What Is the Role of Palmitoylethanolamide Co-Ultramicronized with Luteolin on the Symptomatology Reported by Patients Suffering from Long COVID? A Retrospective Analysis Performed by a Group of General Practitioners in a Real-Life Setting
Abstract
:1. Introduction
2. Materials and Methods
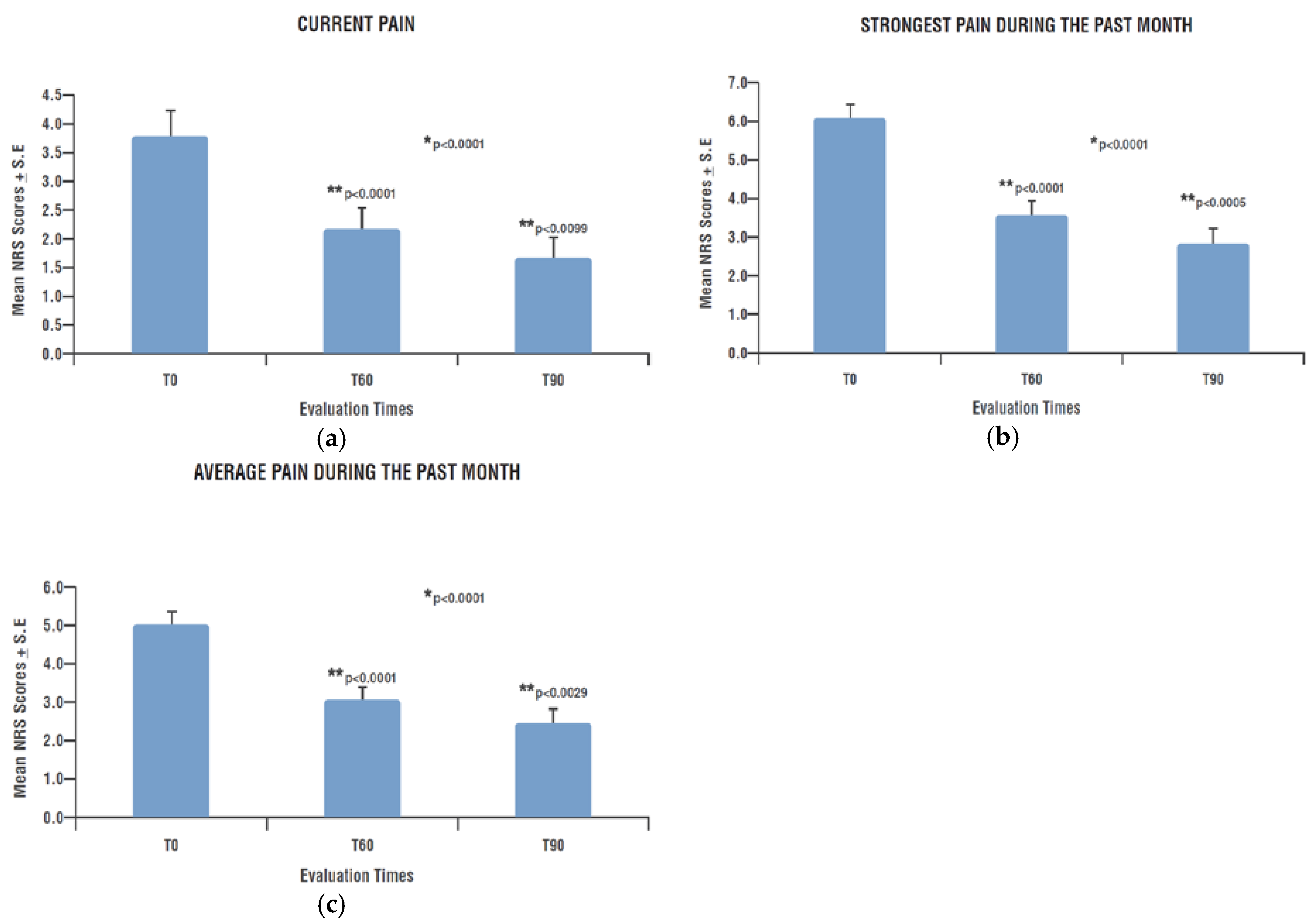
- Intensity and type of painful symptoms by means of the Pain Detect Questionnaire (PD-Q), a validated tool for the screening of neuropathic pain in various clinical conditions, and subsequently also for the monitoring of patients clinical course [52]. PD-Q is a self-reported questionnaire divided into four sections: pain intensity, pain course pattern, presence/absence of radiating pain and sensory symptoms evaluation. Pain intensity is evaluated by three questions about pain at the moment “current pain”, the strongest “last month pain” and the mean “last month pain”. Each one is assigned a score by an 0–10 NRS (Numeric Rating Scale) where 0 represents “no pain” and 10 a “maximum intensity pain”. Pain course pattern is evaluated by four graphs that represent the four possible options (only one answer possible): persistent pain with slight fluctuations (0 points), persistent pain with pain attacks (−1 point), pain attacks without pain between them (+1 point) and pain attacks with pain between them (+1 point). Radiating pain evaluation is a unique yes/no question about the presence (+2 points) or absence (0 points) of pain radiations; a body chart drawing allows a patient to indicate the direction in which the pain radiates. Sensory symptoms evaluation consists of seven questions about seven items: burning, tingling or prickling sensation, dynamic mechanical allodynia, electric shock, thermal hyperalgesia, numbness and static (pressure) allodynia. Each of them is evaluated by a 0–5 scale where 0 indicates “never noticed” and 5 “very strong”. The score of this section varies from 0 to 35 and represents the PD-Q Total Score. Adding to this Total Score the points obtained from the previous two sections: pain course pattern (−1, 0 or +1) and radiating pain (0 or +2), it is possible to calculate the PD-Q Final Score, used for discriminate the type of pain from “nociceptive” (0–12), “unclear” (13–18) and “neuropathic” (19–38) [53].
