Dietary Supplements for Erectile Dysfunction: Analysis of Marketed Products, Systematic Review, Meta-Analysis and Rational Use
Abstract
1. Introduction
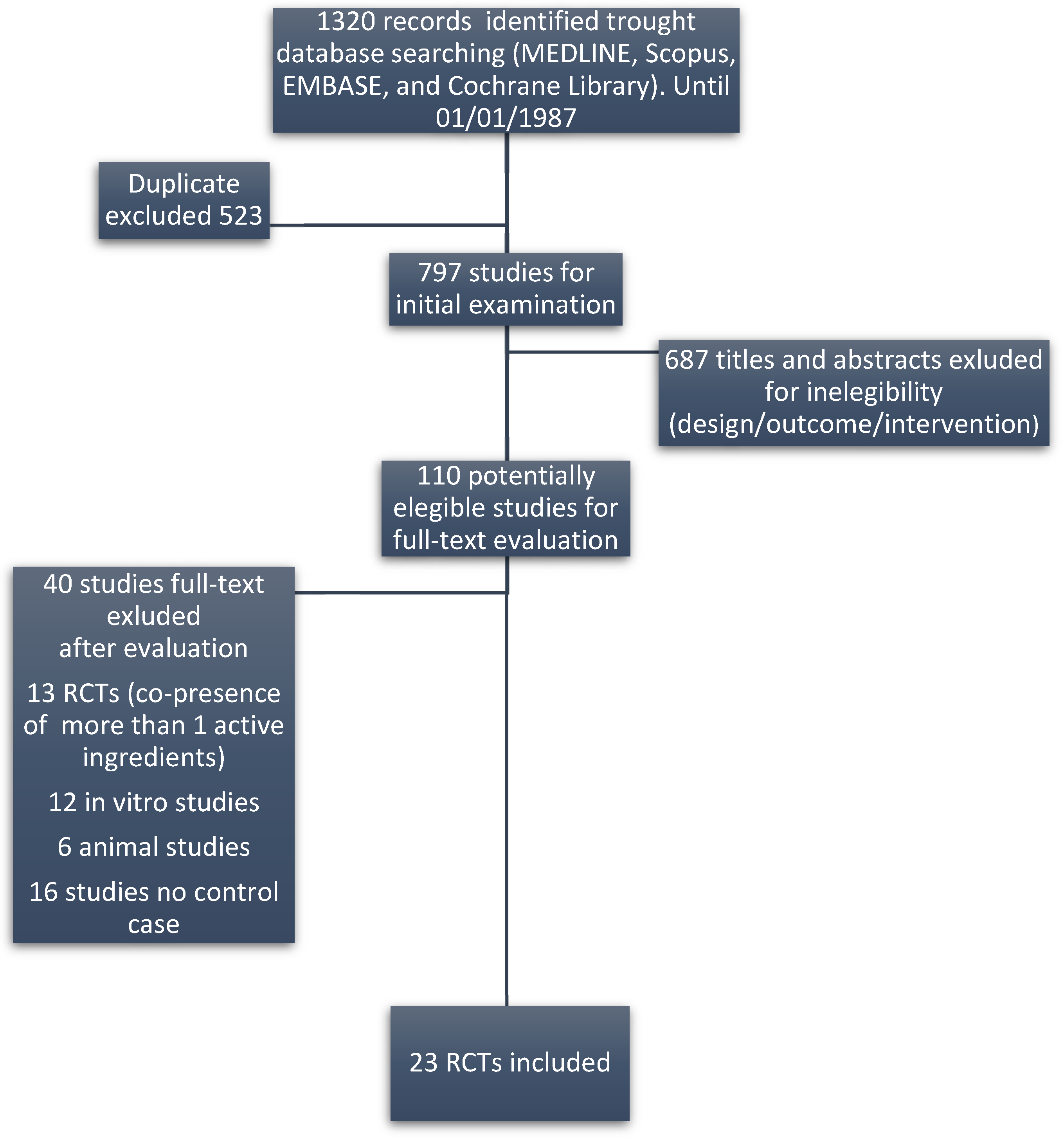
2. Materials and Methods
2.1. Data Analysis
2.2. Risk of Bias
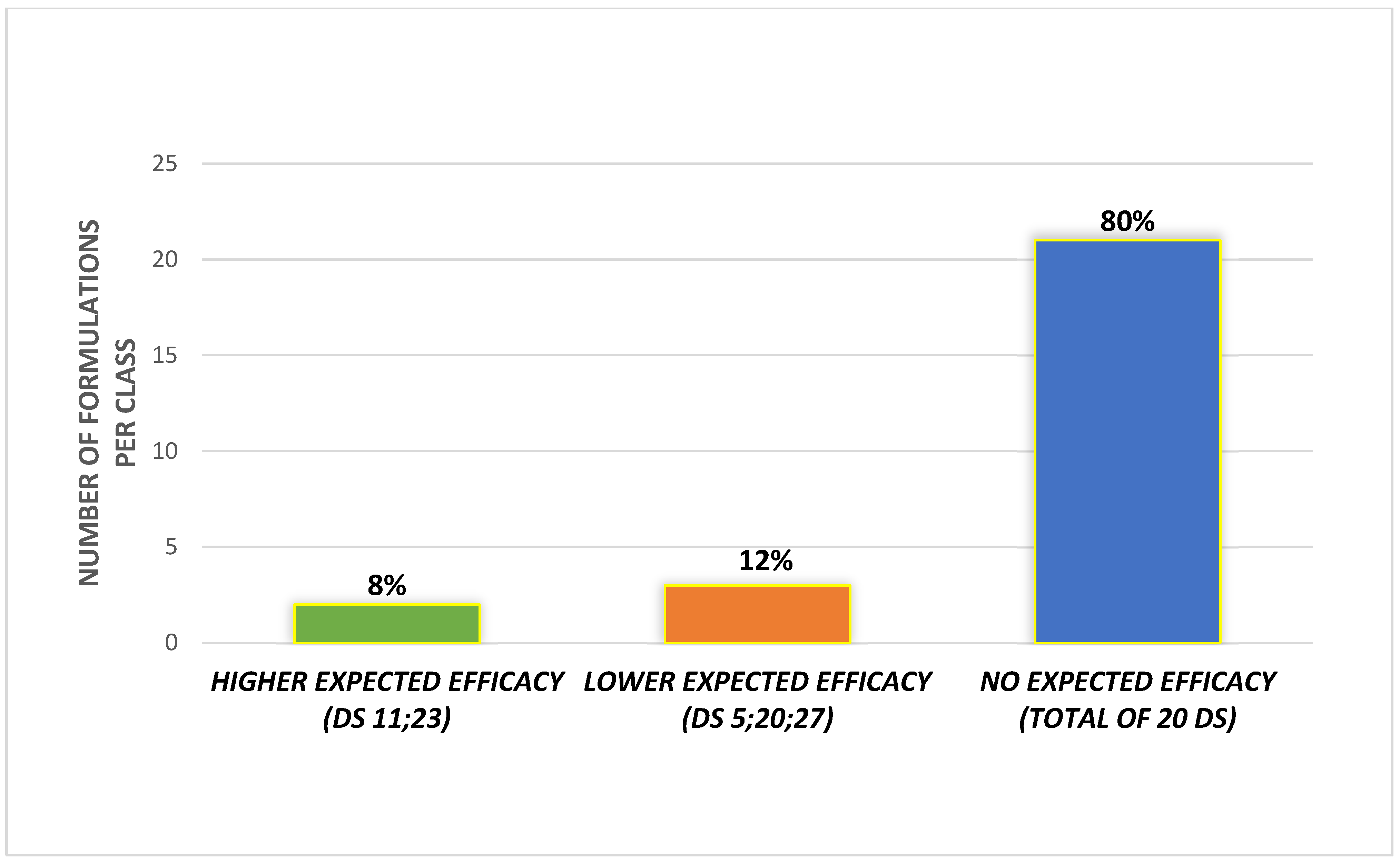
2.3. Definition of Potential Active Ingredients, Minimal Effective Dose and Evaluation of Commercial DS Formulation
3. Results
Meta-Analysis
- (I)
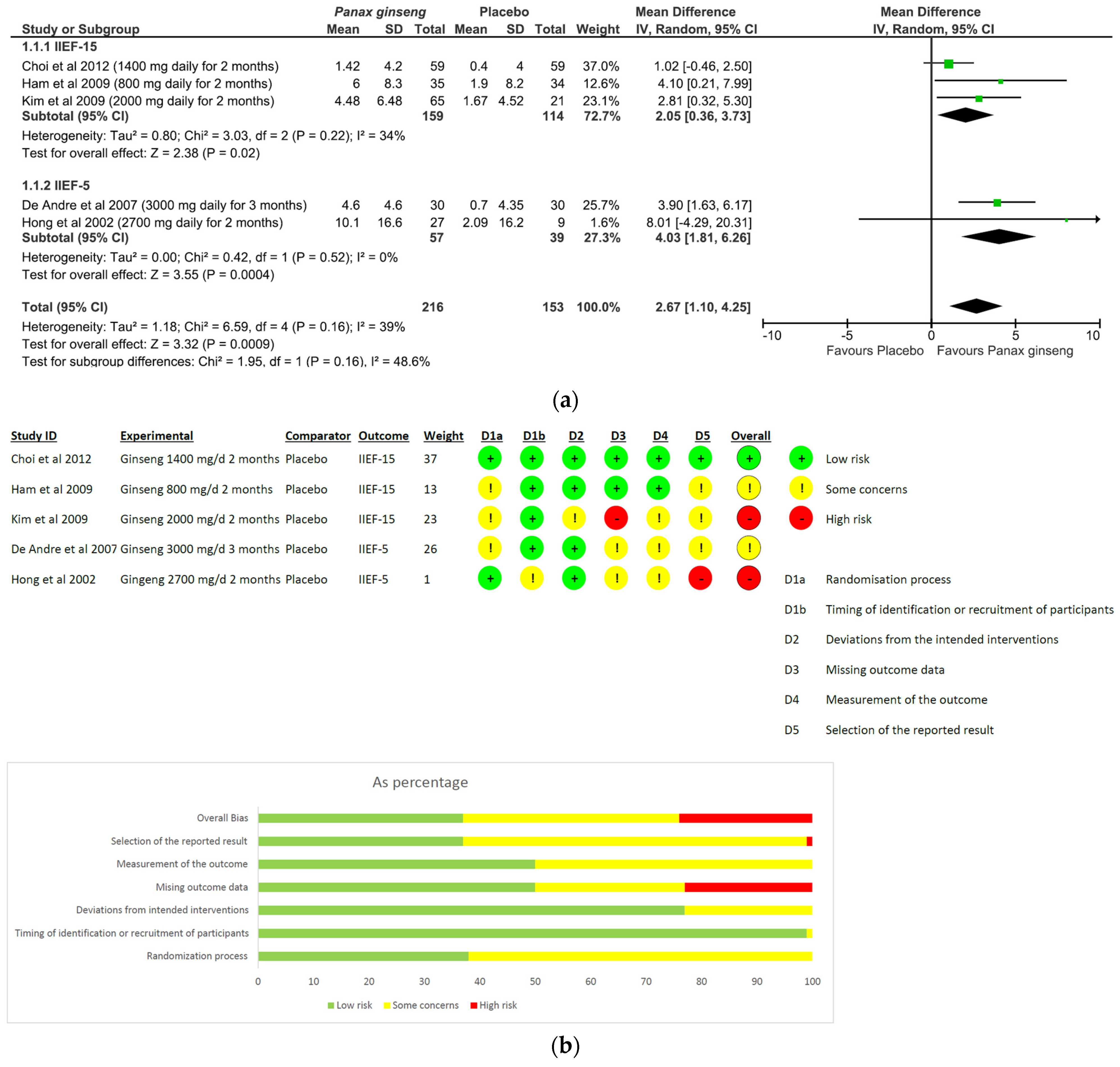
- Panax ginseng: A total of five studies and 369 patients were included in the analysis (intervention group N = 216 patients; control group N = 153 patients). The intervention was associated with a significant improvement in erectile performance as assessed using IIEF-15 and IIEF-5 (pooled MD = 2.67, [95% CI 