Exploring Perceptions and Needs of Mobile Health Interventions for Nutrition, Anemia, and Preeclampsia among Pregnant Women in Underprivileged Indian Communities: A Cross-Sectional Survey
Abstract
1. Introduction
2. Materials and Method
2.1. Ethics Statement
2.2. Study Site
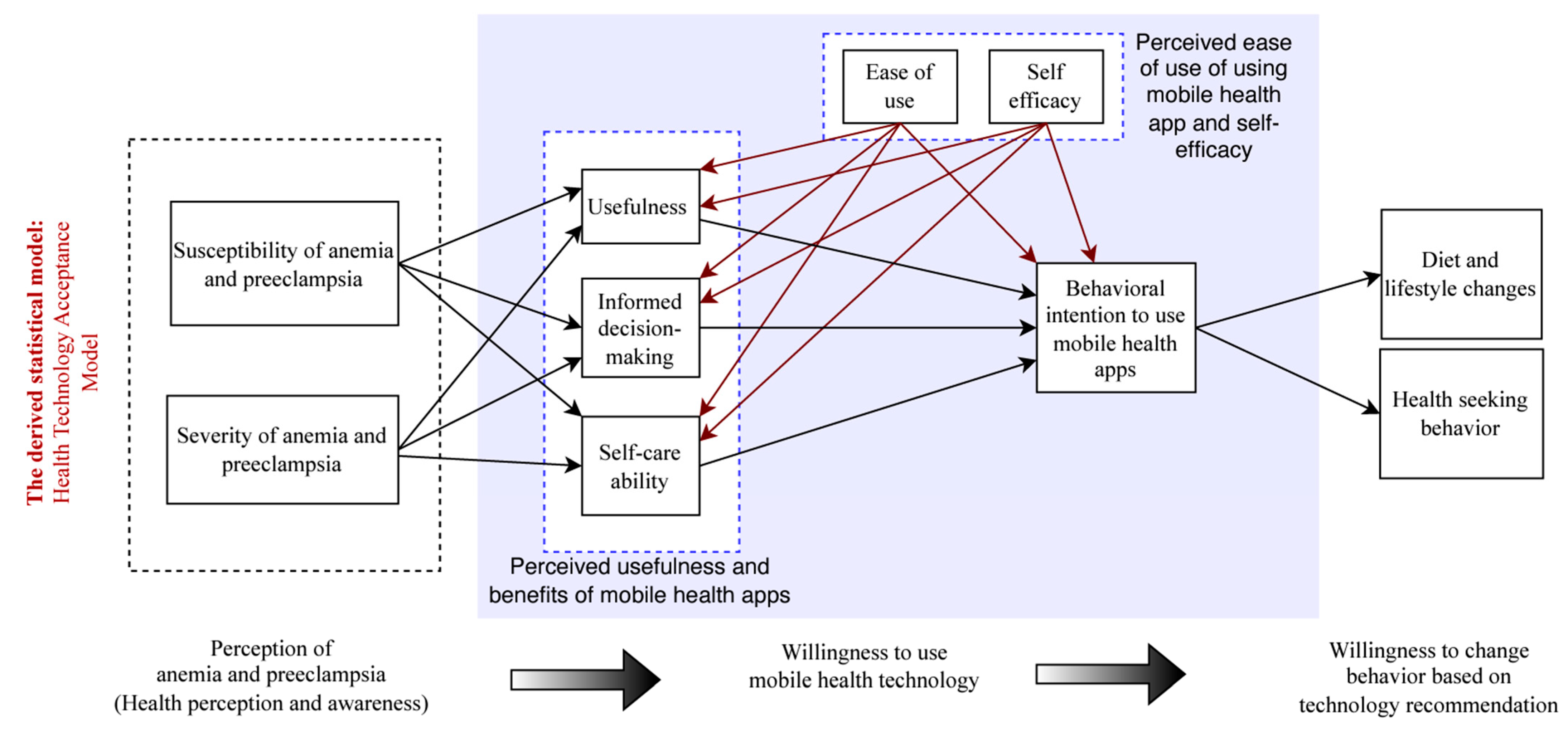
2.3. Health Technology Acceptance Model
2.4. Data Collection
2.5. Survey Instrument
2.5.1. Survey Development and Design
2.5.2. Measuring Maternal Health Awareness and Knowledge
2.5.3. A Comprehensive Survey Based on Health Technology Acceptance Model
2.6. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Smartphone Access
3.3. Smartphone Use
3.4. Willingness to Use mHealth Apps
3.5. Perceived Knowledge of Nutritional Requirements during Pregnancy
3.6. Actual Knowledge of Nutritional Requirements during Pregnancy
3.7. Awareness of Anemia and Preeclampsia during Pregnancy
3.8. Health Technology Acceptance Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bilano, V.L.; Ota, E.; Ganchimeg, T.; Mori, R.; Souza, J.P. Risk Factors of Pre-Eclampsia/Eclampsia and Its Adverse Outcomes in Low- and Middle-Income Countries: A WHO Secondary Analysis. PLoS ONE 2014, 9, e91198. [Google Scholar] [CrossRef]
- Wu, P.; Haththotuwa, R.; Kwok, C.S.; Babu, A.; Kotronias, R.A.; Rushton, C.; Zaman, A.; Fryer, A.A.; Kadam, U.; Chew-Graham, C.A.; et al. Preeclampsia and future cardiovascular health: A systematic review and meta-analysis. Circ. Cardiovasc. Qual. Outcomes 2017, 10, e003497. [Google Scholar] [CrossRef] [PubMed]
- Stevens, G.A.; Finucane, M.M.; De-Regil, L.M.; Paciorek, C.J.; Flaxman, S.R.; Branca, F.; Peña-Rosas, J.P.; Bhutta, Z.A.; Ezzati, M.; on behalf of Nutrition Impact Model Study Group (Anaemia). Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: A systematic analysis of population-representative data. Lancet Glob. Health 2013, 1, e16–e25. [Google Scholar] [CrossRef] [PubMed]
- Braunthal, S.; Brateanu, A. Hypertension in pregnancy: Pathophysiology and treatment. SAGE Open Med. 2019, 7, 843700. [Google Scholar] [CrossRef]
- Say, L.; Chou, D.; Gemmill, A.; Tunçalp, Ö.; Moller, A.-B.; Daniels, J.; Gülmezoglu, A.M.; Temmerman, M.; Alkema, L. Global causes of maternal death: A WHO systematic analysis. Lancet Glob. Health 2014, 2, E323–E333. [Google Scholar] [CrossRef]
- Petry, N.; Olofin, I.; Hurrell, R.F.; Boy, E.; Wirth, J.P.; Moursi, M.; Angel, M.D.; Rohner, F. The Proportion of Anemia Associated with Iron Deficiency in Low, Medium, and High Human Development Index Countries: A Systematic Analysis of National Surveys. Nutrients 2016, 8, 693. [Google Scholar] [CrossRef]
- Ghosh, P.; Dasgupta, A.; Paul, B.; Roy, S.; Biswas, A.; Yadav, A. A cross-sectional study on prevalence and determinants of anemia among women of reproductive age in a rural community of West Bengal. J. Fam. Med. Prim. Care 2020, 9, 5547–5553. [Google Scholar] [CrossRef]
- Little, M.; Humphries, S.; Dodd, W.; Patel, K.; Dewey, C. Socio-demographic patterning of the individual-level double burden of malnutrition in a rural population in South India: A cross-sectional study. BMC Public Heal. 2020, 20, 675. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.; Min, H.; Kim, H.; Jeong, H.-S. Determining factors for the prevalence of anemia in women of reproductive age in Nepal: Evidence from recent national survey data. PLoS ONE 2019, 14, e0218288. [Google Scholar] [CrossRef]