- Depression and anxiety symptoms by means of the Hamilton Rating Scale for Depression (HAM-D) and anxiety (HAM-A). HAM-D is a questionnaire which assesses the presence and severity of depressive symptoms through the evaluation of 21 items. A score <7 indicates absence of depression; 8–17 correspond to mild depression, 18–24 to moderate and >25 severe [54]. HAM-A is a questionnaire consisting of 14 symptom-defined items, scored on a scale of 0 (not present) to 4 (severe), with a total score range of 0–56 where <17 indicates mild severity, 18–24 mild to moderate and 25–30 moderate to severe [55].
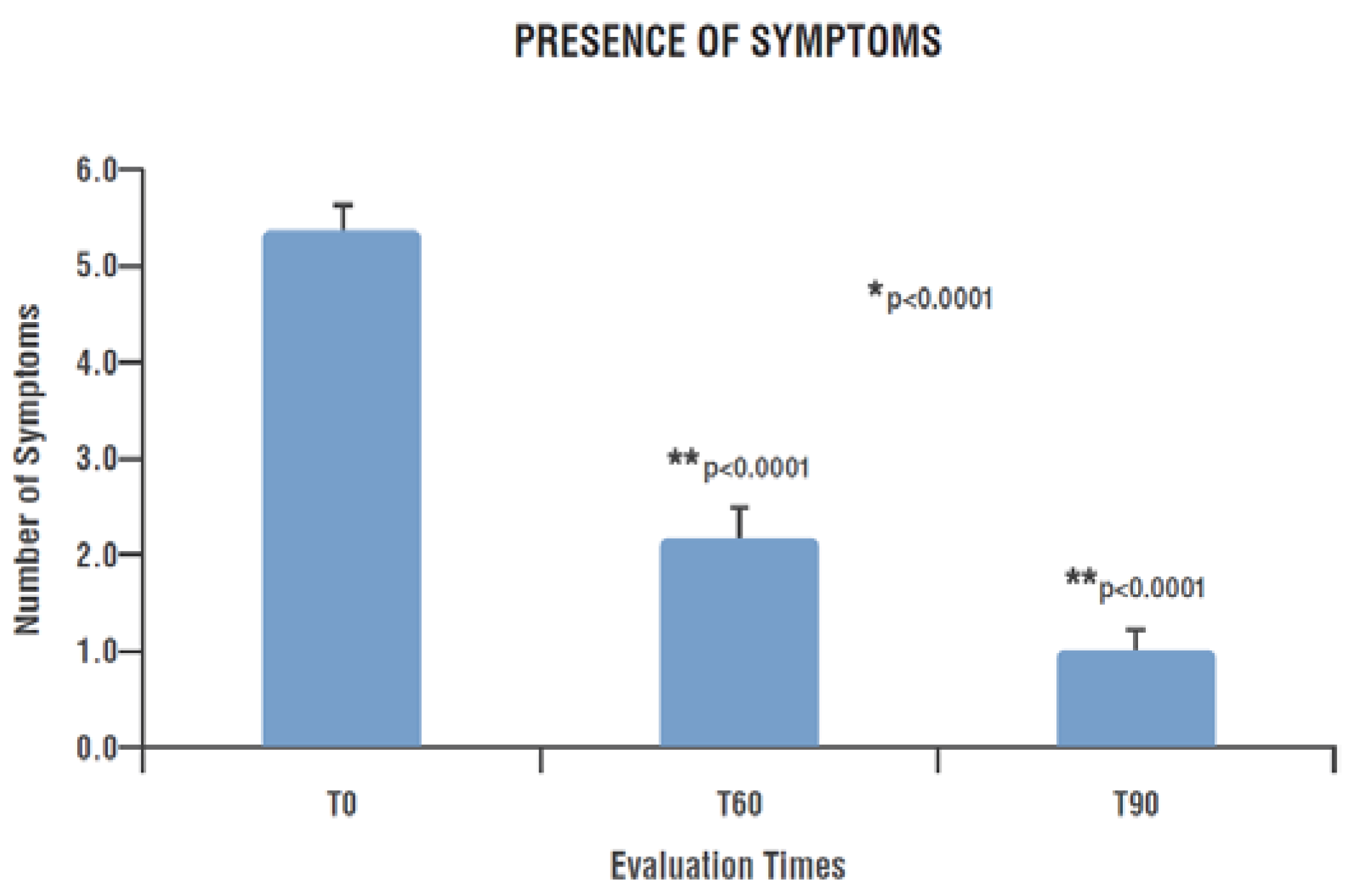
- Long COVID symptomatology by evaluating the persistence or the new onset of clinical signs/symptoms recognized as sequalae of COVID-19 pathology, like brain fog, difficulty multitasking, fatigue, irritability, inability to find the right word, memory loss and weakness [56], in addition to the recognized olfactory and gustatory alteration anosmia and dysgeusia [57].