1.10, 4.25], p = 0.0009, I2 = 39%) (Figure 3a). The overall risk of bias was about 35% high, 40% with some concerns and the remaining percentage was low (Figure 3b).
- (II)
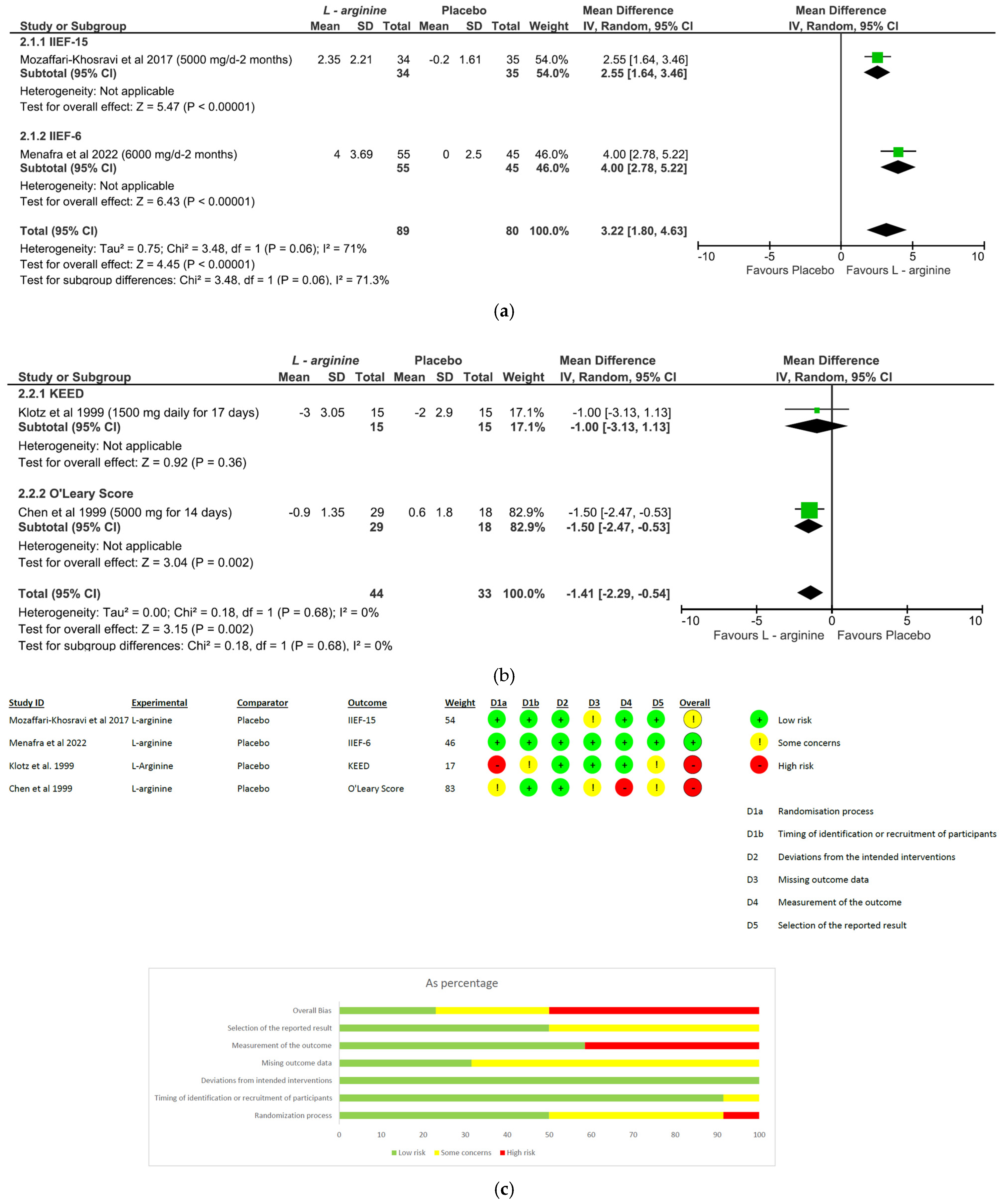
- L-arginine: The analysis included a total of four studies and 246 patients (intervention group N = 133 patients; control group N = 113 patients). The intervention was associated with a significant improvement in erectile performance as assessed using IIEF-15 and IIEF-6 (pooled MD = 3.22, [95% CI 1.80, 4.63], p < 0.00001, I2 = 71%; Figure 4a), as well as the KEED and O’Leary scores (pooled MD = −1.41, [95% CI −2.29, −0.54], p = 0.002, I2 = 0%; Figure 4b). The overall risk of bias was 50% high, about 37% unclear and low for the remaining percentage (Figure 4c).