- Agrawal, S.; Fledderjohann, J.; Vellakkal, S.; Stuckler, D. Adequately Diversified Dietary Intake and Iron and Folic Acid Supplementation during Pregnancy Is Associated with Reduced Occurrence of Symptoms Suggestive of Pre-Eclampsia or Eclampsia in Indian Women. PLoS ONE 2015, 10, e0119120. [Google Scholar] [CrossRef]
- Cherian, A.G.; Aabidha, P.M.; Paul, E.; Helan, J. Maternal and fetal outcome in pre-eclampsia in a secondary care hospital in South India. J. Fam. Med. Prim. Care 2015, 4, 257–260. [Google Scholar] [CrossRef]
- Skjaerven, R.; Wilcox, A.J.; Klungsøyr, K.; Irgens, L.M.; Vikse, B.E.; Vatten, L.J.; Lie, R.T. Cardiovascular mortality after pre-eclampsia in one child mothers: Prospective, population based cohort study. BMJ 2012, 345, e7677. [Google Scholar] [CrossRef]
- Lee, S.E.; Talegawkar, S.A.; Merialdi, M.; Caulfield, L.E. Dietary intakes of women during pregnancy in low- and middle-income countries. Public Health Nutr. 2012, 16, 1340–1353. [Google Scholar] [CrossRef]
- Victora, C.G.; Christian, P.; Vidaletti, L.P.; Gatica-Domínguez, G.; Menon, P.; Black, R.E. Revisiting maternal and child undernutrition in low-income and middle-income countries: Variable progress towards an unfinished agenda. Lancet 2021, 397, 1388–1399. [Google Scholar] [CrossRef]
- Lander, R.L.; Hambidge, K.M.; Westcott, J.E.; Tejeda, G.; Diba, T.S.; Mastiholi, S.C.; Khan, U.S.; Garcés, A.; Figueroa, L.; Tshefu, A.; et al. Pregnant Women in Four Low-Middle Income Countries Have a High Prevalence of Inadequate Dietary Intakes That Are Improved by Dietary Diversity. Nutrients 2019, 11, 1560. [Google Scholar] [CrossRef]
- Sondaal, S.F.V.; Browne, J.L.; Amoakoh-Coleman, M.; Borgstein, A.; Miltenburg, A.S.; Verwijs, M.; Klipstein-Grobusch, K. Assessing the Effect of mHealth Interventions in Improving Maternal and Neonatal Care in Low- and Middle-Income Countries: A Systematic Review. PLoS ONE 2016, 11, e0154664. [Google Scholar] [CrossRef] [PubMed]
- Bourassa, M.W.; Osendarp, S.J.; Adu-Afarwuah, S.; Ahmed, S.; Ajello, C.; Bergeron, G.; Black, R.; Christian, P.; Cousens, S.; Pee, S.; et al. Review of the evidence regarding the use of antenatal multiple micronutrient supplementation in low- and middle-income countries. Ann. N. Y. Acad. Sci. 2019, 1444, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Catherin, N.; Rock, B.; Roger, V.; Ankita, C.; Ashish, G.; Delwin, P.; Deeepthi, S.; Goud, B.R. Beliefs and practices regarding nutrition during pregnancy and lactation in a rural area in Karnataka, India: A qualitative study. Int. J. Community Med. Public Health 2015, 2, 116. [Google Scholar] [CrossRef]
- Chakona, G.; Shackleton, C. Food Taboos and Cultural Beliefs Influence Food Choice and Dietary Preferences among Pregnant Women in the Eastern Cape, South Africa. Nutrients 2019, 11, 2668. [Google Scholar] [CrossRef] [PubMed]
- Kariuki, L.W.; Lambert, C.; Purwestri, R.C.; Maundu, P.; Biesalski, H.K. Role of food taboos in energy, macro and micronutrient intake of pregnant women in western Kenya. Nutr. Food Sci. 2017, 47, 795–807. [Google Scholar] [CrossRef]
- Martínez Pérez, G.; Pascual García, A. Nutritional taboos among the Fullas in Upper River region, the Gambia. J. Anthropol. 2013, 2013, 873612. [Google Scholar] [CrossRef]
- Ugwa, E. Nutritional practices and taboos among pregnant women attending antenatal care at general hospital in Kano, Northwest Nigeria. Ann. Med Health Sci. Res. 2016, 6, 109–114. [Google Scholar] [CrossRef]
- Oni, O.A.; Tukur, J. Identifying pregnant women who would adhere to food taboos in a rural community: A community-based study. Afr. J. Reprod. Health 2012, 16, 67–75. [Google Scholar]