- Patients subjective improvement at the end of the study by the Patient Global Impression of Change (PGIC). PGIC is a validated tool easy to apply in daily clinical practice for the non-research clinician to quantify and monitor patient progress and treatment response over time [58]. PGIC evaluates the variation of the health status perceived by each patient through seven possible options ranging from “extremely worse” to “extremely improved” [59].
3. Results
3.1. Pain Evaluation by Pain Detect Questionnaire (PD-Q)
3.1.1. Pain Intensity
3.1.2. Pain Course Pattern and Radiating Pain Evaluation
3.2. Depression and Anxiety Symptoms by Hamilton Rating Scale for Depression (HAM-D) and Anxiety (HAM-A)
3.3. Long COVID Symptomatology
- -
- -
- -
3.4. Patients Subjective Improvement (PGIC Scale)
4. Discussion
5. Conclusions
- -
- the pathogenetic role of “non-resolving” neuroinflammation in Long COVID;
- -
- the importance of neuroinflammation modulation through a substance such as umPEA;
- -
- the primary role of the General Practitioner and the real-life setting in the early diagnosis and treatment of this pathology;
- -
- the advantage of recognizing this key figure and the territorial setting as the most appropriate for the management of Long COVID syndrome.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Kessel, S.A.M.; Olde Hartman, T.C.; Lucassen, P.L.B.J.; van Jaarsveld, C.H.M. Post-acute and Long COVID-19 symptoms in patients with mild diseases: A systematic review. Fam. Pract. 2022, 39, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Alkodaymi, M.S.; Omrani, O.A.; Fawzy, N.A.; Shaar, B.A.; Almamlouk, R.; Riaz, M.; Obeidat, M.; Obeidat, Y.; Gerberi, D.; Taha, R.M.; et al. Prevalence of post-acute COVID-19 syndrome symptoms at different follow-up periods: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2022, 28, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V. WHO Clinical Case Definition Working Group on Post-COVID-19 Condition. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 2022, 22, e102–e107. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings. mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef]
- Chen, C.; Haupert, S.R.; Zimmermann, L.; Shi, X.; Fritsche, L.G.; Mukherjee, B. Global Prevalence of Post-Coronavirus Disease 2019 (COVID-19) Condition or Long COVID: A Meta-Analysis and Systematic Review. J. Infect. Dis. 2022, 226, 1593–1607. [Google Scholar] [CrossRef]
- National Institute for Health Research. Living with COVID-19—Second Review. A Dynamic Review of the Evidence around Ongoing COVID-19 Symptoms (Often Called Long COVID). Available online: https://evidence.nihr.ac.uk/themedreview/living-with-covid19-second-review/ (accessed on 16 May 2023).