- (III)
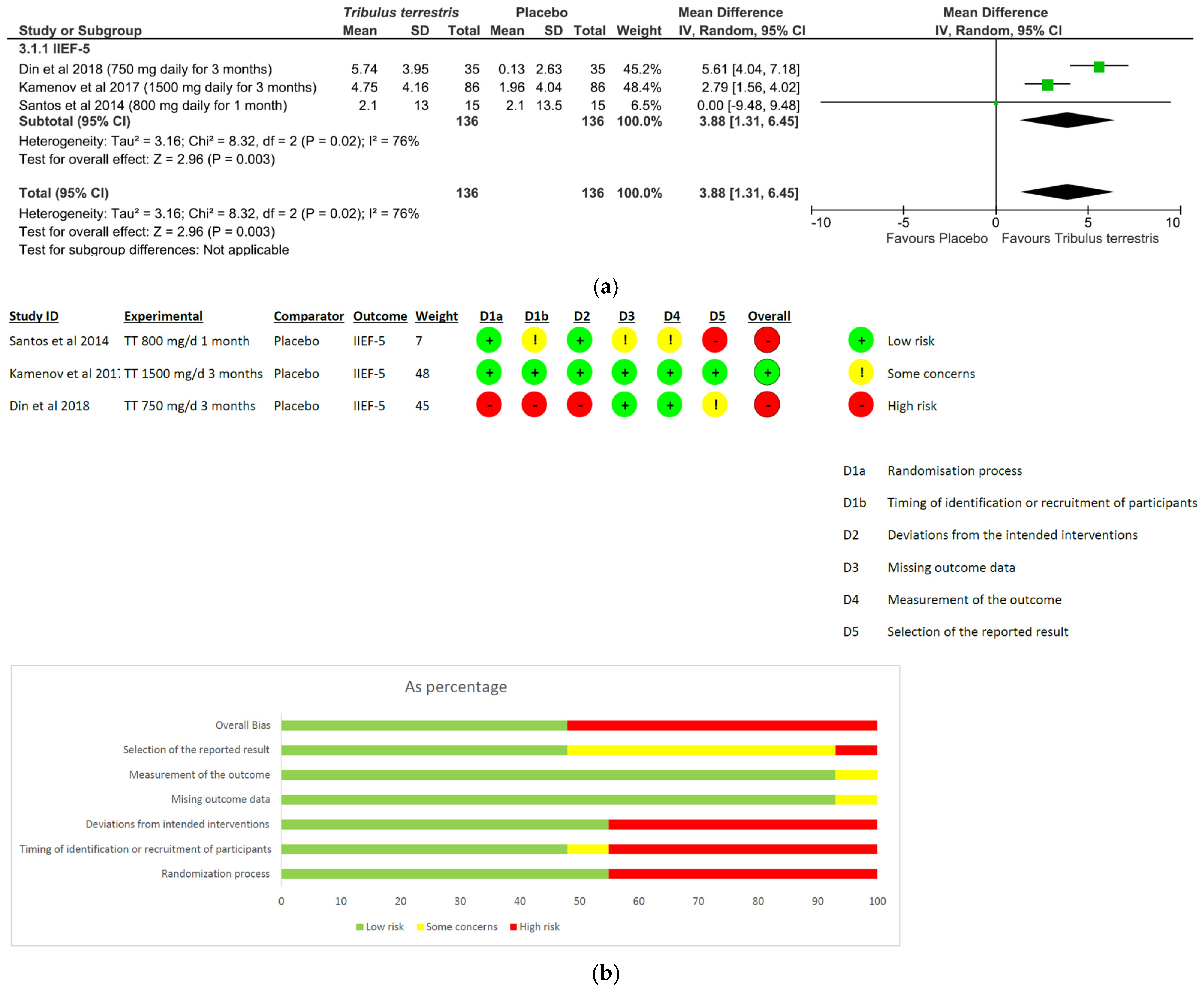
- Tribulus terrestris: A total of three studies and 272 patients were included in the analysis (intervention group N = 136 patients; control group N = 136 patients). The intervention was associated with a significant improvement in erectile performance as assessed using IIEF-5 (pooled MD = 3.88, [95% CI 1.31, 6.45], p = 0.003, I2 = 76%; Figure 5a). The overall risk of bias was 50% high and 50% low (Figure 5b).
- (IV)
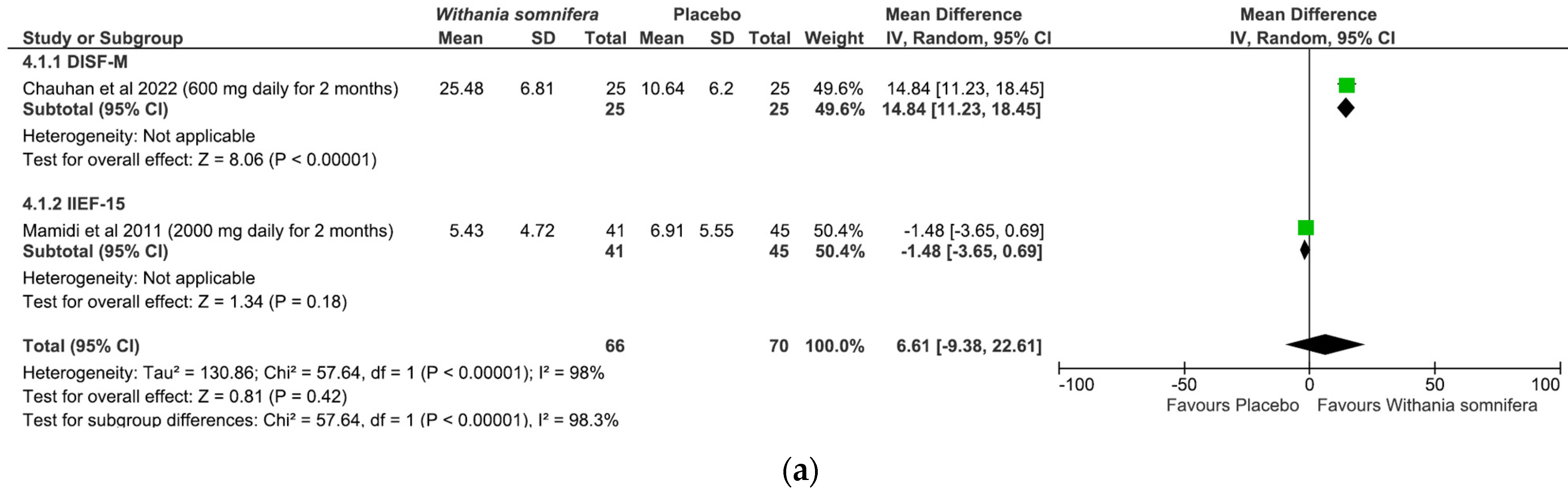
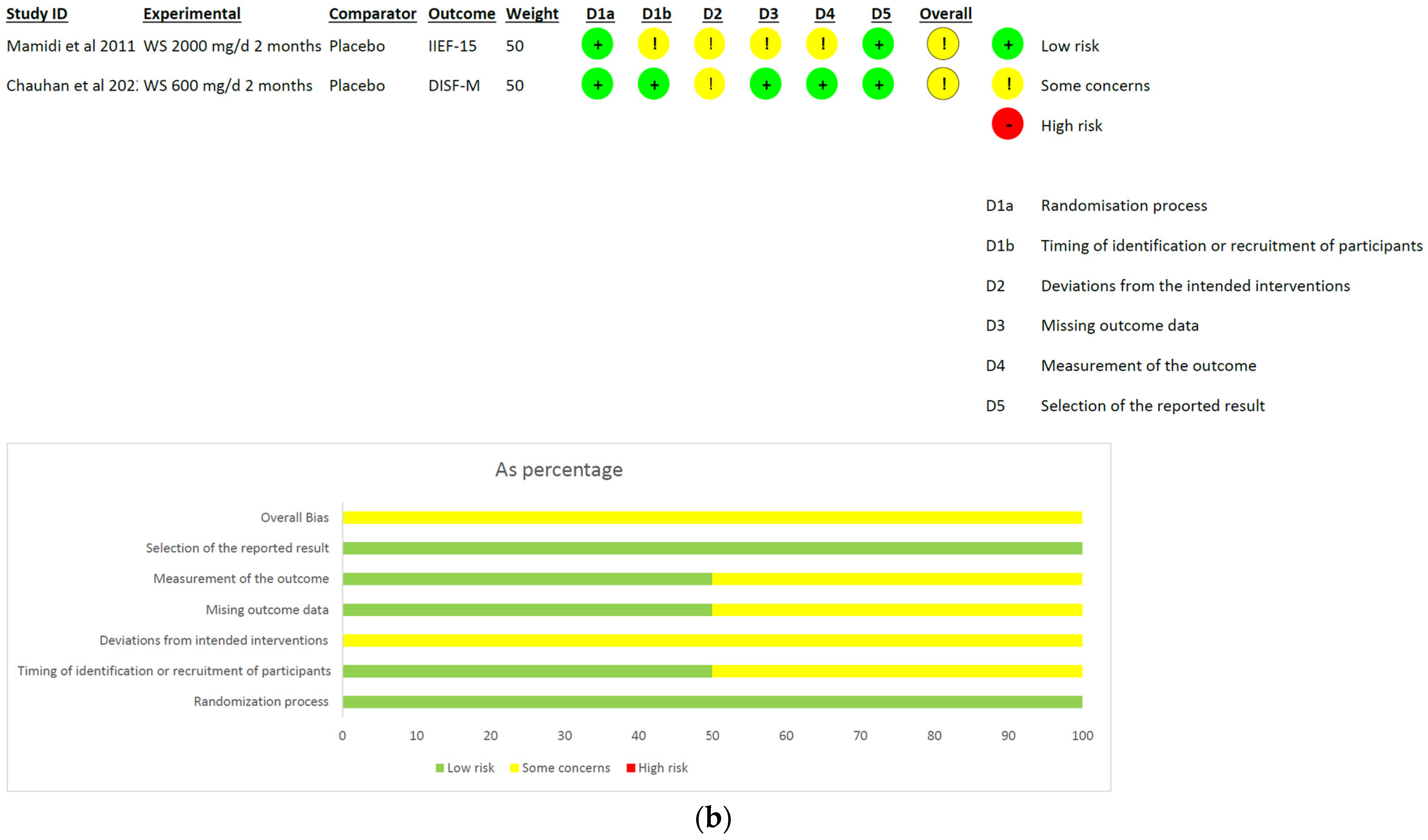
- Withania somnifera: The analysis included a total of two studies and 136 patients (intervention group N = 66 patients; control group N = 70 patients). No difference was found between the comparators when pooling data about erectile performance as assessed with both DISF-M and IIEF-15 (pooled MD = 6.61, [95% CI −9.38, 22.61], p = 0.42, I2 = 98%; Figure 6a). Notably, a significant difference between subgroups was found (p < 0.00001), highlighting a beneficial effect of the intervention in terms of DISF-M (p < 0.00001) but not IIEF-15 (p = 0.18). Furthermore, in the case of Mamidi et al. (IIEF-15), there were patients suffering from psychogenic ED, and therefore, at an organic level, there was no disorder, which was maybe why the active ingredient did not have a positive response. The overall risk of bias was unclear, especially in relation to the study by Mamidi et al., which presented four out of six participants who were uncertain (Figure 6b).