- Tsegaye, D.; Tamiru, D.; Belachew, T. Food-related taboos and misconceptions during pregnancy among rural communities of Illu Aba Bor zone, Southwest Ethiopia. A community based qualitative cross-sectional study. BMC Pregnancy Childbirth 2021, 21, 309. [Google Scholar] [CrossRef] [PubMed]
- Ramulondi, M.; de Wet, H.; Ntuli, N.R. Traditional food taboos and practices during pregnancy, postpartum recovery, and infant care of Zulu women in northern KwaZulu-Natal. J. Ethnobiol. Ethnomed. 2021, 17, 15. [Google Scholar] [CrossRef] [PubMed]
- Craig, H.C.; Jeyanthi, R.; Pelto, G.; Willford, A.C.; Stoltzfus, R.J. Using a cultural-ecological framework to explore dietary beliefs and practices during pregnancy and lactation among women in Adivasi communities in the Nilgiris Biosphere Reserve, India. Ecol. Food Nutr. 2018, 57, 165–186. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Chakrabarti, A. Food taboos in pregnancy and early lactation among women living in a rural area of West Bengal. J. Fam. Med. Prim. Care 2019, 8, 86–90. [Google Scholar] [CrossRef]
- Andersen, L.T.; Thilsted, S.H.; Nielsen, B.B.; Rangasamy, S. Food and nutrient intakes among pregnant women in rural Tamil Nadu, South India. Public Health Nutr. 2003, 6, 131–137. [Google Scholar] [CrossRef][Green Version]
- Vidler, M.; Charantimath, U.; Katageri, G.; Ramadurg, U.; Karadiguddi, C.; Sawchuck, D.; Qureshi, R.; Dharamsi, S.; von Dadelszen, P.; Derman, R.; et al. Community perceptions of pre-eclampsia in rural Karnataka State, India: A qualitative study. Reprod. Health 2016, 13, 45–53. [Google Scholar] [CrossRef]
- Dreyfuss, M.L.; Stoltzfus, R.J.; Shrestha, J.B.; Pradhan, E.K.; LeClerq, S.C.; Khatry, S.K.; Shrestha, S.R.; Katz, J.; Albonico, M.; West, K.P., Jr. Hookworms, malaria and vitamin A deficiency contribute to anemia and iron deficiency among pregnant women in the plains of Nepal. J. Nutr. 2000, 130, 2527–2536. [Google Scholar] [CrossRef]
- Varghese, J.S.; Stein, A.D. Malnutrition among women and children in India: Limited evidence of clustering of underweight, anemia, overweight, and stunting within individuals and households at both state and district levels. Am. J. Clin. Nutr. 2019, 109, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.K.; Manandhar, N. Prevalence of Anemia among Women: A Hospital-Based Study in Eastern Nepal. Janaki Med. Coll. J. Med. Sci. 2020, 8, 43–47. [Google Scholar] [CrossRef]
- Maka, S.S.; Tondare, S.B.; Tondare, M.B. Study of impact of anemia on pregnancy. Int. J. Reprod. Contracept. Obstet. Gynecol. 2017, 6, 4847. [Google Scholar] [CrossRef][Green Version]
- Chalise, B.; Aryal, K.K.; Mehta, R.K.; Dhimal, M.; Sapkota, F.; Mehata, S.; Karki, K.B.; Madjdian, D.; Patton, G.; Sawyer, S. Prevalence and correlates of anemia among adolescents in Nepal: Findings from a nationally representative cross-sectional survey. PLoS ONE 2018, 13, e0208878. [Google Scholar] [CrossRef]
- Barua, A.; Mundle, S.; Bracken, H.; Easterling, T.; Winikoff, B. Facility and personnel factors influencing magnesium sulfate use for eclampsia and pre-eclampsia in 3 Indian hospitals. Int. J. Gynecol. Obstet. 2011, 115, 231–234. [Google Scholar] [CrossRef]
- James, K.S.; Singh, S.K.; Shekhar, C.; Dwivedi, L.K.; Arnold, F. National Family Health Survey (NFHS-5); Government of India: Delhi, India, 2022. [Google Scholar]
- Perappadan, B.S. Explained|Why Is India Rethinking Its Anaemia Policy? Hindu 2023. Available online: https://www.thehindu.com/sci-tech/health/explained-why-is-india-rethinking-its-anaemia-policy/article66928573.ece (accessed on 1 July 2023).