- Theoharides, T.C.; Conti, P. COVID-19 and multisystem inflammatory syndrome, or is it mast cell activation syndrome? J. Biol. Regul. Homeost. Agents 2020, 34, 1633–1636. [Google Scholar]
- Moldofsky, H.; Patcai, J. Chronic widespread musculoskeletal pain, fatigue, depression and disordered sleep in chronic post-SARS syndrome; a case-controlled study. BMC Neurol. 2011, 11, 37. [Google Scholar] [CrossRef]
- Kingstone, T.; Taylor, A.K.; O’Donnell, C.A.; Atherton, H.; Blane, D.N.; Chew-Graham, C.A. Finding the ‘right’ GP: A qualitative study of the experiences of people with Long COVID. BJGP Open 2020, 4, bjgpopen20X101143. [Google Scholar] [CrossRef]
- Almeria, M.; Cejudo, J.C.; Sotoca, J.; Deus, J.; Krupinski, J. Cognitive profile following COVID-19 infection: Clinical predictors leading to neuropsychological impairment. Brain Behav. Immun. Health 2020, 9, 100163. [Google Scholar] [CrossRef]
- Noce, A.; Albanese, M.; Marrone, G.; Di Lauro, M.; Pietroboni Zaitseva, A.; Palazzetti, D.; Guerriero, C.; Paolino, A.; Pizzenti, G.; Di Daniele, F.; et al. Ultramicronized Palmitoylethanolamide (um-PEA): A New Possible Adjuvant Treatment in COVID-19 patients. Pharmaceuticals 2021, 14, 336. [Google Scholar] [CrossRef] [PubMed]
- Afrin, L.B.; Ackerley, M.B.; Bluestein, L.S.; Brewer, J.H.; Brook, J.B.; Buchanan, A.D.; Cuni, J.R.; Davey, W.P.; Dempsey, T.T.; Dorff, S.R.; et al. Diagnosis of mast cell activation syndrome: A global “consensus-2”. Diagnosis 2020, 8, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Giannetti, A.; Filice, E.; Caffarelli, C.; Ricci, G.; Pession, A. Mast Cell Activation Disorders. Medicina 2021, 57, 124. [Google Scholar] [CrossRef]
- Xanthos, D.N.; Sandkühler, J. Neurogenic neuroinflammation: Inflammatory CNS reactions in response to neuronal activity. Nat. Rev. Neurosci. 2014, 15, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Conti, P. Coronavirus-19 (SARS-CoV-2) induces acute severe lung inflammation via IL-1 causing cytokine storm in COVID-19: A promising inhibitory strategy. J. Biol. Regul. Homeost. Agents 2020, 34, 1971–1975. [Google Scholar]
- Steardo, L.; Steardo, L., Jr.; Zorec, R.; Verkhratsky, A. Neuroinfection may potentially contribute to pathophysiology and clinical manifestations of COVID-19. Acta Physiol. 2020, 229, e13473. [Google Scholar] [CrossRef]
- Xu, J.; Zhong, S.; Liu, J.; Li, L.; Li, Y.; Wu, X.; Li, Z.; Deng, P.; Zhang, J.; Zhong, N.; et al. Detection of severe acute respiratory syndrome coronavirus in the brain: Potential role of the chemokine mig in pathogenesis. Clin. Infect. Dis. 2015, 41, 1089–1096. [Google Scholar] [CrossRef]
- Gigante, A.; Aquili, A.; Farinelli, L.; Caraffa, A.; Ronconi, G.; Gallenga, C.E.; Tetè, G.; Kritas, S.K.; Conti, P. Sodium Chromo-Glycate and Palmitoylethanolamide: A Possible Strategy to Treat Mast Cellinduced Lung Inflammation in COVID-19. Med. Hypotheses 2020, 143, 109856. [Google Scholar] [CrossRef]
- Afrin, L.B.; Weinstock, L.B.; Molderings, G.J. Covid-19 hyperinflammation and post-COVID-19 illness may be rooted in mast cell activation syndrome. Int. J. Infect. Dis. 2020, 100, 327–332. [Google Scholar] [CrossRef]
- Liu, Y.H.; Wang, Y.R.; Wang, Q.H.; Chen, Y.; Chen, X.; Li, Y.; Cen, Y.; Xu, C.; Hu, T.; Liu, X.D.; et al. Post-infection cognitive impairments in a cohort of elderly patients with COVID-19. Mol. Neurodegener. 2021, 16, 48. [Google Scholar] [CrossRef]
- Mansell, V.; Hall Dykgraaf, S.; Kidd, M.; Goodyear-Smith, F. Long COVID and older people. Lancet Healthy Longev. 2022, 3, e849–e854. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Klein, S.L.; Garibaldi, B.T.; Li, H.; Wu, C.; Osevala, N.M.; Li, T.; Margolick, J.B.; Pawelec, G.; Leng, S.X. Aging in COVID-19: Vulnerability, immunity and intervention. Ageing Res. Rev. 2021, 65, 101205. [Google Scholar] [CrossRef] [PubMed]