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kouidrat, Y.; Pizzol, D.; Cosco, T.; Thompson, T.; Carnaghi, M.; Bertoldo, A.; Solmi, M.; Stubbs, B.; Veronese, N. High Prevalence of Erectile Dysfunction in Diabetes: A Systematic Review and Meta-Analysis of 145 Studies. Diabet. Med. 2017, 34, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- Franco, J.V.; Jung, J.H.; Imamura, M.; Borofsky, M.; Omar, M.I.; Escobar Liquitay, C.M.; Young, S.; Golzarian, J.; Veroniki, A.A.; Garegnani, L.; et al. Minimally Invasive Treatments for Lower Urinary Tract Symptoms in Men with Benign Prostatic Hyperplasia: A Network Meta-Analysis. Cochrane Database Syst. Rev. 2021, 2021, CD013656. [Google Scholar] [CrossRef]
- Fan, Y.; Hu, B.; Man, C.; Cui, F. Erectile Dysfunction and Risk of Cardiovascular and All-Cause Mortality in the General Population: A Meta-Analysis of Cohort Studies. World J. Urol. 2018, 36, 1681–1689. [Google Scholar] [CrossRef]
- Shamloul, R.; Ghanem, H. Erectile Dysfunction. Lancet 2013, 381, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Najari, B.B.; Kashanian, J.A. Erectile Dysfunction. JAMA 2016, 316, 1838. [Google Scholar] [CrossRef]
- Mykoniatis, I.; Pyrgidis, N.; Sokolakis, I.; Ouranidis, A.; Sountoulides, P.; Haidich, A.-B.; van Renterghem, K.; Hatzichristodoulou, G.; Hatzichristou, D. Assessment of Combination Therapies vs Monotherapy for Erectile Dysfunction. JAMA Netw. Open 2021, 4, e2036337. [Google Scholar] [CrossRef]
- Salonia, A.; Bettocchi, C.; Boeri, L.; Capogrosso, P.; Carvalho, J.; Cilesiz, N.C.; Cocci, A.; Corona, G.; Dimitropoulos, K.; Gül, M.; et al. EAU Working Group on Male Sexual and Reproductive Health. European Association of Urology Guidelines on Sexual and Reproductive Health-2021 Update: Male Sexual Dysfunction. Eur. Urol. 2021, 80, 333–357. [Google Scholar] [CrossRef]
- Kuchakulla, M.; Narasimman, M.; Soni, Y.; Leong, J.Y.; Patel, P.; Ramasamy, R. A Systematic Review and Evidence-Based Analysis of Ingredients in Popular Male Testosterone and Erectile Dysfunction Supplements. Int. J. Impot. Res. 2021, 33, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, F.; Colalto, C.; Delfino, D.V.; Iriti, M.; Izzo, A.A. Herbal Dietary Supplements for Erectile Dysfunction: A Systematic Review and Meta-Analysis. Drugs 2018, 78, 643–673. [Google Scholar] [CrossRef]
- Corona, G.; Cucinotta, D.; Di Lorenzo, G.; Ferlin, A.; Giagulli, V.A.; Gnessi, L.; Isidori, A.M.; Maiorino, M.I.; Miserendino, P.; Murrone, A.; et al. The Italian Society of Andrology and Sexual Medicine (SIAMS), along with Ten Other Italian Scientific Societies, Guidelines on the Diagnosis and Management of Erectile Dysfunction. J. Endocrinol. Investig. 2023, 46, 1241–1274. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.; Balasubramaniam, K.; Jarbøl, D.E.; Søndergaard, J.; Haastrup, P.F. Socioeconomic status and barriers for contacting the general practitioner when bothered by erectile dysfunction: A population-based cross-sectional study. BMC Fam. Pract. 2020, 16, 21–166. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, A.; Thirumavalavan, N.; Srivatsav, A.; Yu, J.; Hotaling, J.M.; Lipshultz, L.I.; Pastuszak, A.W. An Analysis of Popular Online Erectile Dysfunction Supplements. J. Sex. Med. 2019, 16, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Ministero Della Salute. Available online: https://www.salute.gov.it/portale/temi/documenti/integratori/registro_integratori_per_prodotto.pdf (accessed on 5 May 2023).
- Garolla, A.; Petre, G.C.; Francini-Pesenti, F.; De Toni, L.; Vitagliano, A.; Di Nisio, A.; Grande, G.; Foresta, C. Systematic Review and Critical Analysis on Dietary Supplements for Male Infertility: From a Blend of Ingredients to a Rationale Strategy. Front. Endocrinol. 2022, 12, 824078. [Google Scholar] [CrossRef]
- Vitagliano, A.; Petre, G.C.; Francini-Pesenti, F.; De Toni, L.; Di Nisio, A.; Grande, G.; Foresta, C.; Garolla, A. Dietary Supplements for Female Infertility: A Critical Review of Their Composition. Nutrients 2021, 13, 3552. [Google Scholar] [CrossRef]
- Budoff, M.J.; Achenbach, S.; Blumenthal, R.S.; Carr, J.J.; Goldin, J.G.; Greenland, P.; Guerci, A.D.; Lima, J.A.C.; Rader, D.J.; Rubin, G.D.; et al. Assessment of Coronary Artery Disease by Cardiac Computed Tomography. Circulation 2006, 114, 1761–1791. [Google Scholar] [CrossRef]
- Panel, E.; Sources, N.; Ans, F. Scientific Opinion on the Evaluation of the Safety in Use of Yohimbe (Pausinystalia Yohimbe (K. Schum.) Pierre Ex Beille). EFSA J. 2013, 11, 3302. [Google Scholar] [CrossRef]
- Leitão, A.E.; de Souza Vieira, M.C.; Pelegrini, A.; da Silva, E.L.; de Azevedo Guimarães, A.C. A 6-Month, Double-Blind, Placebo-Controlled, Randomized Trial to Evaluate the Effect of Eurycoma Longifolia (Tongkat Ali) and Concurrent Training on Erectile Function and Testosterone Levels in Androgen Deficiency of Aging Males (ADAM). Maturitas 2021, 145, 78–85. [Google Scholar] [CrossRef]
- Ismail, S.B.; Wan Mohammad, W.M.Z.; George, A.; Nik Hussain, N.H.; Musthapa Kamal, Z.M.; Liske, E. Randomized Clinical Trial on the Use of PHYSTA Freeze-Dried Water Extract of Eurycoma Longifolia for the Improvement of Quality of Life and Sexual Well-Being in Men. Evid.-Based Complement. Altern. Med. 2012, 2012, 429268. [Google Scholar] [CrossRef] [PubMed]
- Ham, W.S.; Kim, W.T.; Lee, J.S.; Ju, H.J.; Kang, S.J.; Oh, J.H.; Her, Y.; Chung, J.Y.; Park, K.; Choi, Y.D. Efficacy and Safety of Red Ginseng Extract Powder in Patients with Erectile Dysfunction: Multicenter, Randomized, Double-Blind, Placebo-Controlled Study. Korean J. Urol. 2009, 50, 159. [Google Scholar] [CrossRef]
- Choi, Y.D.; Park, C.W.; Jang, J.; Kim, S.H.; Jeon, H.Y.; Kim, W.G.; Lee, S.J.; Chung, W.S. Effects of Korean Ginseng Berry Extract on Sexual Function in Men with Erectile Dysfunction: A Multicenter, Placebo-Controlled, Double-Blind Clinical Study. Int. J. Impot. Res. 2013, 25, 45–50. [Google Scholar] [CrossRef]
