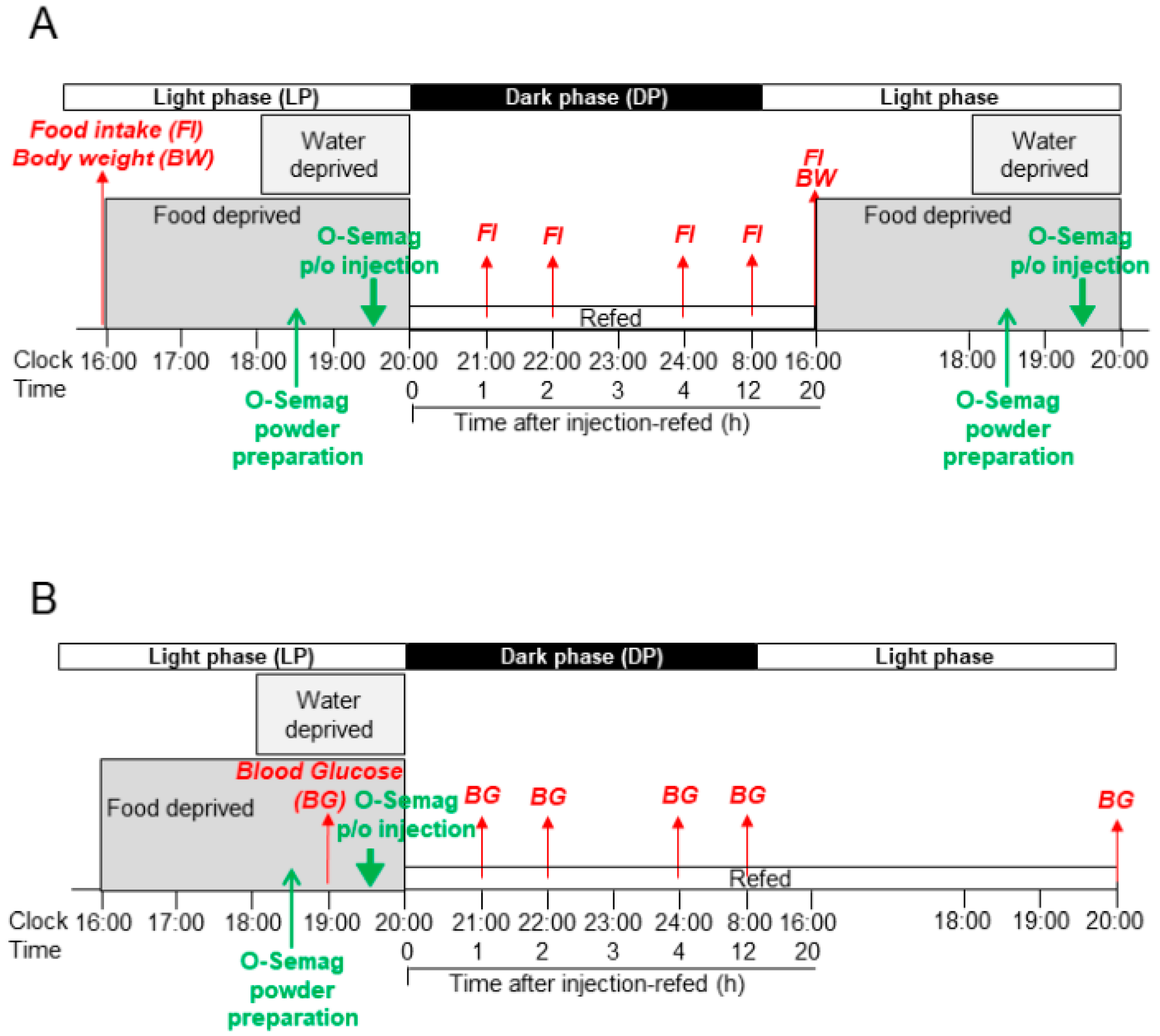
2.4. Oral Semaglutide Dose Selection, Preparation and Protocol of p/o Injection
2.5. Measurement of Blood Glucose, Food Intake, and Body Weight
2.6. Statistical Analysis
3. Results