- Aloe, L.; Leon, A.; Levi-Montalcini, R. A proposed autacoidmechanism controlling mastocyte behaviour. Agents Actions 1993, 39, 145–147. [Google Scholar] [CrossRef]
- Petrosino, S.; Schiano Moriello, A.; Cerrato, S.; Fusco, M.; Puigdemont, A.; De Petrocellis, L.; Di Marzo, V. The anti-inflammatory mediator palmitoylethanolamide enhances the levels of 2-arachidonoyl-glycerol and potentiates its actions at transient receptor potential vanilloid type-1 channels. Br. J. Pharmacol. 2016, 173, 1154–1162. [Google Scholar] [CrossRef]
- Facci, L.; Dal Toso, R.; Romanello, S.; Buriani, A.; Skaper, S.D.; Leon, A. Mast cells express a peripheral cannabinoid receptor with differentialsensitivity to anandamide and palmitoylethanolamide. Proc. Natl. Acad. Sci. USA 1995, 92, 3376–3380. [Google Scholar] [CrossRef] [PubMed]
- Cerrato, S.; Brazis, P.; della Valle, M.F.; Miolo, A.; Puigdemont, A. Effects of palmitoylethanolamide on immunologically induced histamine, PGD2 and TNFalpha release from canine skin mast cells. Vet. Immunol. Immunopathol. 2010, 133, 9–15. [Google Scholar] [CrossRef]
- Skaper, S.D.; Facci, L.; Fusco, M.; Della Valle, M.F.; Zusso, M.; Costa, B.; Giusti, P. Palmitoylethanolamide, a naturally occurring disease-modifying agent in neuropathic pain. Inflammopharmacology 2014, 22, 79–94. [Google Scholar]
- Skaper, S.D.; Facci, L.; Zusso, M.; Giusti, P. An Inflammation-Centric View of Neurological Disease: Beyond the Neuron. Front. Cell. Neurosci. 2018, 12, 72. [Google Scholar]
- Beggiato, S.; Tomasini, M.C.; Cassano, T.; Ferraro, L. Chronic Oral Palmitoylethanolamide Administration Rescues Cognitive Deficit and Reduces Neuroinflammation, Oxidative Stress, and Glutamate Levels in A Transgenic Murine Model of Alzheimer’s Disease. J. Clin. Med. 2020, 9, 428. [Google Scholar] [CrossRef]
- Petrosino, S.; Cordaro, M.; Verde, R.; Schiano Moriello, A.; Marcolongo, G.; Schievano, C.; Siracusa, R.; Piscitelli, F.; Peritore, A.F.; Crupi, R.; et al. Oral Ultramicronized Palmitoylethanolamide: Plasma and Tissue Levels and Spinal Anti-hyperalgesic Effect. Front. Pharmacol. 2018, 9, 249. [Google Scholar] [CrossRef]
- Impellizzeri, D.; Bruschetta, G.; Cordaro, M.; Crupi, R.; Siracusa, R.; Esposito, E.; Cuzzocrea, S. Micronized/ultramicronized palmitoylethanolamide displays superior oral efficacy compared to nonmicronized palmitoylethanolamide in a rat model of inflammatory pain. J. Neuroinflammation 2014, 28, 136. [Google Scholar] [CrossRef] [PubMed]
- Crupi, R.; Impellizzeri, D.; Cordaro, M.; Siracusa, R.; Casili, G.; Evangelista, M.; Cuzzocrea, S. N-palmitoylethanolamide Prevents Parkinsonian Phenotypes in Aged Mice. Mol. Neurobiol. 2018, 55, 8455–8472. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Gugliandolo, E.; Campolo, M.; Evangelista, M.; Di Paola, R.; Cuzzocrea, S. Effect of a new formulation of micronized and ultramicronized N-palmitoylethanolamine in a tibia fracture mouse model of complex regional pain syndrome. PLoS ONE 2017, 12, e0178553, Erratum in: PLoS ONE 2018, 13, e0201501. [Google Scholar] [CrossRef] [PubMed]
- Impellizzeri, D.; Peritore, A.F.; Cordaro, M.; Gugliandolo, E.; Siracusa, R.; Crupi, R.; D’Amico, R.; Fusco, R.; Evangelista, M.; Cuzzocrea, S.; et al. The neuroprotective effects of micronized PEA (PEA-m) formulation on diabetic peripheral neuropathy in mice. FASEB J. 2019, 33, 11364–11380. [Google Scholar] [CrossRef]
- Siracusa, R.; Fusco, R.; Cordaro, M.; Peritore, A.F.; D’Amico, R.; Gugliandolo, E.; Crupi, R.; Genovese, T.; Evangelista, M.; Di Paola, R.; et al. The Protective Effects of Pre- and Post-Administration of Micronized Palmitoylethanolamide Formulation on Postoperative Pain in Rats. Int. J. Mol. Sci. 2020, 21, 7700. [Google Scholar] [CrossRef]