- Kim, T.-H.; Jeon, S.H.; Hahn, E.-J.; Paek, K.-Y.; Park, J.K.; Youn, N.Y.; Lee, H.-L. Effects of Tissue-Cultured Mountain Ginseng (Panax Ginseng CA Meyer) Extract on Male Patients with Erectile Dysfunction. Asian J. Androl. 2009, 11, 356–361. [Google Scholar] [CrossRef] [PubMed]
- HONG, B.; JI, Y.H.; HONG, J.H.; NAM, K.I.Y.; AHN, T.Y. A Double-Blind Crossover Study Evaluating the Efficacy of Korean Red Ginseng in Patients with Erectile Dysfunction: A Preliminary Report. J. Urol. 2002, 168, 2070–2073. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, E.; de Mesquita, A.A.; de Almeida Claro, J.; de Andrade, P.M.; Ortiz, V.; Paranhos, M.; Srougi, M.; Erdogrun, T. Study of the Efficacy of Korean Red Ginseng in the Treatment of Erectile Dysfunction. Asian J. Androl. 2007, 9, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Mozaffari-Khosravi, H.; Fallahi, M.; Afkhami-Ardekani, M. Effect of Oral Supplementation of L-Arginine on Sexual Function in Men with Type 2 Diabetes: A Double-Blind Clinical Trial. J. Nutr. Food Secur. 2017, 2, 165–172. [Google Scholar]
- Menafra, D.; de Angelis, C.; Garifalos, F.; Mazzella, M.; Galdiero, G.; Piscopo, M.; Castoro, M.; Verde, N.; Pivonello, C.; Simeoli, C.; et al. Long-Term High-Dose l-Arginine Supplementation in Patients with Vasculogenic Erectile Dysfunction: A Multicentre, Double-Blind, Randomized, Placebo-Controlled Clinical Trial. J. Endocrinol. Invest. 2022, 45, 941–961. [Google Scholar] [CrossRef]
- Klotz, T.; Mathers, M.J.; Braun, M.; Bloch, W.; Engelmann, U. Effectiveness of Oral L-Arginine in First-Line Treatment of Erectile Dysfunction in a Controlled Crossover Study. Urol. Int. 1999, 63, 220–223. [Google Scholar] [CrossRef]
- Chen; Wollman; Chernichovsky; Iaina; Sofer; Matzkin. Effect of Oral Administration of High-Dose Nitric Oxide Donor l-Arginine in Men with Organic Erectile Dysfunction: Results of a Double-Blind, Randomized, Placebo-Controlled Study. BJU Int. 2001, 83, 269–273. [Google Scholar] [CrossRef]
- Reid, K.; Morales, A.; Harris, C.; Surridge, D.H.C.; Condra, M.; Owen, J.; Fenemore, J. Double-blind trial of yohimbine in treatment of psychogenic impotence. Lancet 1987, 330, 421–423. [Google Scholar] [CrossRef]
- Vogt, H.-J.; Brandl, P.; Kockott, G.; Schmitz, J.; Wiegand, M.; Schadrack, J.; Gierend, M. Double-Blind, Placebo-Controlled Safety and Efficacy Trial with Yohimbine Hydrochloride in the Treatment of Nonorganic Erectile Dysfunction. Int. J. Impot. Res. 1997, 9, 155–161. [Google Scholar] [CrossRef]
- Morales, A.; Condra, M.; Owen, J.A.; Surridge, D.H.; Fenemore, J.; Harris, C. Is Yohimbine Effective in the Treatment of Organic Impotence? Results of a Controlled Trial. J. Urol. 1987, 137, 1168–1172. [Google Scholar] [CrossRef]
- Kunelius, P.; Häkkinen, J.; Lukkarinen, O. Is High-Dose Yohimbine Hydrochloride Effective in the Treatment of Mixed-Type Impotence? A Prospective, Randomized, Controlled Double-Blind Crossover Study. Urology 1997, 49, 441–444. [Google Scholar] [CrossRef]
- GamalEl Din, S.F.; Abdel Salam, M.A.; Mohamed, M.S.; Ahmed, A.R.; Motawaa, A.T.; Saadeldin, O.A.; Elnabarway, R.R. Tribulus Terrestris versus Placebo in the Treatment of Erectile Dysfunction and Lower Urinary Tract Symptoms in Patients with Late-Onset Hypogonadism: A Placebo-Controlled Study. Urol. J. 2019, 86, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Kamenov, Z.; Fileva, S.; Kalinov, K.; Jannini, E.A. Evaluation of the Efficacy and Safety of Tribulus Terrestris in Male Sexual Dysfunction—A Prospective, Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Maturitas 2017, 99, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.A.; Reis, L.O.; Destro-Saade, R.; Luiza-Reis, A.; Fregonesi, A. Tribulus Terrestris versus Placebo En El Tratamiento de La Disfunción Eréctil: Un Estudio Aleatorizado, Prospectivo y Doble Ciego. Actas. Urol. Esp. 2014, 38, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Modabbernia, A.; Sohrabi, H.; Nasehi, A.-A.; Raisi, F.; Saroukhani, S.; Jamshidi, A.; Tabrizi, M.; Ashrafi, M.; Akhondzadeh, S. Effect of Saffron on Fluoxetine-Induced Sexual Impairment in Men: Randomized Double-Blind Placebo-Controlled Trial. Psychopharmacology 2012, 223, 381–388. [Google Scholar] [CrossRef]
- Chauhan, S.; Srivastava, M.K.; Pathak, A.K. Effect of Standardized Root Extract of Ashwagandha (Withania Somnifera) on Well-being and Sexual Performance in Adult Males: A Randomized Controlled Trial. Health Sci. Rep. 2022, 5, e741. [Google Scholar] [CrossRef]
- Mamidi, P.; Thakar, A. Efficacy of Ashwagandha (Withania Somnifera Dunal. Linn.) in the Management of Psychogenic Erectile Dysfunction. Int. Q. J. Res. Ayurveda 2011, 32, 322. [Google Scholar] [CrossRef]
- Duračková, Z.; Trebatický, B.; Novotný, V.; Žitňanová, I.; Breza, J. Lipid Metabolism and Erectile Function Improvement by Pycnogenol®, Extract from the Bark of Pinus Pinaster in Patients Suffering from Erectile Dysfunction—A Pilot Study. Nutr. Res. 2003, 23, 1189–1198. [Google Scholar] [CrossRef]
- Trebaticky, B.; Muchova, J.; Ziaran, S.; Bujdak, P.; Breza, J.; Durackova, Z. Natural Polyphenols Improve Erectile Function and Lipid Profile in Patients Suffering from Erectile Dysfunction. Bratisl. Med. J. 2019, 120, 941–944. [Google Scholar] [CrossRef]
- Leisegang, K.; Finelli, R. Alternative Medicine and Herbal Remedies in the Treatment of Erectile Dysfunction: A Systematic Review. Arab. J. Urol. 2021, 19, 323–339. [Google Scholar] [CrossRef]
- Srivatsav, A.; Balasubramanian, A.; Pathak, U.I.; Rivera-Mirabal, J.; Thirumavalavan, N.; Hotaling, J.M.; Lipshultz, L.I.; Pastuszak, A.W. Efficacy and Safety of Common Ingredients in Aphrodisiacs Used for Erectile Dysfunction: A Review. Sex. Med. Rev. 2020, 8, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Kiani, A.K.; Bonetti, G.; Medori, M.C.; Caruso, P.; Manganotti, P.; Fioretti, F.; Nodari, S.; Connelly, S.T.; Bertelli, M. Dietary Supplements for Improving Nitric-Oxide Synthesis. J. Prev. Med. Hyg. 2022, 63, E239–E245. [Google Scholar] [CrossRef] [PubMed]
- Stanislavov, R.; Nikolova, V. Treatment of Erectile Dysfunction with Pycnogenol and L-Arginine. J. Sex. Marital. Ther. 2003, 29, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Nik-Ahd, F.; Shindel, A.W. Pharmacotherapy for Erectile Dysfunction in 2021 and Beyond. Urol. Clin. N. Am. 2022, 49, 209–217. [Google Scholar] [CrossRef]