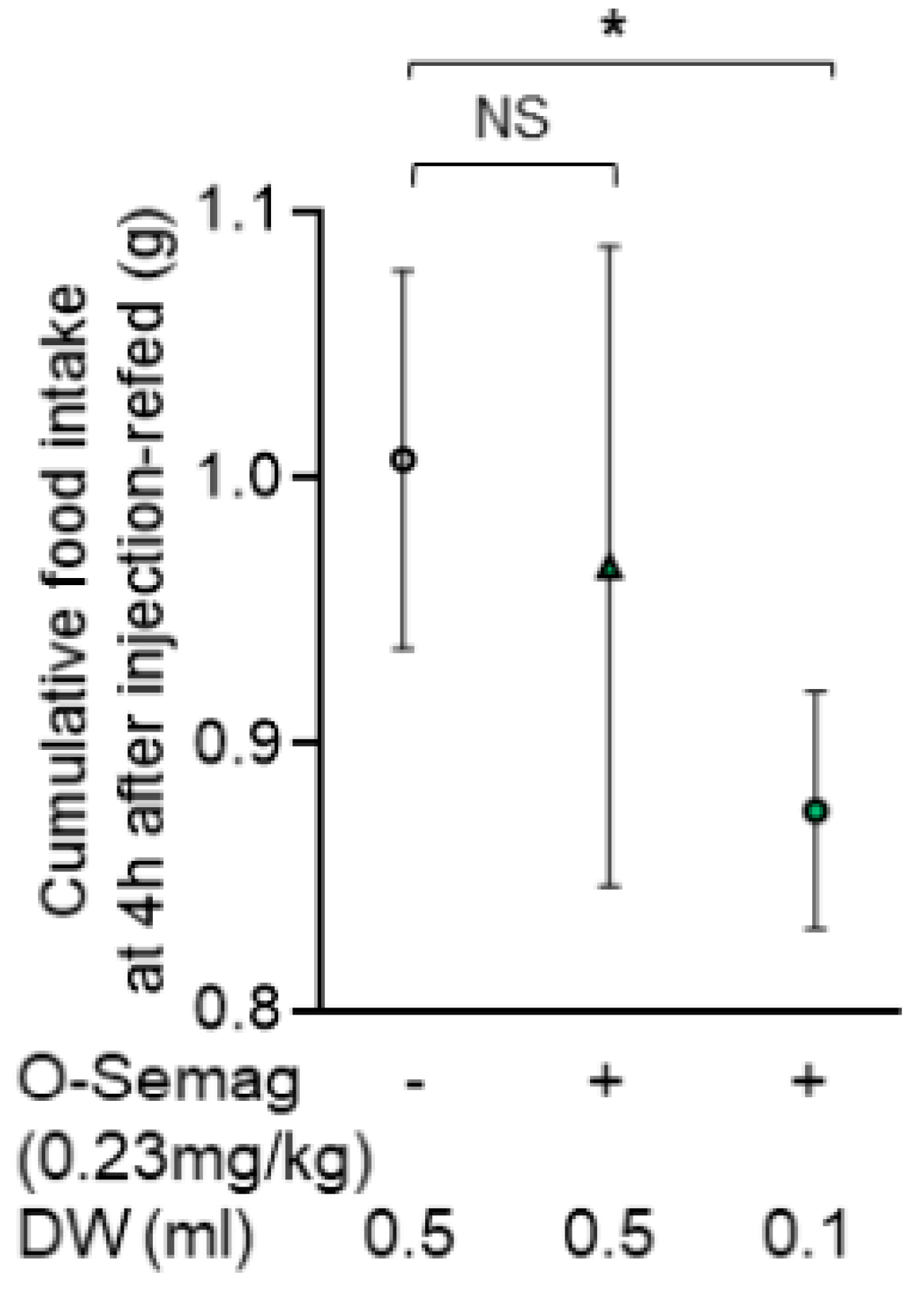
3.1. Effect of Oral Semaglutide with Different Water Volumes on Food Intake
3.2. Acute Effect of Oral Semaglutide 0.23 mg/kg (Eq. 14 mg) on Blood Glucose in DIO Mice
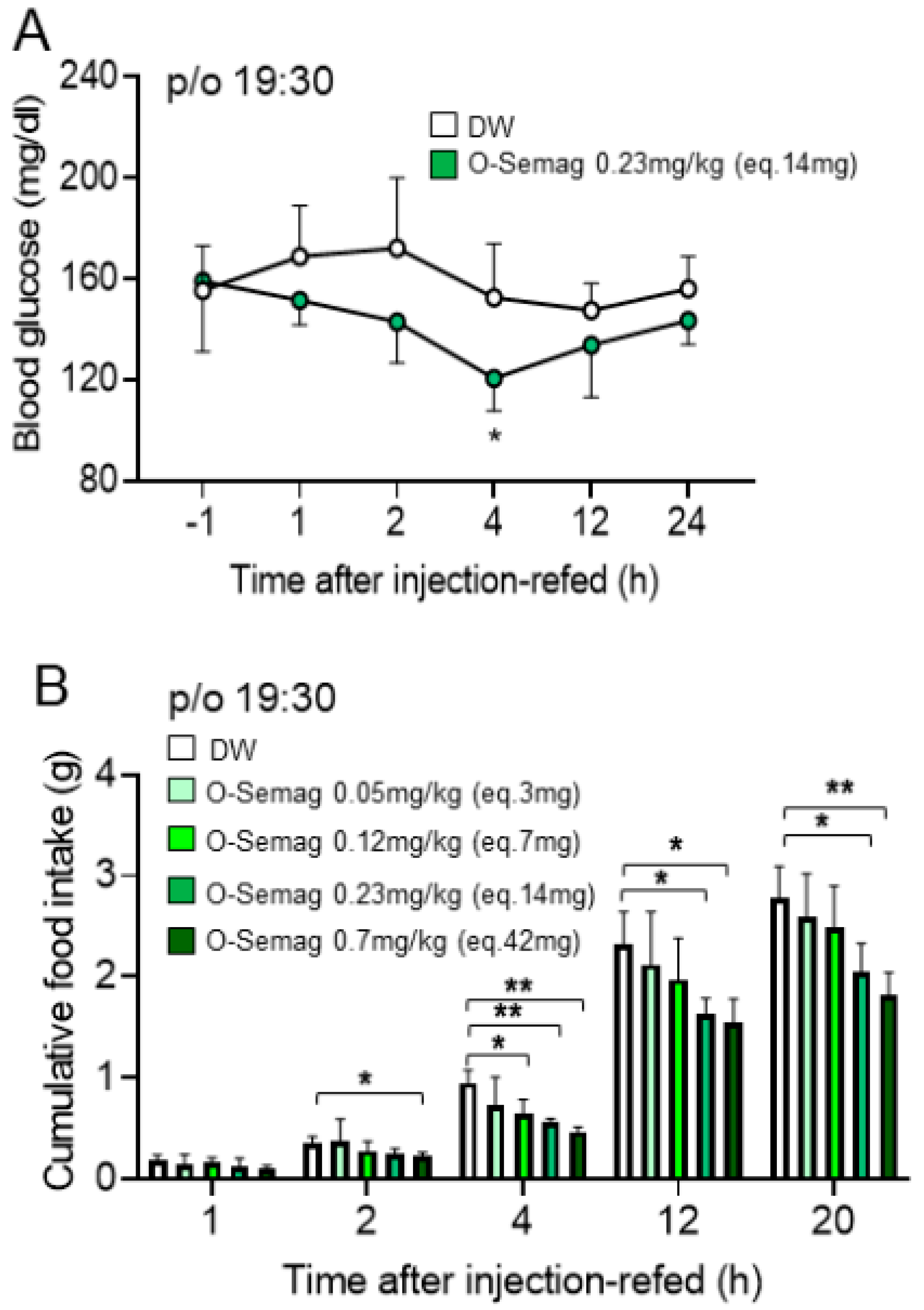