- Bhadoria, A.S.; Dhinwa, M.; Gawande, K.; Jha, N.; Anjali, M.; Sinha, S. Prevalence of hypertensive disorders of pregnancy in India: A systematic review and meta-analysis. J. Med. Evid. 2021, 2, 105. [Google Scholar] [CrossRef]
- Bankar, S.; Ghosh, D. Accessing Antenatal Care (ANC) services during the COVID-19 first wave: Insights into decision-making in rural India. Reprod. Health 2022, 19, 158. [Google Scholar] [CrossRef] [PubMed]
- Yilma, H.; Sedlander, E.; Rimal, R.N.; Pant, I.; Munjral, A.; Mohanty, S. The reduction in anemia through normative innovations (RANI) project: Study protocol for a cluster randomized controlled trial in Odisha, India. BMC Public Health 2020, 20, 203. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, P.Y.; Bhawalkar, J.S.; Jadhav, A. Anemia control program in India needs to be more comprehensive. Indian J. Public Health 2022, 66, 358–361. [Google Scholar] [CrossRef]
- Sedlander, E.; Long, M.W.; Mohanty, S.; Munjral, A.; Bingenheimer, J.B.; Yilma, H.; Rimal, R.N. Moving beyond individual barriers and identifying multi-level strategies to reduce anemia in Odisha India. BMC Public Health 2020, 20, 457. [Google Scholar] [CrossRef]
- Jeyakumar, A.; Chalwadi, S.; Madhu, P.; Ghugre, P. Sustainability of integrated anaemia prevention activities implemented through non-government organizations and schools, and its effect on haemoglobin status of adolescent girls in urban slums of Pune, in Maharashtra, India. Nutr. Health 2021, 28, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Diamond-Smith, N.; Holton, A.E.; Francis, S.; Bernard, D. Addressing anemia among women in India—An informed intervention using Facebook Ad Manager. Mhealth 2020, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Olney, D.K.; Pedehombga, A.; Ruel, M.T.; Dillon, A. A 2-Year Integrated Agriculture and Nutrition and Health Behavior Change Communication Program Targeted to Women in Burkina Faso Reduces Anemia, Wasting, and Diarrhea in Children 3–12.9 Months of Age at Baseline: A Cluster-Randomized Controlled Trial. J. Nutr. 2015, 145, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, A.; Choudhury, M. Mobile for Mothers mHealth Intervention to Augment Maternal Health Awareness and Behavior of Pregnant Women in Tribal Societies: Randomized Quasi-Controlled Study. JMIR mHealth uHealth 2022, 10, e38368. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, A.; Asan, O.; Choudhury, M.M. Mobile health technology to improve maternal health awareness in tribal populations: Mobile for mothers. J. Am. Med. Inform. Assoc. 2021, 28, 2467–2474. [Google Scholar] [CrossRef]
- Ilozumba, O.; Van Belle, S.; Dieleman, M.; Liem, L.; Choudhury, M.; Broerse, J.E.W. The Effect of a Community Health Worker Utilized Mobile Health Application on Maternal Health Knowledge and Behavior: A Quasi-Experimental Study. Front. Public Health 2018, 6, 133. [Google Scholar] [CrossRef]
- Ben-Zeev, D.; Brian, R.M.; Jonathan, G.; Razzano, L.; Pashka, N.; Carpenter-Song, E.; Drake, R.E.; Scherer, E.A. Mobile Health (mHealth) Versus Clinic-Based Group Intervention for People with Serious Mental Illness: A Randomized Controlled Trial. Psychiatr. Serv. 2018, 69, 978–985. [Google Scholar] [CrossRef]
- Benski, A.C.; Schmidt, N.C.; Viviano, M.; Stancanelli, G.; Soaroby, A.; Reich, M.R. Improving the Quality of Antenatal Care Using Mobile Health in Madagascar: Five-Year Cross-Sectional Study. JMIR mHealth uHealth 2020, 8, e18543. [Google Scholar] [CrossRef]
- Vilaplana, E.G.; Petignat, P.; Benski, A.-C.; Soaroby, A.; Sormani, J.; Vassilakos, P.; Schmidt, N.C. Description of Maternal Morbidities Amongst 1000 Women During Pregnancy in Ambanja, Madagascar—Opportunities and Challenges of Using an mHealth System. Int. J. Women’s Health 2020, 12, 823–833. [Google Scholar] [CrossRef]
- Breymann, C.; Auerbach, M. Iron deficiency in gynecology and obstetrics: Clinical implications and management. Hematology 2017, 2017, 152–159. [Google Scholar] [CrossRef]
- Lim, J.; Cloete, G.; Dunsmuir, D.T.; Payne, B.A.; Scheffer, C.; von Dadelszen, P.; Dumont, G.A.; Ansermino, J.M. Usability and Feasibility of PIERS on the Move: An mHealth App for Pre-Eclampsia Triage. JMIR mHealth uHealth 2015, 3, e37. [Google Scholar] [CrossRef]