- Schoonees, A.; Visser, J.; Musekiwa, A.; Volmink, J. Pycnogenol® for the Treatment of Chronic Disorders. In Cochrane Database of Systematic Reviews; Volmink, J., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2012. [Google Scholar]
- Ștefănescu, R.; Farczadi, L.; Huțanu, A.; Ősz, B.E.; Mărușteri, M.; Negroiu, A.; Vari, C.E. Tribulus Terrestris Efficacy and Safety Concerns in Diabetes and Erectile Dysfunction, Assessed in an Experimental Model. Plants 2021, 10, 744. [Google Scholar] [CrossRef] [PubMed]
- Neychev, V.; Mitev, V. Pro-Sexual and Androgen Enhancing Effects of Tribulus Terrestris L.: Fact or Fiction. J. Ethnopharmacol. 2016, 179, 345–355. [Google Scholar] [CrossRef]
- Semwal, P.; Painuli, S.; Abu-Izneid, T.; Rauf, A.; Sharma, A.; Daştan, S.D.; Kumar, M.; Alshehri, M.M.; Taheri, Y.; Das, R.; et al. Diosgenin: An Updated Pharmacological Review and Therapeutic Perspectives. Oxid. Med. Cell Longev. 2022, 2022, 1035441. [Google Scholar] [CrossRef]
- Mancuso, C.; Santangelo, R. Panax Ginseng and Panax Quinquefolius: From Pharmacology to Toxicology. Food Chem. Toxicol. 2017, 107, 362–372. [Google Scholar] [CrossRef]
- Jin, Y.; Cui, R.; Zhao, L.; Fan, J.; Li, B. Mechanisms of Panax Ginseng Action as an Antidepressant. Cell Prolif. 2019, 52, e12696. [Google Scholar] [CrossRef]
- Lee, H.W.; Lee, M.S.; Kim, T.-H.; Alraek, T.; Zaslawski, C.; Kim, J.W.; Moon, D.G. Ginseng for Erectile Dysfunction. Cochrane Database Syst. Rev. 2021, 2021, CD012654. [Google Scholar] [CrossRef]
- Akhgarjand, C.; Asoudeh, F.; Bagheri, A.; Kalantar, Z.; Vahabi, Z.; Shab-bidar, S.; Rezvani, H.; Djafarian, K. Does Ashwagandha Supplementation Have a Beneficial Effect on the Management of Anxiety and Stress? A Systematic Review and Meta-analysis of Randomized Controlled Trials. Phytother. Res. 2022, 36, 4115–4124. [Google Scholar] [CrossRef]
- Choudhary, D.; Bhattacharyya, S.; Bose, S. Efficacy and Safety of Ashwagandha (Withania Somnifera (L.) Dunal) Root Extract in Improving Memory and Cognitive Functions. J. Diet. Suppl. 2017, 14, 599–612. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, A.L.; Smith, S.J.; Malvi, H.; Kodgule, R. An Investigation into the Stress-Relieving and Pharmacological Actions of an Ashwagandha (Withania Somnifera) Extract. Medicine 2019, 98, e17186. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.J.; Lopresti, A.L.; Teo, S.Y.M.; Fairchild, T.J. Examining the Effects of Herbs on Testosterone Concentrations in Men: A Systematic Review. Adv. Nutr. 2021, 12, 744–765. [Google Scholar] [CrossRef] [PubMed]
- Santos, H.O.; Howell, S.; Teixeira, F.J. Beyond Tribulus (Tribulus Terrestris L.): The Effects of Phytotherapics on Testosterone, Sperm and Prostate Parameters. J. Ethnopharmacol. 2019, 235, 392–405. [Google Scholar] [CrossRef]
- Ambiye, V.R.; Langade, D.; Dongre, S.; Aptikar, P.; Kulkarni, M.; Dongre, A. Clinical Evaluation of the Spermatogenic Activity of the Root Extract of Ashwagandha (Withania somnifera) in Oligospermic Males: A Pilot Study. Evid.-Based Complement. Altern. Med. 2013, 2013, 571420. [Google Scholar] [CrossRef]
- Corona, G.; Goulis, D.G.; Huhtaniemi, I.; Zitzmann, M.; Toppari, J.; Forti, G.; Vanderschueren, D.; Wu, F.C.; Corona, G.; Goulis, D.G.; et al. European Academy of Andrology (EAA) Guidelines on Investigation, Treatment and Monitoring of Functional Hypogonadism in Males. Andrology 2020, 8, 970–987. [Google Scholar] [CrossRef]
- Tucker, J.; Fischer, T.; Upjohn, L.; Mazzera, D.; Kumar, M. Unapproved Pharmaceutical Ingredients Included in Dietary Supplements Associated with US Food and Drug Administration Warnings. JAMA Netw. Open 2018, 1, e183337. [Google Scholar] [CrossRef]
| Reached mED | ||
|---|---|---|
| RCT Characteristics | Yes | Not |
| ≥2 + RCTs | A | B |
| 1 + RCT | B | C |
| ≥2+/≤1 − RCT | B | C |
| ≥2+/> 1 − RCT | C | D |
| No evidence | D | D |
| Active Ingredient | Reference | Participant Characteristics (Number; Age; Type of ED) | Duration of Treatment | Evaluated Outcomes (Baseline Value; End of Treatment vs. Baseline) | Employed Daily Dose | Minimal Effective Dose (mED) |
|---|---|---|---|---|---|---|
| Eurycoma longifolia | [18] [19] | T: 18; 47.38 ± 5.03; all types P: 19; 47.38 ± 5.03; all types T: 52; 43.6 ± 6.52; all types P: 50; 42.8 ± 6.73; all types | 6 months 3 months | T: ↑ IIEF-15; 18.66 ± 2.43; ∆2.82 * ± 2.20 (p < 0.05) P: ↑ IIEF-15; 20.50 ± 2,10; ∆1.00 ± 2.33 T: ↑ IIEF-15; 25.36 ± 0.47; ∆1.43 * ± 0.46 (p < 0.001) P: no significant differences | 200 mg 300 mg | 200 mg |
| Panax ginseng | [20] [21] [22] [23] [24] | T: 35; 53.20 ± 9.70; all types P: 34; 50.80 ± 8.00; all types T: 59; 57.49 ± 7.94; all types P: 59; 57.32 ± 8.41; all types T: 65; 57.51 ± 1.24; all types P: 21; 57.51 ± 2.02; all types T: 27; 54.00; all types P: 9; 54.00; all types T: 30; 52.60; all types P: 30; 54.30; all types | 2 months 2 months 2 months 2 months 3 months | T: ↑ IIEF-15; 17.20 ± 9.4; ∆6.00 * ± 8.30 (p = 0.003) P: ↑ IIEF-15; 17.70 ± 8.2; ∆1.90 ± 8.20 T: ↑ IIEF-15; 17.17 ± 2.57; ∆1.42 * ± 4.20 (p < 0.05) P: ↑ IIEF-15; 17.56 ± 2.89; ∆0.40 ± 4.00 T: ↑ IIEF-15; 11.89 ± 5.89; ∆4.48 * ± 6.48 (p < 0.001) P: ↑ IIEF-15; 11.38 ± 4.78; ∆1.67 ± 4.52 T: ↑ IIEF-5; 10.60 ± 7.41; ∆10.10 * ± 16.60 (p < 0.01) P: ↑ IIEF-15; 10,60 ± 7.41; ∆2.09 ± 16.20 T: ↑ IIEF-5; 16.40 ± 2.90; ∆4.60 * ± 4.60 (p < 0.01) P: ↑ IIEF-5; 17.00 ± 3.10; ∆0.70 ± 04.35 | 800 mg 1400mg 2000 mg 2700 mg 3000 mg | 800 mg |
| L-arginine | [25] [26] | T: 34; 51.58 ± 2.67; vasculogenic P: 35; 51.31 ± 2.65; vasculogenic T: 55; 50.00 ± 14; vasculogenic P: 45; 53.00 ± 10; vasculogenic | 2 months 3 months | T: ↑ IIEF-15; 16.32 ± 1.36; ∆2.35 * ± 2.21 (p < 0.001) P: ↓ IIEF-15; 16,11 ± 1.30; ∆ −0.20 ± 1.61 T: ↑ IIEF-6; 20,00 ± 6.30; ∆4.00 * ± 3.69 (p < 0.0001) P: ↔ IIEF-6; 20,00 ± 5,00; ∆0.00 ± 2.50 | 5000 mg 6000 mg | 6000 mg |