- Evangelista, M.; Cilli, V.; De Vitis, R.; Militerno, A.; Fanfani, F. Ultra-micronized Palmitoylethanolamide Effects on Sleep-wake Rhythm and Neuropathic Pain Phenotypes in Patients with Carpal Tunnel Syndrome: An Open-label, Randomized Controlled Study. CNS Neurol. Disord. Drug Targets 2018, 17, 291–298. [Google Scholar] [CrossRef]
- Petrosino, S.; Schiano Moriello, A. Palmitoylethanolamide: A Nutritional Approach to Keep Neuroinflammation within Physiological Boundaries-A Systematic Review. Int. J. Mol. Sci. 2020, 21, 9526. [Google Scholar] [CrossRef] [PubMed]
- Colizzi, M.; Bortoletto, R.; Colli, C.; Bonomo, E.; Pagliaro, D.; Maso, E.; Di Gennaro, G.; Balestrieri, M. Therapeutic effect of palmitoylethanolamide in cognitive decline: A systematic review and preliminary meta-analysis of preclinical and clinical evidence. Front. Psychiatry 2022, 13, 1038122. [Google Scholar] [CrossRef]
- Landolfo, E.; Cutuli, D.; Petrosini, L.; Caltagirone, C. Effects of Palmitoylethanolamide on Neurodegenerative Diseases: A Review from Rodents to Humans. Biomolecules 2022, 12, 667. [Google Scholar] [CrossRef]
- Scuteri, D.; Guida, F.; Boccella, S.; Palazzo, E.; Maione, S.; Rodríguez-Landa, J.F.; Martínez-Mota, L.; Tonin, P.; Bagetta, G.; Corasaniti, M.T. Effects of Palmitoylethanolamide (PEA) on Nociceptive, Musculoskeletal and Neuropathic Pain: Systematic Review and Meta-Analysis of Clinical Evidence. Pharmaceutics 2022, 14, 1672. [Google Scholar] [CrossRef]
- Albanese, M.; Marrone, G.; Paolino, A.; Di Lauro, M.; Di Daniele, F.; Chiaramonte, C.; D’Agostini, C.; Romani, A.; Cavaliere, A.; Guerriero, C.; et al. Effects of Ultramicronized Palmitoylethanolamide (um-PEA) in COVID-19 Early Stages: A Case-Control Study. Pharmaceuticals 2022, 15, 253. [Google Scholar] [CrossRef]
- Petrosino, S.; Di Marzo, V. The pharmacology of Palmitoylethanolamide and first data on the therapeutic efficacy of some of its new formulations. Br. J. Pharmacol. 2017, 174, 1349–1365. [Google Scholar]
- Peritore, A.F.; Siracusa, R.; Crupi, R.; Cuzzocrea, S. Therapeutic Efficacy of Palmitoylethanolamide and Its New Formulations in Synergy with Different Antioxidant Molecules Present in Diets. Nutrients 2019, 11, 2175. [Google Scholar]
- Di Paola, R.; Fusco, R.; Gugliandolo, E.; Crupi, R.; Evangelista, M.; Granese, R.; Cuzzocrea, S. Co-micronized Palmitoylethanolamide/Polydatin Treatment Causes Endometriotic Lesion Regression in a Rodent Model of Surgically Induced Endometriosis. Front. Pharmacol. 2016, 7, 382. [Google Scholar] [CrossRef]
- Paterniti, I.; Impellizzeri, D.; Di Paola, R.; Navarra, M.; Cuzzocrea, S.; Esposito, E. A new co-ultramicronized composite including palmitoylethanolamide and luteolin to prevent neuroinflammation in spinal cord injury. J. Neuroinflamm. 2013, 23, 91. [Google Scholar] [CrossRef] [PubMed]
- Cordaro, M.; Impellizzeri, D.; Paterniti, I.; Bruschetta, G.; Siracusa, R.; De Stefano, D.; Cuzzocrea, S.; Esposito, E. Neuroprotective effects of PEALUT on secondary inflammatory process and autophagy involved in traumatic brain injury. J. Neurotrauma 2016, 33, 132–146. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, R.; Impellizzeri, D.; Cordaro, M.; Crupi, R.; Esposito, E.; Petrosino, S.; Cuzzocrea, S. Anti-inflammatory and neuroprotective effects of PEALUT in a mouse model of vascular dementia. Front. Neurol. 2017, 8, 233. [Google Scholar] [CrossRef] [PubMed]
- Di Stadio, A.; D’Ascanio, L.; Vaira, L.A.; Cantone, E.; De Luca, P.; Cingolani, C.; Motta, G.; De Riu, G.; Vitelli, F.; Spriano, G.; et al. Ultramicronized Palmitoylethanolamide and Luteolin Supplement Combined with Olfactory Training to Treat Post-COVID-19 Olfactory Impairment: A Multi-Center Double-Blinded Randomized Placebo- Controlled Clinical Trial. Curr. Neuropharmacol. 2022, 20, 2001–2012. [Google Scholar] [CrossRef] [PubMed]