- Dunsmuir, D.T.; Payne, B.A.; Cloete, G.; Petersen, C.L.; Gorges, M.; Lim, J.; von Dadelszen, P.; Dumont, G.A.; Ansermino, J.M. Development of mHealth Applications for Pre-Eclampsia Triage. IEEE J. Biomed. Health Informatics 2014, 18, 1857–1864. [Google Scholar] [CrossRef]
- White, A.; Crowther, S.; Lee, S. Supporting rural midwifery practice using a mobile health (mHealth) intervention: A qualitative descriptive study. Rural Remote Health 2019, 19, 5294. [Google Scholar] [CrossRef]
- Charanthimath, U.; Katageri, G.; Kinshella, M.-L.W.; Mallapur, A.; Goudar, S.; Ramadurg, U.; Vidler, M.; Sharma, S.; Derman, R.; Magee, L.A.; et al. Community Health Worker Evaluation of Implementing an mHealth Application to Support Maternal Health Care in Rural India. Front. Glob. Women's Health 2021, 2, 645690. [Google Scholar] [CrossRef] [PubMed]
- Boene, H.; Valá, A.; Kinshella, M.-L.W.; La, M.; Sharma, S.; Vidler, M.; Magee, L.A.; von Dadelszen, P.; Sevene, E.; Munguambe, K.; et al. Implementation of the PIERS on the Move mHealth Application from the Perspective of Community Health Workers and Nurses in Rural Mozambique. Front. Glob. Women's Health 2021, 2, 659582. [Google Scholar] [CrossRef]
- Aranda-Jan, C. The Mobile Disability Gap Report 2020; The Mobile Disability Gap Report; GSMA: London, UK, 2020. [Google Scholar]
- Bhawan, M.D.; Marg, J.L.N. Telecom Regulatory Authority of India. Resource 2019, 7, 12. [Google Scholar]
- Tomlinson, M.; Rotheram-Borus, M.J.; Swartz, L.; Tsai, A.C. Scaling up mHealth: Where is the evidence? PLoS Med. 2013, 10, e1001382. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. mHealth: New Horizons for Health through Mobile Technologies; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Jonas, S.M.; Deserno, T.M.; Buhimschi, C.S.; Makin, J.; Choma, M.A.; Buhimschi, I.A. Smartphone-based diagnostic for preeclampsia: An mHealth solution for administering the Congo Red Dot (CRD) test in settings with limited resources. J. Am. Med. Inform. Assoc. 2015, 23, 166–173. [Google Scholar] [CrossRef][Green Version]
- Archibong, E.; Konnaiyan, K.R.; Kaplan, H.; Pyayt, A. A mobile phone-based approach to detection of hemolysis. Biosens. Bioelectron. 2017, 88, 204–209. [Google Scholar] [CrossRef]
- Hacker, F.M.; Jeyabalan, A.; Quinn, B.; Hauspurg, A. Implementation of a universal postpartum blood pressure monitoring program: Feasibility and outcomes. Am. J. Obstet. Gynecol. MFM 2022, 4, 100613. [Google Scholar] [CrossRef]
- Hauspurg, A.; Lemon, L.S.; Quinn, B.A.; Binstock, A.; Larkin, J.; Beigi, R.H.; Watson, A.R.; Simhan, H.N. A Postpartum Remote Hypertension Monitoring Protocol Implemented at the Hospital Level. Obstet. Gynecol. 2019, 134, 685–691. [Google Scholar] [CrossRef]
- Kantorowska, A.; Cohen, K.; Oberlander, M.; Jaysing, A.R.; Akerman, M.B.; Wise, A.-M.; Mann, D.M.; Testa, P.A.; Chavez, M.R.; Vintzileos, A.M.; et al. Remote patient monitoring for management of diabetes mellitus in pregnancy is associated with improved maternal and neonatal outcomes. Am. J. Obstet. Gynecol. 2023, 228, 726.e1–726.e11. [Google Scholar] [CrossRef] [PubMed]
- Von Dadelszen, P.; Bhutta, Z.A.; Sharma, S.; Bone, J.; Singer, J.; Wong, H.; Bellad, M.B.; Goudar, S.S.; Lee, T.; Li, J.; et al. The Community-Level Interventions for Pre-eclampsia (CLIP) cluster randomised trials in Mozambique, Pakistan, and India: An individual participant-level meta-analysis. Lancet 2020, 396, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Ashok, S.; Nguyen, P.H.; Singh, S.K.; Sarwal, R.; Bhatia, N.; Johnston, R.; Joe, W.; Sarswat, E.; Menon, P. State Nutrition Profile: Jharkhand; International Food Policy Research Institute: Delhi, India, 2022. [Google Scholar]
- Horwood, G.; Opondo, C.; Choudhury, S.S.; Rani, A.; Nair, M. Risk factors for maternal mortality among 1.9 million women in nine empowered action group states in India: Secondary analysis of Annual Health Survey data. BMJ Open 2020, 10, e038910. [Google Scholar] [CrossRef] [PubMed]
- Mishra, C.K. Guidance Note on Prevention and Management of Postpartum Haemorrhage; Ministry of Health and Family Welfare, Division MH: New Delhi, India, 2015. [Google Scholar]
- Executive Summary. Government of Jharkhand. 2021. Available online: https://finance.jharkhand.gov.in/pdf/Economic_Survey_2020_21/JES_Executive_Summary_2020_21.pdf (accessed on 26 August 2022).