3.3. Dose-Dependent Effect of Oral Semaglutide on Cumulative Food Intake in DIO Mice
3.4. Sub-Chronic Effect of Oral Semaglutide on Food Intake and Body Weight in DIO Mice

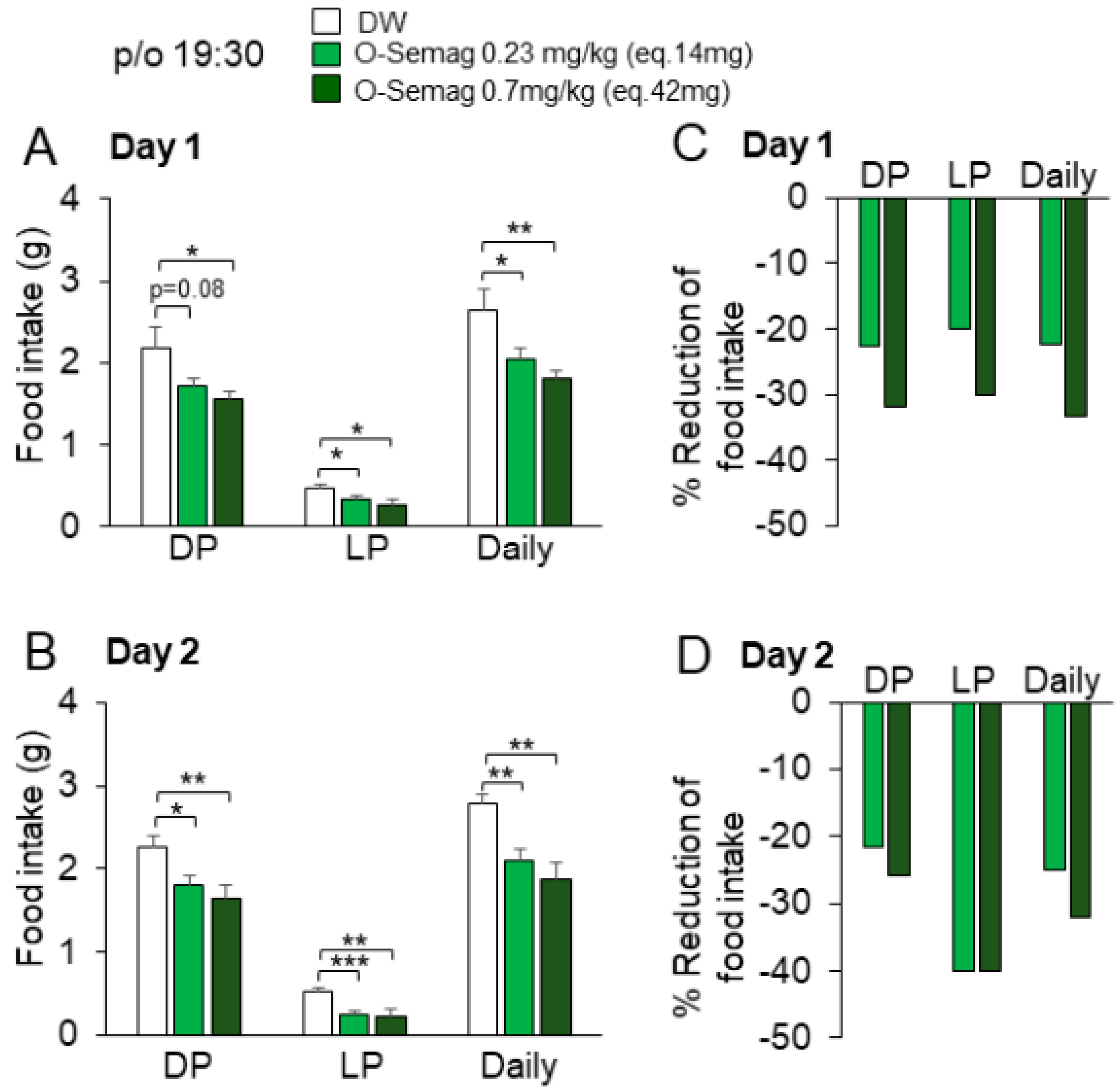
3.5. Effect of Oral Semaglutide on Diurnal Food Intake in DIO Mice
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Novo Nordisk. RYBELSUS (Semaglutide) Tablets, for Oral Use Package Insert. Novo Nordisk. Available online: https://www.rybelsus.com (accessed on 8 October 2019).