- D’Ascanio, L.; Vitelli, F.; Cingolani, C.; Maranzano, M.; Brenner, M.J.; Di Stadio, A. Randomized clinical trial “olfactory dysfunction after COVID-19: Olfactory rehabilitation therapy vs. intervention treatment with Palmitoylethanolamide and Luteolin”: Preliminary results. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 4156–4162. [Google Scholar] [PubMed]
- De Luca, P.; Camaioni, A.; Marra, P.; Salzano, G.; Carriere, G.; Ricciardi, L.; Pucci, R.; Montemurro, N.; Brenner, M.J.; Di Stadio, A. Effect of Ultra-Micronized Palmitoylethanolamide and Luteolin on Olfaction and Memory in Patients with Long COVID: Results of a Longitudinal Study. Cells 2022, 11, 2552. [Google Scholar] [CrossRef]
- Versace, V.; Ortelli, P.; Dezi, S.; Ferrazzoli, D.; Alibardi, A.; Bonini, I.; Engl, M.; Maestri, R.; Assogna, M.; Ajello, V.; et al. Co-ultramicronized palmitoylethanolamide/luteolin normalizes GABAB-ergic activity and cortical plasticity in long COVID-19 syndrome. Clin. Neurophysiol. 2023, 145, 81–88. [Google Scholar]
- Freynhagen, R.; Tölle, T.R.; Gockel, U.; Baron, R. The painDETECT project—Far more than a screening tool on neuropathic pain. Curr. Med. Res Opin. 2016, 32, 1033–1057. [Google Scholar] [CrossRef] [PubMed]
- Haefeli, M.; Elfering, A. Pain assessment. Eur. Spine J. 2006, 15, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Sharp, R. The Hamilton Rating Scale for Depression. Occup. Med. 2015, 65, 340. [Google Scholar] [CrossRef] [PubMed]
- Thompson, E. Hamilton Rating Scale for Anxiety (HAM-A). Occup. Med. 2015, 65, 601. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Cholevas, C.; Polyzoidis, K.; Politis, A. Long COVID syndrome-associated brain fog and chemofog: Luteolin to the rescue. Biofactors 2021, 47, 232–241. [Google Scholar] [CrossRef]
- Raciti, L.; De Luca, R.; Raciti, G.; Arcadi, F.A.; Calabrò, R.S. The Use of Palmitoylethanolamide in the Treatment of Long COVID: A Real-Life Retrospective Cohort Study. Med. Sci. 2022, 10, 37. [Google Scholar] [CrossRef]
- Busner, J.; Targum, S.D. The clinical global impressions scale: Applying a research tool in clinical practice. Psychiaty 2007, 4, 28–37. [Google Scholar]
- Rampakakis, E.; Ste-Marie, P.A.; Sampalis, J.S.; Karellis, A.; Shir, Y.; Fitzcharles, M.A. Real-life assessment of the validity of patient global impression of change in fibromyalgia. RMD Open 2015, 1, e000146. [Google Scholar] [CrossRef]
- Is ‘long COVID’ Worsening the Labor Shortage? Available online: https://www.brookings.edu/articles/is-long-covid-worsening-the-labor-shortage/ (accessed on 16 May 2013).
- The Economic Cost of Long COVID: An Update—David Cutler. Available online: https://www.hks.harvard.edu/centers/mrcbg/programs/growthpolicy/economic-cost-long-covid-update-david-cutler (accessed on 16 May 2023).
- We Need an Operation Warp Speed for Long COVID. Available online: https://www.scientificamerican.com/article/we-need-an-operation-warp-speed-for-long-covid/ (accessed on 16 May 2023).
- Spudich, S.; Nath, A. Nervous system consequences of COVID-19. Science 2022, 375, 267–269. [Google Scholar] [CrossRef]
- Guo, P.; Benito Ballesteros, A.; Yeung, S.P.; Liu, R.; Saha, A.; Curtis, L.; Kaser, M.; Haggard, M.P.; Cheke, L.G. COVCOG 2: Cognitive and Memory Deficits in Long COVID: A Second Publication From the COVID and Cognition Study. Front. Aging Neurosci. 2022, 14, 804937. [Google Scholar] [CrossRef]
- Raciti, L.; Arcadi, F.A.; Calabrò, R.S. Could Palmitoylethanolamide Be an Effective Treatment for Long COVID-19? Hypothesis and Insights in Potential Mechanisms of Action and Clinical Applications. Innov. Clin. Neurosci. 2022, 19, 19–25. [Google Scholar] [PubMed]
- Thompson, D.R.; Al-Jabr, H.; Windle, K.; Ski, C.F. Long COVID: Supporting people through the quagmire. Br. J. Gen Pract. 2021, 71, 561. [Google Scholar] [CrossRef] [PubMed]