- Champion, V.L.; Skinner, C.S. The health belief model. Health Behav. Health Educ. Theory Res. Pract. 2008, 4, 45–65. [Google Scholar]
- Silva, P. Davis’ technology acceptance model (TAM) (1989). Inf. Seek. Behav. Technol. Adopt. Theor. Trends 2015, 205–219. [Google Scholar] [CrossRef]
- Rosenstock, I.M. The Health Belief Model and Preventive Health Behavior. Health Educ. Monogr. 1974, 2, 354–386. [Google Scholar] [CrossRef]
- Davis, F.D. Perceived Usefulness, Perceived Ease of Use, and User Acceptance of Information Technology. MIS Q. 1989, 13, 319–340. [Google Scholar] [CrossRef]
- Davis, F.D. A Technology Acceptance Model for Empirically Testing New End-User Information Systems: Theory and Results. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 1985. [Google Scholar]
- Rosenstock, I.M.; Strecher, V.J.; Becker, M.H. Social Learning Theory and the Health Belief Model. Health Educ. Q. 1988, 15, 175–183. [Google Scholar] [CrossRef]
- Ajzen, I. The theory of planned behavior. Organ. Behav. Hum. Decis. Process. 1991, 50, 179–211. [Google Scholar] [CrossRef]
- Hair, J.F., Jr.; Hult, G.T.M.; Ringle, C.M.; Sarstedt, M.; Danks, N.P.; Ray, S. The SEMinR Package. Partial Least Squares Structural Equation Modeling (PLS-SEM) Using R: A Workbook; Springer: Berlin/Heidelberg, Germany, 2021; pp. 49–74. [Google Scholar]
- Ihaka, R.; Gentleman, R. R: A language for data analysis and graphics. J. Comput. Graph. Stat. 1996, 5, 299–314. [Google Scholar]
- Cohen, J.; Cohen, P.; West, S.G.; Aiken, L.S. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences; Routledge: London, UK, 2013; ISBN 1134801017. [Google Scholar]
- Streukens, S.; Leroi-Werelds, S. Bootstrapping and PLS-SEM: A step-by-step guide to get more out of your bootstrap results. Eur. Manag. J. 2016, 34, 618–632. [Google Scholar] [CrossRef]
- Bhattacherjee, A. Understanding Information Systems Continuance: An Expectation-Confirmation Model. MIS Q. 2001, 25, 351–370. [Google Scholar] [CrossRef]
- Hua, T.V.; Hou, Y. Factors That Influence the Intention to Use Self-Diagnosis Apps in Vietnam. J. Health Med. Nurs. 2020, 72, 47–56. [Google Scholar] [CrossRef][Green Version]
- Chib, A.; van Velthoven, M.H.; Car, J. mHealth Adoption in Low-Resource Environments: A Review of the Use of Mobile Healthcare in Developing Countries. J. Health Commun. 2014, 20, 4–34. [Google Scholar] [CrossRef] [PubMed]
- Labrique, A.B.; Vasudevan, L.; Kochi, E.; Fabricant, R.; Mehl, G. mHealth innovations as health system strengthening tools: 12 common applications and a visual framework. Glob. Health Sci. Pract. 2013, 1, 160–171. [Google Scholar] [CrossRef] [PubMed]
- James, D.C.S.; Harville, C. Smartphone Usage, Social Media Engagement, and Willingness to Participate in mHealth Weight Management Research Among African American Women. Health Educ. Behav. 2017, 45, 315–322. [Google Scholar] [CrossRef]



| Variable Names | Survey Questions | n (%) | |
|---|---|---|---|
| Self-care ability (SA) | Q1. How likely is it that using a mobile health (mHealth) app for anemia and preeclampsia management would improve your ability to care for your health during pregnancy? | Extremely unlikely | 6 (4.58) |
| Unlikely | 13 (9.92) | ||
| Neutral | 50 (38.17) | ||
| Likely | 43 (32.82) | ||
| Extremely likely | 19 (14.50) | ||
| Informed decision-making (IDM) | Q2. How useful do you think a mHealth app for anemia and preeclampsia management would be in helping you make informed decisions about your health during pregnancy? | Not useful at all | 9 (6.87) |
| Slightly useful | 55 (41.98) | ||
| Moderately useful | 42 (32.06) | ||
| Very useful | 21 (16.03) | ||
| Extremely useful | 4 (3.05) | ||
| Perceived usefulness of mobile app (PU) | Q3. How effective do you believe using a mHealth app for anemia and preeclampsia management would be in reducing the risk of complications during pregnancy? | Not effective at all | 15 (11.45) |
| Slightly effective | 41 (31.30) | ||
| Moderately effective | 43 (32.82) | ||
| Very effective | 30 (22.90) | ||
| Extremely effective | 2 (1.53) | ||