- Gibbons, C.; Blundell, J.; Hoff, S.T.; Dahl, K.; Bauer, R.; Bækdal, T. MSc2 Effects of oral semaglutide on energy intake, food preference, appetite, control of eating and body weight in subjects with type 2 diabetes. Diabetes Obes. Metab. 2021, 23, 581–588. [Google Scholar] [CrossRef]
- Buckley, S.T.; Bækdal, T.A.; Vegge, A.; Maarbjerg, S.J.; Pyke, C.; Ahnfelt-Rønne, J.; Madsen, K.G.; Schéele, S.G.; Alanentalo, T.; Kirk, R.K. Transcellular stomach absorption of a derivatized glucagon-like peptide-1 receptor agonist. Sci. Transl. Med. 2018, 10, 467. [Google Scholar] [CrossRef]
- Anderson, S.L.; Beutel, T.R.; Trujillo, J.M. Oral semaglutide in type 2 diabetes. J. Diabetes Complicat. 2020, 34, 107520. [Google Scholar] [CrossRef]
- Pratley, R.; Amod, A.; Hoff, S.T.; Kadowaki, T.; Lingvay, I.; Nauck, M.; Pedersen, K.B.; Saugstrup, T.; Meier, J.J.; PIONEER 4 Investigators. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): A randomised, double-blind, phase 3A trial. Lancet 2019, 394, 39–50. [Google Scholar] [CrossRef]
- Yabe, D.; Nakamura, J.; Kaneto, H.; Deenadayalan, S.; Navarria, A.; Gislum, M.; Inagaki, N.; Arisaka, T.; Asakura, T.; Azuma, N.; et al. Safety and efficacy of oral semaglutide versus dulaglutide in Japanese patients with type 2 diabetes (PIONEER 10): An open-label, randomised, active-controlled, phase 3a trial. Lancet Diabetes Endocr. 2020, 8, 392–406. [Google Scholar] [CrossRef]
- Andersen, A.; Knop, F.K.; Vilsbøll, T.A. Pharmacological and Clinical Overview of Oral Semaglutide for the Treatment of Type 2 Diabetes. Drugs 2021, 81, 1003–1030. [Google Scholar] [CrossRef]
- Baekdal, T.A.; Borregaard, J.; Donsmark, M.; Breitschaft, A.; Sondergaard, F.L. Evaluation of the effects of water volume with dosing and post-dose fasting period on pharmacokinetics of oral semaglutide. Diabetes 2017, 66, A315. [Google Scholar]
- Kararli, T.T. Comparison of the gastrointestinal Anatomy, Physiology, and Biochemistry of humans and commonly used laboratory animals. Biopharm. Drug Dispos. 1995, 16, 351–380. [Google Scholar] [CrossRef]
- Kohsaka, A.; Laposky, A.D.; Ramsey, K.M.; Estrada, C.; Joshu, C.; Kobayashi, Y.; Turek, F.W.; Bass, J. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007, 6, 414–421. [Google Scholar] [CrossRef]
- Hatori, M.; Vollmers, C.; Zarrinpar, A.; DiTacchio, L.; Bushong, E.A.; Gill, S.; Leblanc, M.; Chaix, A.; Joens, M.; Fitzpatrick, J.A.J.; et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012, 15, 848–860. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Sendo, M.; Dezaki, K.; Hira, T.; Sato, T.; Nakata, M.; Goswami, C.; Aoki, R.; Arai, T.; Yada, T.; et al. GLP-1 release and vagal afferent activation mediate the beneficial metabolic and chronotherapeutic effects of D-allulose. Nat. Commun. 2018, 9, 113. [Google Scholar] [CrossRef]
- Zhou, D.; Chen, L.; Mou, X. Acarbose ameliorates spontaneous type-2 diabetes in db/db mice by inhibiting PDX-1 methylation. Mol. Med. Rep. 2021, 23, 72. [Google Scholar] [CrossRef]
- Muramoto, A.; Matsushita, M.; Kato, A.; Yamamoto, N.; Koike, G.; Nakamura, M.; Numata, T.; Tamakoshi, A.; Tsushita, K. Three percent weight reduction is the minimum requirement to improve health hazards in obese and overweight people in Japan. Obes. Res. Clin. Pract. 2014, 8, e466–e475. [Google Scholar] [CrossRef]
- Overgaard, R.V.; Hertz, C.L.; Ingwersen, S.H.; Navarria, A.; Drucker, D.J. Levels of circulating semaglutide determine reductions in HbA1c and body weight in people with type 2 diabetes. Cell Rep. Med. 2021, 2, 100387. [Google Scholar] [CrossRef]