- Hastie, C.E.; Lowe, D.J.; McAuley, A.; Mills, N.L.; Winter, A.J.; Black, C.; Scott, J.T.; O’Donnell, C.A.; Blane, D.N.; Browne, S.; et al. Natural history of Long COVID in a nationwide, population cohort study. Nat. Commun. 2023, 14, 3504. [Google Scholar] [CrossRef] [PubMed]



| No. of Patients | Sex | Age (Mean ± S.D. *) |
|---|---|---|
| 49 outpatients | 18 males; 31 females | 53.4 ± 12.0 |
| Pre-Existing Pathologies | No. of Patients |
|---|---|
| Hypertension | 10 |
| Obesity | 3 |
| Hyperlipidemia | 8 |
| Diabetes Mellitus | 5 |
| Hypothyroidism | 6 |
| Anxiety | 4 |
| Depression | 4 |
| Chronic obstructive pulmonary disease | 3 |
| Osteoartritis (Diffuse, other than chronic low back) | 6 |
| Osteoporosis | 7 |
| Chronic Low Back Pain | 6 |
| Gastroesophageal reflux disease | 4 |
| Diverticulosis | 2 |
| Ulcerative colitis | 1 |
| Mieloma | 1 |
| Glaucoma | 1 |
| Pain Course Pattern | T0 (N = 49) | T60 (N = 46) | T90 (N = 42) | |
|---|---|---|---|---|
 | “Persistent pain with slight fluctuations” | 11 (22.4) | 13 (28.3) | 9 (21.4) |
 | “Persistent pain with pain attacks” | 8 (16.3) | 3 (6.5) | 2 (4.8) |
 | “Pain attacks without pain between them” | 17 (34.7) | 11 (23.9) | 13 (31.0) |
 | “Pain attacks with pain between them” | 9 (18.4) | 5 (10.9) | 4 (9.5) |
| No pain | 4 (8.2) | 14 (30.4) | 14 (33.3) | |
| Symptoms Distribution | T0 (N = 47) | T60 (N = 46) | T90 (N = 45) | p |
|---|---|---|---|---|
| Fatigue | 43 (91.5) | 16 (34.8) | 10 (22.2) | <0.0001 |
| Weakness | 35 (74.5) | 17 (37.0) | 7 (15.6) | <0.0001 |
| Brain fog | 30 (63.8) | 8 (17.4) | 5 (11.1) | <0.0001 |
| Irritability | 30 (63.8) | 14 (30.4) | 7 (15.6) | <0.0001 |
| Difficulty multitasking | 28 (59.6) | 13 (28.3) | 5 (11.1) | <0.0031 |
| Memory loss | 24 (51.1) | 14 (30.4) | 5 (11.1) | <0.0002 |
| Anosmia | 22 (46.8) | 2 (4.3) | 0 | <0.0001 |
| Dysgeusia | 20 (42.6) | 2 (4.3) | 1 (2.2) | <0.0001 |
| Inability to find the right word | 20 (42.6) | 14 (30.4) | 5 (11.1) | <0.0025 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pirro, M.; Ferri, L.; Piccioni, L.; Bellucci, A.M.; Bartolucci, F.; Russo, A.; Piga, A.; Ciaramaglia, P.L.; Lucangeli, M.; Russo, A.M.; et al. What Is the Role of Palmitoylethanolamide Co-Ultramicronized with Luteolin on the Symptomatology Reported by Patients Suffering from Long COVID? A Retrospective Analysis Performed by a Group of General Practitioners in a Real-Life Setting. Nutrients 2023, 15, 3701. https://doi.org/10.3390/nu15173701
Pirro M, Ferri L, Piccioni L, Bellucci AM, Bartolucci F, Russo A, Piga A, Ciaramaglia PL, Lucangeli M, Russo AM, et al. What Is the Role of Palmitoylethanolamide Co-Ultramicronized with Luteolin on the Symptomatology Reported by Patients Suffering from Long COVID? A Retrospective Analysis Performed by a Group of General Practitioners in a Real-Life Setting. Nutrients. 2023; 15(17):3701. https://doi.org/10.3390/nu15173701
Chicago/Turabian StylePirro, Maurizio, Luana Ferri, Licia Piccioni, Anna Maria Bellucci, Federica Bartolucci, Arianna Russo, Andrea Piga, Paola Lucia Ciaramaglia, Marco Lucangeli, Anna Maria Russo, and et al. 2023. "What Is the Role of Palmitoylethanolamide Co-Ultramicronized with Luteolin on the Symptomatology Reported by Patients Suffering from Long COVID? A Retrospective Analysis Performed by a Group of General Practitioners in a Real-Life Setting" Nutrients 15, no. 17: 3701. https://doi.org/10.3390/nu15173701
APA StylePirro, M., Ferri, L., Piccioni, L., Bellucci, A. M., Bartolucci, F., Russo, A., Piga, A., Ciaramaglia, P. L., Lucangeli, M., Russo, A. M., Cuzzocrea, S., & Evangelista, M. (2023). What Is the Role of Palmitoylethanolamide Co-Ultramicronized with Luteolin on the Symptomatology Reported by Patients Suffering from Long COVID? A Retrospective Analysis Performed by a Group of General Practitioners in a Real-Life Setting. Nutrients, 15(17), 3701. https://doi.org/10.3390/nu15173701