| [27] [28] | Cross-over; 15; 51.60; all types T: 29; range 55–75; all types P: 18; range 55–75; all types | 17 days 2 weeks | T: ↓ KEED; 21.9 ± 2.7; −∆3.0 ± 3.0 P: ↓ KEED; 21.9 ± 2.7; −∆2.0 ± 2.9 T: ↓ O’Leary score; 18.6 ± 1.3; −∆0.9 ± 1.3 P: ↑ O’Leary score; 19.2 ± 1.7; ∆0.6 ± 1.8 | 1500 mg 5000 mg | ||
| Corynanthe yohimbe | [29] [30] | Cross-over; 48; 18 to 70; psychogenic T: 41; 53.9; all types P: 42; 51.3; all types | 10 weeks 2 months | ↑ NPT in 62% of patients (p < 0.05) T: ↑ NPT in 71% of treated (p = 0.01) P: ↑ NPT in 45% of patients | 18 mg 30 mg | 18 mg |
| [31] [32] | Cross-over; 100; 56; all types Cross-over; range 25–51; 51; all types | 10 weeks 25 days | ↑ NPT in 42.6% of patients (p = 0.42) ↑ Rigiscan in 44% of patients | 18 mg 36 mg | ||
| Tribulus terrestris | [33] [34] | T: 35; 55.69 ± 9.35; all types P: 35; 58.38 ± 9.71; all types T: 86; 44.11 ± 12.37; all types P: 86; 41.18 ± 12.36; all types | 3 months 3 months | T: ↑ IIEF-5; 10.71 ± 3.01; ∆5.74 * ± 3.95 (p < 0.001) P: ↓ IIEF-5; 10.75 ± 3.01; −∆13.00 ± 2.63 T: ↑ IIEF-5; 18.01 ± 3.21; ∆4.75 * ± 4.16 (p < 0.001) P: ↑ IIEF-5; 18.22 ± 3.44; ∆1.96 ± 4.04 | 750 mg 1500 mg | 750 mg |
| [35] | T: 15; 60 ± 9.40; all types P: 15; 63 ± 7.90; all types | 1 month | T: ↑ IIEF-5; 13.20 ± 13.00; ∆2.10 * ± 13.00 (p = 0.0004) P: ↑ IIEF-5; 11.60 ± 13.50; ∆2.10 * ± 13.50 (p = 0.0004) | 800 mg | ||
| Crocus sativus | [36] | T: 15; 36.60 ± 8.30; SSRi related ED P: 15; 40.05 ± 9.40; SSRi related ED | 1 month | T: ↑ IIEF-15; 20.70 ± 4.30; ∆4.50 * ± 2.50 (p < 0.001) P: ↓ IIEF-15; 21.20 ± 3.10; ∆−2.50 ± 4.60 | 30 mg | 30 mg |
| Withania somnifera | [37] | T: 25; 34.32 ± 3.21; all types P:25; 35.20 ± 3.66; all types | 2 months | T: ↑DISF-M; 62.92 ± 4.75; ∆9.8 * ± 9.80 (p < 0.0001) P: no significant differences | 600 mg | 600 mg |
| [38] | T: 41; 21 to 40; psychogenic P: 45; 21 to 40; psychogenic | 2 months | T: ↑ IIEF-15; 38.80 ± 4.72; ∆5.43 * ± 4.72 (p < 0.01) P: ↑ IIEF-15; 38.97 ± 5.55; ∆6.91 * ± 5.55 (p < 0.01) | 2000 mg | ||
| Pinus pinaster | [39] [40] | T: 21; 46.50 ± 12.5; all types P: not declared T: 32; 49.00 ± 12.50; vasculogenic P: 21; 50.75 ± 8.20; vasculogenic | 3 months 4 months | T: ↑ IIEF-5; 12.60 ± 1.10; ∆4.20 * ± 0.95 (p < 0.019) P: ↓ IIEF-5: 11.30 ± 1.30; ∆−2.40 * ± 1.25 (p < 0.01) T: ↑ IIEF-5; 9,0; ∆1.85 * (p < 0.019) P: ↓ IIEF-5; 10.8; ∆−1.25 | 120 mg 120 mg | 120 mg |
| Active Ingredient | DS 1 | DS 2 | DS 3 | DS 4 | DS5 | DS 6 | DS 7 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S = −0.67 | S = −0.50 | S = −0.50 | S = −0.58 | S = 0.50 | S = −0.67 | S = −0.46 | ||||||||
| Daily Dose | EV | Daily Dose | EV | Daily Dose | EV | Daily Dose | EV | Daily Dose | EV | Daily Dose | EV | Daily Dose | EV | |
| Tribulus terrestris | 200 mg | C | 200 mg | C | 1200 mg | B | 2000 mg | B | ||||||
| Crocus sativus | ||||||||||||||
| Panax ginseng | 100 mg | B | 200 mg | B | 200 mg | B | 960 mg | A | 125 mg | B | ||||
| L-arginine | 100 mg | C | 1000 mg | C | 600 mg | C | 100 mg | C | 800 mg | C | ||||
| Withania somnifera | ||||||||||||||
| Eurycoma longifolia | ||||||||||||||
| Pinus pinaster | ||||||||||||||
| Corynanthe yohimbe | ||||||||||||||
| L-taurine | 200 mg | D | 200 mg | D | 300 mg | D | 180 mg | D | 200 mg | D | ||||
| L-carnitine | 100 mg | D | ||||||||||||
| L-citrulline | 150 mg | D | 210 mg | D | 70 mg | D | 1000 mg | D | ||||||
| Muira puama | 100 mg | D | 100 mg | D | 100 mg | D | 600 mg | D | ||||||
| Trigonella foenum-graecum | 150 mg | D | 100 mg | D | 400 mg | D | ||||||||
| Ginkgo biloba | 200 mg | D | ||||||||||||
| Hawthorn berry | 200 mg | D | ||||||||||||
| Punica granatum | 30 mg | D | ||||||||||||
| Cuscuta chinensis | 20 mg | D | ||||||||||||
| Black Pepper | 15 mg | D | 225 mg | D | ||||||||||
| Zingiber officinale | 50 mg | D | ||||||||||||
| Turnera diffusa | ||||||||||||||
| Vitamin C | 50 mg | D | 30 mg | D | 50 mg | D | ||||||||
| Vitamin B6 | 9.5 mg | D | 5.7 mg | D | 2 mg | D | ||||||||
| Vitamin B9 | 200 mcg | D | ||||||||||||
| Vitamin E | 6 mg | D | 15 mg | D | 9 mg | D | 15 mg | D | ||||||
| Vitamin D | 5 mg | D | ||||||||||||
| Vitamin B1 | ||||||||||||||
| Vitamin H | ||||||||||||||
| Vitamin B12 | ||||||||||||||
| Vitamin B3 | ||||||||||||||
| Magnesium | 100 mg | D | 60 mg | D | ||||||||||
| Zinc | 7.5 mg | D | 5 mg | D | 15 mg | D | 15 mg | D | 9 mg | D | 14 mg | D | 12 mg | D |
| Selenium | ||||||||||||||
| Alpha lipoic acid | ||||||||||||||
| Aspartic acid | ||||||||||||||
| Bambusa arundinacea | ||||||||||||||
| Epimedium acuminatum | ||||||||||||||
| Capsicum annuum | ||||||||||||||
| Caffeine | ||||||||||||||
| Paullinia capuana | ||||||||||||||
| Chlorella pyrenoidosa | ||||||||||||||
| Lepidium meyenii | 150 mg | D | 200 mg | D | 200 mg | D | 200 mg | D | 1200 mg | D | 200 mg | D | 200 mg | D |
| Active Ingredient | DS 8 | DS 9 | DS 10 | DS 11 | DS 12 | DS 13 | DS 14 | |||||||
| S = −1 | S = −0.33 | S = −0.66 | S = 2 | S = −0.67 | S = −0.78 | S = −0.38 | ||||||||
| Daily Dose | EV | Daily Dose | EV | Daily Dose | EV | Daily Dose | EV | Daily Dose | EV | Daily Dose | EV | Daily Dose | EV | |
| Tribulus terrestris | 75 mg | C | ||||||||||||
| Crocus sativus | ||||||||||||||
| Panax ginseng | 200 mg | B | 50 mg | B | ||||||||||
| L-arginine | 2500 mg | C | 60 mg | C | 124 mg | C | 114 mg | C | ||||||
| Withania somnifera | 300 mg | B | ||||||||||||
| Eurycoma longifolia | ||||||||||||||
| Pinus pinaster | ||||||||||||||
| Corynanthe yohimbe | ||||||||||||||
| L-taurine | 50 mg | D | 100 mg | D | ||||||||||
| L-carnitine | 160 mg | D | ||||||||||||
| L-citrulline | ||||||||||||||
| Muira puama | 200 mg | D | 200 mg | D | 100 mg | D | ||||||||
| Trigonella foenum-graecum | ||||||||||||||
| Ginkgo biloba | 30 mg | D | ||||||||||||
| Hawthorn berry | ||||||||||||||
| Punica granatum | ||||||||||||||
| Cuscuta chinensis | ||||||||||||||
| Black Pepper | ||||||||||||||
| Zingiber officinale | 20 mg | D | ||||||||||||
| Turnera diffusa | 100 mg | D | 200 mg | D | 50 mg | D | ||||||||
| Vitamin C | 16 mg | D | ||||||||||||
| Vitamin B6 | 0.42 mg | D | ||||||||||||
| Vitamin B9 | 50 mcg | D | ||||||||||||
| Vitamin E | ||||||||||||||
| Vitamin D | ||||||||||||||