| Perceived ease of use of using mobile health apps (PEU) | Q4. How easy or difficult do you think it would be to use a mHealth app for anemia and preeclampsia management? | Extremely difficult | 7 (5.34) |
| Difficult | 17 (12.98) | ||
| Neutral | 72 (54.96) | ||
| Easy | 30 (22.90) | ||
| Extremely easy | 5 (3.82) | ||
| Self-efficacy of using mobile health apps (SE) | Q5. How confident are you in your ability to use a mHealth app for anemia and preeclampsia management to improve your health during pregnancy? | Not confident at all | 23 (17.56) |
| Slightly confident | 50 (38.17) | ||
| Moderately confident | 30 (22.90) | ||
| Very confident | 22 (16.79) | ||
| Extremely confident | 6 (4.58) | ||
| Behavioral intention to use mobile health apps (IU) | Q6. If a mHealth app for anemia and preeclampsia management were available, how likely would you be to use it during your pregnancy? | Extremely unlikely | 7 (5.34) |
| Unlikely | 17 (12.98) | ||
| Neutral | 44 (33.59) | ||
| Likely | 56 (42.75) | ||
| Extremely likely | 7 (5.34) | ||
| Perceived susceptibility of anemia and preeclampsia (PSus) | Q7. How likely do you think it is for pregnant individuals to develop anemia and preeclampsia during pregnancy? | Extremely unlikely | 7 (5.34) |
| Unlikely | 17 (12.98) | ||
| Neutral | 43 (32.82) | ||
| Likely | 57 (43.51) | ||
| Extremely likely | 7 (5.34) | ||
| Perceived severity of anemia and preeclampsia (PSer) | Q8. How serious do you think the consequences of anemia and preeclampsia are for pregnant individuals and their babies? | Not serious at all | 17 (12.98) |
| Slightly serious | 55 (41.98) | ||
| Moderately serious | 39 (29.77) | ||
| Very serious | 18 (13.74) | ||
| Extremely serious | 2 (1.53) | ||
| Diet and lifestyle changes (DL) | Q9. If you were to use a mHealth app for anemia and preeclampsia management, how likely is it that you would make changes to your diet and lifestyle based on the app’s recommendations? | Extremely unlikely | 10 (7.63) |
| Unlikely | 10 (7.63) | ||
| Neutral | 29 (22.14) | ||
| Likely | 54 (41.22) | ||
| Extremely likely | 28 (21.37) | ||
| Health-seeking behavior (HB) | Q10. After using a mHealth app for anemia and preeclampsia management, how likely would you be to seek medical advice or treatment for any symptoms or concerns related to anemia or preeclampsia? | Extremely unlikely | 8 (6.11) |
| Unlikely | 10 (7.63) | ||
| Neutral | 37 (28.24) | ||
| Likely | 55 (41.98) | ||
| Extremely likely | 21 (16.03) | ||
| Categories | Sub-Categories | Respondents n(%) |
|---|---|---|
| Age group (years) | 25–34 years | 72 (55) |
| 18–24 years | 28 (21) | |
| 35–44 years | 28 (21) | |
| Under 18 years | 1 (<1) | |
| 45–54 years | 2 (<1) | |
| Employment | Homemakers | 92 (70) |
| Employed part-time | 30 (23) | |
| Self-employed | 8 (6) | |
| Unemployed | 1 (<1) | |
| Religion | Hindu | 116 (89) |
| Muslim | 15 (11) | |
| Level of education | Secondary (7–12 years) | 57 (44) |
| Primary (1–6 years) | 27 (21) | |
| No formal education | 28 (21) | |
| Bachelor’s degree | 14 (11) | |
| Other | 4 (<2) | |
| Linguistic proficiency in Hindi | Speak, read, and write | 48 (37) |
| Speak only | 30 (23) | |
| Speak and read | 26 (30) | |
| No understanding | 25 (19) | |
| Read only | 2 (2) |
| Paths | Direct Path | Total Path | ||||
|---|---|---|---|---|---|---|
| β | SD | CI | β | SD | CI | |
| PSus → PU | 0.120 | 0.072 | [−0.019, 0.261] | 0.120 | 0.072 | [−0.019, 0.261] |
| PSus → SA | 0.025 | 0.096 | [−0.163, 0.213] | 0.025 | 0.096 | [−0.163, 0.213] |
| PSus → IDM | 0.080 | 0.095 | [−0.107, 0.265] | 0.080 | 0.095 | [−0.107, 0.265] |
| PSus → IU | 0.055 | 0.040 | [−0.014, 0.143] | |||
| PSus → DL | 0.023 | 0.018 | [−0.005, 0.066] | |||
| PSus → HB | 0.030 | 0.022 | [−0.007, 0.082] | |||
| PSer → PU | 0.413 | 0.075 | [0.261, 0.552] * | 0.413 | 0.075 | [0.261, 0.552] * |
| PSer → SA | −0.173 | 0.089 | [−0.353, 0.001] | −0.173 | 0.089 | [−0.353, 0.000] |
| PSer → IDM | 0.151 | 0.104 | [−0.049, 0.359] | 0.151 | 0.104 | [−0.049, 0.359] |
| PSer → IU | 0.120 | 0.061 | [0.009, 0.247] * | |||
| PSer → DL | 0.047 | 0.026 | [0.003, 0.104] * | |||