- Ryan, D.H.; Lingvay, I.; Colhoun, H.M.; Deanfield, J.; Emerson, S.S.; Kahn, S.E.; Kushner, R.F.; Marso, S.; Plutzky, J.; Brown-Frandsen, K.; et al. Semaglutide Effects on Cardiovascular Outcomes in People With Overweight or Obesity (SELECT) rationale and design. Am. Heart J. 2020, 229, 61–69. [Google Scholar] [CrossRef]
- Davies, M.; Pieber, T.R.; Hartoft-Nielsen, M.R.; Hansen, O.K.H.; Jabbour, S.; Rosenstock, J. Effect of Oral Semaglutide compared with placebo and subcutaneous Semaglutide on glycemic control in patients with Type 2 Diabetes. JAMA 2017, 318, 1460–1470. [Google Scholar] [CrossRef]
- Singh, G.; Krauthamer, M.; Bjalme-Evans, M. Wegovy (semaglutide): A new weight loss drug for chronic weight management. J. Investig. Med. 2022, 70, 5–13. [Google Scholar] [CrossRef]
- Muramoto, A.; Tsushita, K.; Kato, A.; Ozaki, N.; Tabata, M.; Endo, M.; Oike, Y.; Oiso, Y. Angiopoietin-like protein 2 sensitively responds to weight reduction induced by lifestyle intervention on overweight Japanese men. Nutr. Diabetes 2011, 1, e20. [Google Scholar] [CrossRef]
- Mistlberger, R.E.; Lukman, H.; Nadeau, B.G. Circadian rhythms in the Zucker obese rat: Assessment and intervention. Appetite 1998, 30, 255–267. [Google Scholar] [CrossRef]
- Takeda, K.; Sawazaki, H.; Takahashi, H.; Yeh, Y.S.; Jheng, H.F.; Nomura, W.; Ara, T.; Takahashi, N.; Seno, S.; Osato, N. The dipeptidyl peptidase-4 (DPP-4) inhibitor teneligliptin enhances brown adipose tissue function, thereby preventing obesity in mice. FEBS Open Bio 2018, 8, 1782–1793. [Google Scholar] [CrossRef]
- Norgaard, S.A.; Briand, F.; Sand, F.W.; Galsgaard, E.D.; Søndergaard, H.; Sørensen, D.B.; Sulpice, T. Nephropathy in diabetic db/db mice is accelerated by high protein diet and improved by the SGLT2 inhibitor dapagliflozin. Eur. J. Pharmacol. 2019, 860, 172537. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Fujimoto, H.; Fujita, N.; Hamamatsu, K.; Yabe, D.; Inagaki, N. Association of glucagon-like peptide-1 receptor-targeted imaging probe with in vivo glucagon-like peptide-1 receptor agonist glucose-lowering effects. J. Diabetes Investig. 2020, 11, 1448–1456. [Google Scholar] [CrossRef] [PubMed]
- Niedowicz, D.M.; Ozcan, S.; Nelson, P.T. Glimepiride Administered in Chow Reversibly Impairs Glucose Tolerance in Mice. J. Diabetes Res. 2018, 12, 1251345. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rakhat, Y.; Wang, L.; Han, W.; Rustemova, A.; Kulzhanova, N.; Yamada, Y.; Yabe, D.; Seino, Y.; Yada, T. Oral Semaglutide under Human Protocols and Doses Regulates Food Intake, Body Weight, and Glycemia in Diet-Induced Obese Mice. Nutrients 2023, 15, 3765. https://doi.org/10.3390/nu15173765
Rakhat Y, Wang L, Han W, Rustemova A, Kulzhanova N, Yamada Y, Yabe D, Seino Y, Yada T. Oral Semaglutide under Human Protocols and Doses Regulates Food Intake, Body Weight, and Glycemia in Diet-Induced Obese Mice. Nutrients. 2023; 15(17):3765. https://doi.org/10.3390/nu15173765
Chicago/Turabian StyleRakhat, Yermek, Lei Wang, Wanxin Han, Aktolkyn Rustemova, Nazymgul Kulzhanova, Yuichiro Yamada, Daisuke Yabe, Yutaka Seino, and Toshihiko Yada. 2023. "Oral Semaglutide under Human Protocols and Doses Regulates Food Intake, Body Weight, and Glycemia in Diet-Induced Obese Mice" Nutrients 15, no. 17: 3765. https://doi.org/10.3390/nu15173765
APA StyleRakhat, Y., Wang, L., Han, W., Rustemova, A., Kulzhanova, N., Yamada, Y., Yabe, D., Seino, Y., & Yada, T. (2023). Oral Semaglutide under Human Protocols and Doses Regulates Food Intake, Body Weight, and Glycemia in Diet-Induced Obese Mice. Nutrients, 15(17), 3765. https://doi.org/10.3390/nu15173765