| Vitamin B1 | 0.33 mg | D | ||||||||||||
| Vitamin H | 15 mcg | D | ||||||||||||
| Vitamin B12 | 0.75 mcg | D | ||||||||||||
| Vitamin B3 | 20 mg | D | 48 mg | D | ||||||||||
| Magnesium | 112.5 mg | D | 76 mg | D | ||||||||||
| Zinc | 10 mg | D | 1.5 mg | D | ||||||||||
| Selenium | ||||||||||||||
| Alpha lipoic acid | 10 mg | D | ||||||||||||
| Aspartic acid | 86 mg | D | ||||||||||||
| Bambusa arundinacea | ||||||||||||||
| Epimedium acuminatum | 10 mg | D | ||||||||||||
| Capsicum annuum | 375 mg | D | ||||||||||||
| Caffeine | 25 mg | D | ||||||||||||
| Paullinia capuana | ||||||||||||||
| Chlorella pyrenoidosa | ||||||||||||||
| Lepidium meyenii | 200 mg | D | 300 mg | D | 200 mg | D | 100 mg | D | ||||||
| Active Ingredient | DS 15 | DS 16 | DS 17 | DS 18 | DS 19 | DS 20 | DS 21 | |||||||
| S = −0.20 | S = −0.56 | S = 0 | S = 0.21 | S = −0.38 | S = 0.90 | S = −1 | ||||||||
| Daily Dose | EV | Daily Dose | EV | Daily Dose | EV | Daily Dose | EV | Daily Dose | EV | Daily Dose | EV | Daily Dose | EV | |
| Tribulus terrestris | 200 mg | C | 108 mg | C | 150 mg | C | 200 mg | C | 125 mg | C | ||||
| Crocus sativus | ||||||||||||||
| Panax ginseng | 150 mg | B | 100 mg | B | 80 mg | B | ||||||||
| L-arginine | 500 mg | C | 364 mg | C | 240 mg | C | 200 mg | C | 125 mg | C | ||||
| Withania somnifera | ||||||||||||||
| Eurycoma longifolia | ||||||||||||||
| Pinus pinaster | ||||||||||||||
| Corynanthe yohimbe | ||||||||||||||
| L-taurine | 200 mg | D | 30 mg | D | 200 mg | D | ||||||||
| L-carnitine | ||||||||||||||
| L-citrulline | 63.7 mg | D | 600 mg | D | ||||||||||
| Muira puama | 30 mg | D | 150 mg | D | ||||||||||
| Trigonella foenum-graecum | 300 mg | D | ||||||||||||
| Ginkgo biloba | ||||||||||||||
| Hawthorn berry | ||||||||||||||
| Pomegranate | ||||||||||||||
| Cuscuta chinensis | ||||||||||||||
| Black Pepper | 30 mg | D | 20 mg | D | ||||||||||
| Zingiber officinale | ||||||||||||||
| Turnera diffusa | ||||||||||||||
| Vitamin C | 100 mg | D | ||||||||||||
| Vitamin B6 | 10 mg | D | ||||||||||||
| Vitamin B9 | 400 mcg | D | ||||||||||||
| Vitamin E | 24 mg | D | ||||||||||||
| Vitamin D | 10 mcg | D | ||||||||||||
| Vitamin B1 | ||||||||||||||
| Vitamin H | ||||||||||||||
| Vitamin B12 | 5 mcg | D | ||||||||||||
| Vitamin B3 | ||||||||||||||
| Magnesium | 190 mg | D | ||||||||||||
| Zinc | 15 mg | D | 7.5 mg | D | 10 mg | D | ||||||||
| Selenium | 100 mcg | D | ||||||||||||
| Alpha lipoic acid | ||||||||||||||
| Aspartic acid | 650 mg | D | ||||||||||||
| Bambusa arundinacea | ||||||||||||||
| Epimedium acuminatum | 9 mg | D | ||||||||||||
| Capsicum annuum | ||||||||||||||
| Caffeine | 180 mg | D | ||||||||||||
| Paullinia capuana | 180 mg | D | ||||||||||||
| Chlorella pyrenoidosa | 100 mg | D | ||||||||||||
| Lepidium meyenii | 500 mg | D | 80 mg | D | 200 mg | D | 300 mg | D | 220 mg | D | 3000 mg | D | ||
| Active Ingredient | DS 22 | DS 23 | DS 24 | DS 25 | DS 26 | DS 27 | ||||||||
| S = 0.30 | S = 2 | S = 0.30 | S = 2 | S = 0.30 | S = 1.33 | |||||||||
| Daily Dose | EV | Daily Dose | EV | Daily Dose | EV | Daily Dose | EV | Daily Dose | EV | Daily Dose | EV | |||
| Tribulus terrestris | 30 mg | C | 525 mg | C | 250 mg | C | 2000 mg | B | 250 mg | C | ||||
| Crocus sativus | 14 mg | B | ||||||||||||
| Panax ginseng | 21 mg | B | 150 mg | B | 150 mg | B | ||||||||
| L-arginine | 1350 mg | C | 40 mg | C | 400 mg | C | ||||||||
| Withania somnifera | ||||||||||||||
| Eurycoma longifolia | 50 mg | B | ||||||||||||
| Pinus pinaster | 60 mg | B | ||||||||||||
| Corynanthe yohimbe | 100 mg | B | 200 mg | B | ||||||||||
| L-taurine | ||||||||||||||
| L-carnitine | ||||||||||||||
| L-citrulline | 84.6 mg | D | 50 mg | D | ||||||||||
| Muira puama | 200 mg | D | ||||||||||||
| Trigonella foenum-graecum | ||||||||||||||
| Ginkgo biloba | 120 mg | D | ||||||||||||
| Hawthorn berry | ||||||||||||||
| Punica granatum | ||||||||||||||
| Cuscuta chinensis | ||||||||||||||
| Black Pepper | ||||||||||||||
| Zingiber officinale | ||||||||||||||
| Turnera diffusa | ||||||||||||||
| Vitamin C | ||||||||||||||
| Vitamin B6 | 3 mg | D | ||||||||||||
| Vitamin B9 | ||||||||||||||
| Vitamin E | ||||||||||||||
| Vitamin D | ||||||||||||||
| Vitamin B1 | ||||||||||||||
| Vitamin H | ||||||||||||||
| Vitamin B12 | ||||||||||||||
| Vitamin B3 | ||||||||||||||
| Magnesium | 140 mg | D | ||||||||||||
| Zinc | 20 mg | D | 7 mg | D | 8 mg | D | 14.7 mg | D | ||||||
| Selenium | ||||||||||||||
| Alpha lipoic acid | ||||||||||||||
| Aspartic acid | ||||||||||||||
| Bambusa arundinacea | ||||||||||||||
| Epimedium acuminatum | 500 mg | D | 500 mg | D | ||||||||||
| Capsicum annuum | ||||||||||||||
| Caffeine | ||||||||||||||
| Paullinia capuana | ||||||||||||||
| Chlorella pyrenoidosa | ||||||||||||||
| Ledidium meyenii | 50 mg | D | 250 mg | D | 600 mg | D | 1180 mg | D | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petre, G.C.; Francini-Pesenti, F.; Vitagliano, A.; Grande, G.; Ferlin, A.; Garolla, A. Dietary Supplements for Erectile Dysfunction: Analysis of Marketed Products, Systematic Review, Meta-Analysis and Rational Use. Nutrients 2023, 15, 3677. https://doi.org/10.3390/nu15173677
Petre GC, Francini-Pesenti F, Vitagliano A, Grande G, Ferlin A, Garolla A. Dietary Supplements for Erectile Dysfunction: Analysis of Marketed Products, Systematic Review, Meta-Analysis and Rational Use. Nutrients. 2023; 15(17):3677. https://doi.org/10.3390/nu15173677
Chicago/Turabian StylePetre, Gabriel Cosmin, Francesco Francini-Pesenti, Amerigo Vitagliano, Giuseppe Grande, Alberto Ferlin, and Andrea Garolla. 2023. "Dietary Supplements for Erectile Dysfunction: Analysis of Marketed Products, Systematic Review, Meta-Analysis and Rational Use" Nutrients 15, no. 17: 3677. https://doi.org/10.3390/nu15173677
APA StylePetre, G. C., Francini-Pesenti, F., Vitagliano, A., Grande, G., Ferlin, A., & Garolla, A. (2023). Dietary Supplements for Erectile Dysfunction: Analysis of Marketed Products, Systematic Review, Meta-Analysis and Rational Use. Nutrients, 15(17), 3677. https://doi.org/10.3390/nu15173677