| PSer → HB | 0.063 | 0.032 | [0.005, 0.129] * | |||
| PEU → PU | 0.202 | 0.078 | [0.050, 0.355] * | 0.202 | 0.078 | [0.050, 0.355] * |
| PEU → SA | 0.392 | 0.097 | [0.194, 0.574] * | 0.392 | 0.097 | [0.194, 0.574] * |
| PEU → IDM | 0.139 | 0.103 | [−0.068, 0.338] | 0.139 | 0.103 | [−0.068, 0.338] |
| PEU → IU | 0.166 | 0.103 | [−0.039, 0.363] | 0.315 | 0.087 | [0.139, 0.480] * |
| PEU → DL | 0.127 | 0.051 | [0.038, 0.237] * | |||
| PEU → HB | 0.169 | 0.059 | [0.061, 0.291] * | |||
| SE → PU | 0.144 | 0.085 | [−0.013, 0.322] | 0.144 | 0.085 | [−0.013, 0.322] |
| SE → SA | 0.111 | 0.094 | [−0.072, 0.295] | 0.111 | 0.094 | [−0.072, 0.295] |
| SE → IDM | 0.316 | 0.113 | [0.088, 0.527] * | 0.316 | 0.113 | [0.088, 0.527] * |
| SE → IU | 0.075 | 0.096 | [−0.107,0.268] | 0.165 | 0.099 | [−0.028, 0.358] |
| SE → DL | 0.069 | 0.047 | [−0.008, 0.172] | |||
| SE → HB | 0.091 | 0.059 | [−0.012, 0.216] | |||
| PU → IU | 0.319 | 0.133 | [0.053, 0.562] * | 0.319 | 0.133 | [0.053, 0.562] * |
| PU → DL | 0.126 | 0.061 | [0.019, 0.254] * | |||
| PU → HB | 0.168 | 0.072 | [0.028, 0.309] * | |||
| SA → IU | 0.196 | 0.087 | [0.026, 0.368] * | 0.196 | 0.087 | [0.026, 0.368] * |
| SA → DL | 0.079 | 0.041 | [0.009, 0.167] * | |||
| SA → HB | 0.105 | 0.051 | [0.013, 0.210] * | |||
| IDM → IU | 0.089 | 0.112 | [−0.130, 0.306] | 0.089 | 0.112 | [−0.130, 0.306] |
| IDM → DL | 0.036 | 0.046 | [−0.050, 0.128] | |||
| IDM → HB | 0.048 | 0.060 | [−0.066, 0.167] | |||
| IU → DL | 0.397 | 0.096 | [0.199, 0.577] * | 0.397 | 0.096 | [0.199, 0.577] * |
| IU → HB | 0.530 | 0.083 | [0.357, 0.683] * | 0.530 | 0.083 | [0.357, 0.683] * |
| Age → PU | 0.004 | 0.062 | [−0.118, 0.126] | 0.004 | 0.062 | [−0.118, 0.126] |
| Age → SA | −0.022 | 0.085 | [−0.185, 0.144] | −0.022 | 0.085 | [−0.185, 0.144] |
| Age → IDM | −0.172 | 0.074 | [−0.315, −0.026] * | −0.172 | 0.074 | [−0.315, −0.026] * |
| Age → IU | 0.047 | 0.074 | [−0.093, 0.197] | 0.029 | 0.074 | [−0.111, 0.179] |
| Age → DL | −0.095 | 0.088 | [−0.271, 0.078] | −0.082 | 0.093 | [−0.271, 0.094] |
| Age → HB | −0.003 | 0.079 | [−0.163, 0.145] | 0.012 | 0.085 | [−0.161, 0.173] |
| Education → PU | 0.135 | 0.060 | [0.022, 0.256] * | 0.135 | 0.060 | [0.022, 0.256] * |
| Education → SA | 0.089 | 0.097 | [−0.097, 0.280] | 0.089 | 0.097 | [−0.097, 0.280] |
| Education → IDM | 0.029 | 0.096 | [−0.153, 0.222] | 0.029 | 0.096 | [−0.153, 0.222] |
| Education → IU | −0.035 | 0.104 | [−0.229, 0.177] | 0.031 | 0.117 | [−0.190, 0.264] |
| Education → DL | 0.066 | 0.095 | [−0.116, 0.256] | 0.078 | 0.099 | [−0.111, 0.276] |
| Education → HB | 0.123 | 0.082 | [−0.029, 0.288] | 0.138 | 0.093 | [−0.038, 0.321] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choudhury, A.; Shahsavar, Y.; Sarkar, K.; Choudhury, M.M.; Nimbarte, A.D. Exploring Perceptions and Needs of Mobile Health Interventions for Nutrition, Anemia, and Preeclampsia among Pregnant Women in Underprivileged Indian Communities: A Cross-Sectional Survey. Nutrients 2023, 15, 3699. https://doi.org/10.3390/nu15173699
Choudhury A, Shahsavar Y, Sarkar K, Choudhury MM, Nimbarte AD. Exploring Perceptions and Needs of Mobile Health Interventions for Nutrition, Anemia, and Preeclampsia among Pregnant Women in Underprivileged Indian Communities: A Cross-Sectional Survey. Nutrients. 2023; 15(17):3699. https://doi.org/10.3390/nu15173699
Chicago/Turabian StyleChoudhury, Avishek, Yeganeh Shahsavar, Krishnendu Sarkar, Murari Mohan Choudhury, and Ashish D. Nimbarte. 2023. "Exploring Perceptions and Needs of Mobile Health Interventions for Nutrition, Anemia, and Preeclampsia among Pregnant Women in Underprivileged Indian Communities: A Cross-Sectional Survey" Nutrients 15, no. 17: 3699. https://doi.org/10.3390/nu15173699
APA StyleChoudhury, A., Shahsavar, Y., Sarkar, K., Choudhury, M. M., & Nimbarte, A. D. (2023). Exploring Perceptions and Needs of Mobile Health Interventions for Nutrition, Anemia, and Preeclampsia among Pregnant Women in Underprivileged Indian Communities: A Cross-Sectional Survey. Nutrients, 15(17), 3699. https://doi.org/10.3390/nu15173699






