Hepatocyte Aquaporins AQP8 and AQP9 Are Engaged in the Hepatic Lipid and Glucose Metabolism Modulating the Inflammatory and Redox State in Milk-Supplemented Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Chemicals
2.2. Oral Glucose Tolerance Test and Insulin Tolerance Test
2.3. Hepatic Inflammatory and Oxidative State
2.4. Semiquantitative Reverse Transcription PCR
2.5. Isolation of Liver Mitochondria and Hepatocyte Sinusoidal Membrane Vesicles
2.6. Western Blot Analysis
2.7. Stopped-Flow Light Scattering Measurements of Glycerol Permeability
2.8. Histological Analyses
2.9. Statistical Analysis
3. Results
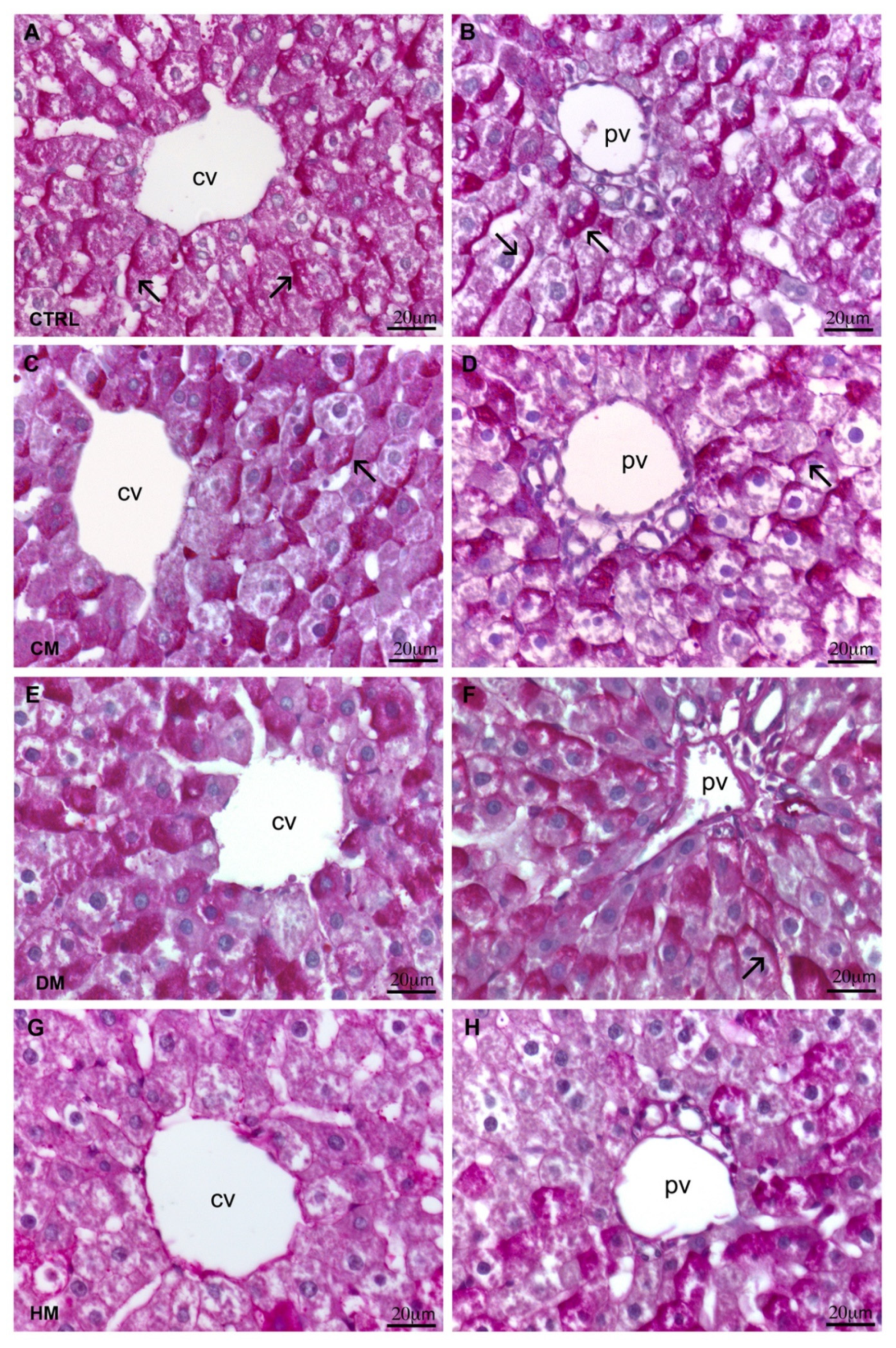
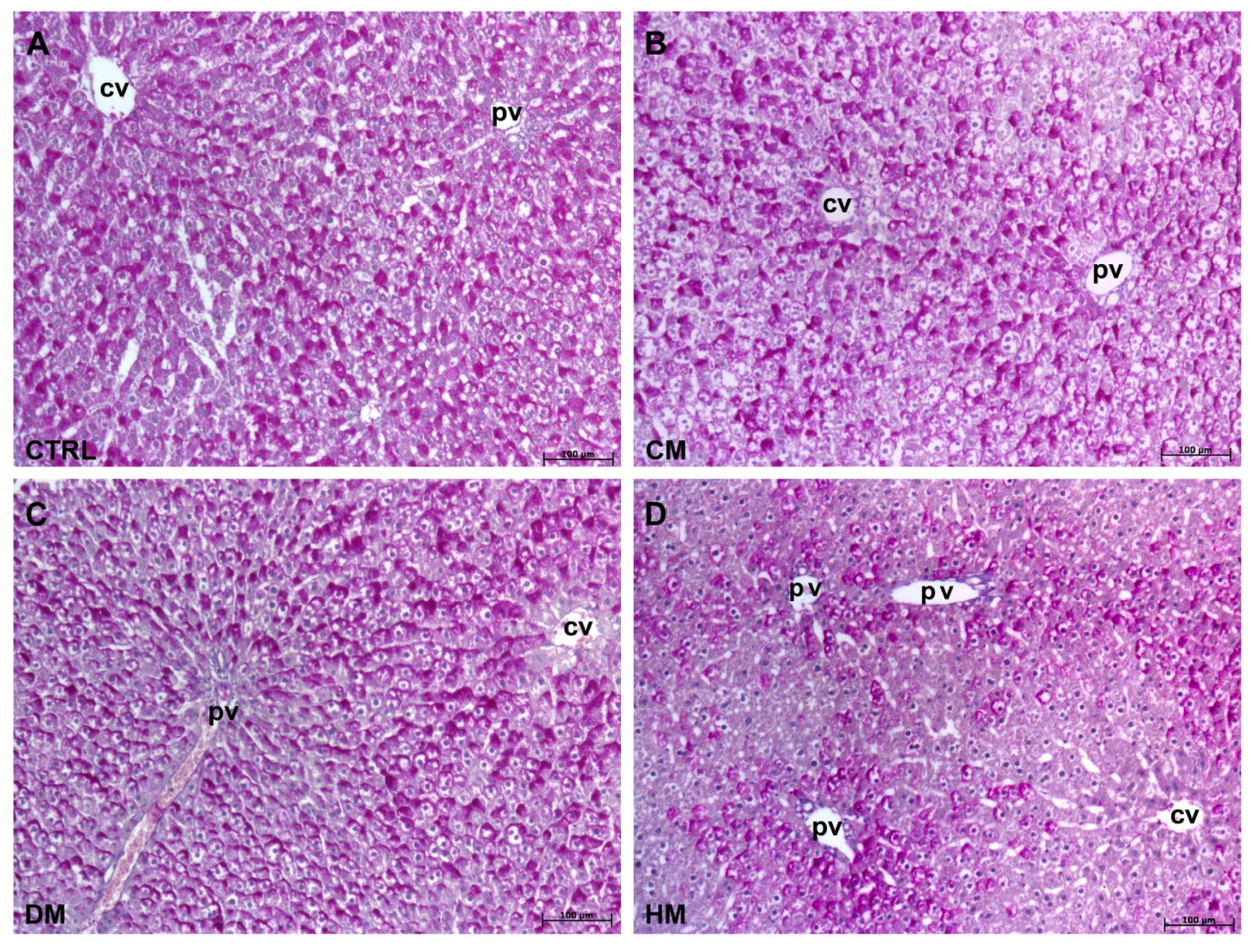
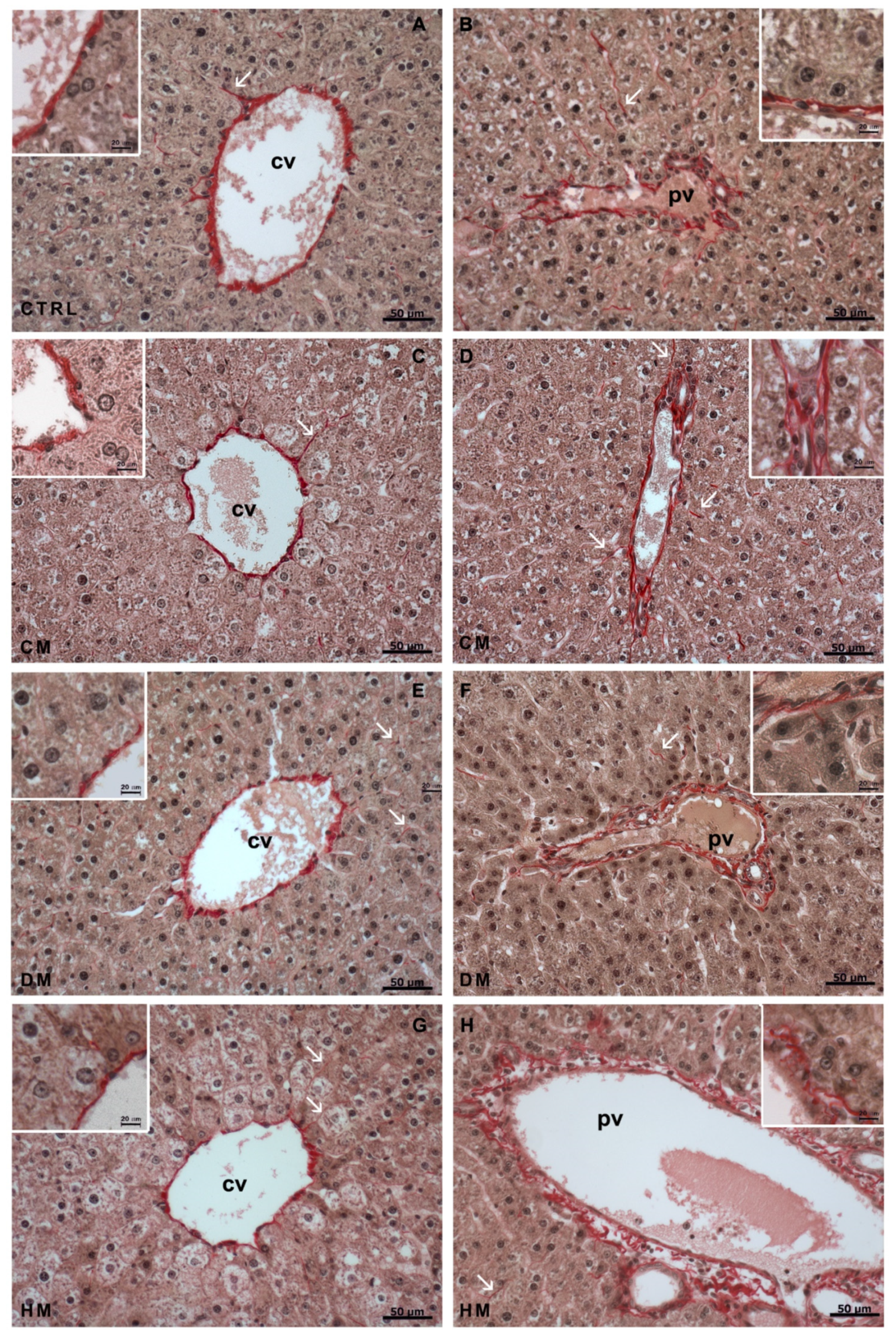
3.1. Histological Analyses
3.2. Glucose Homeostasis
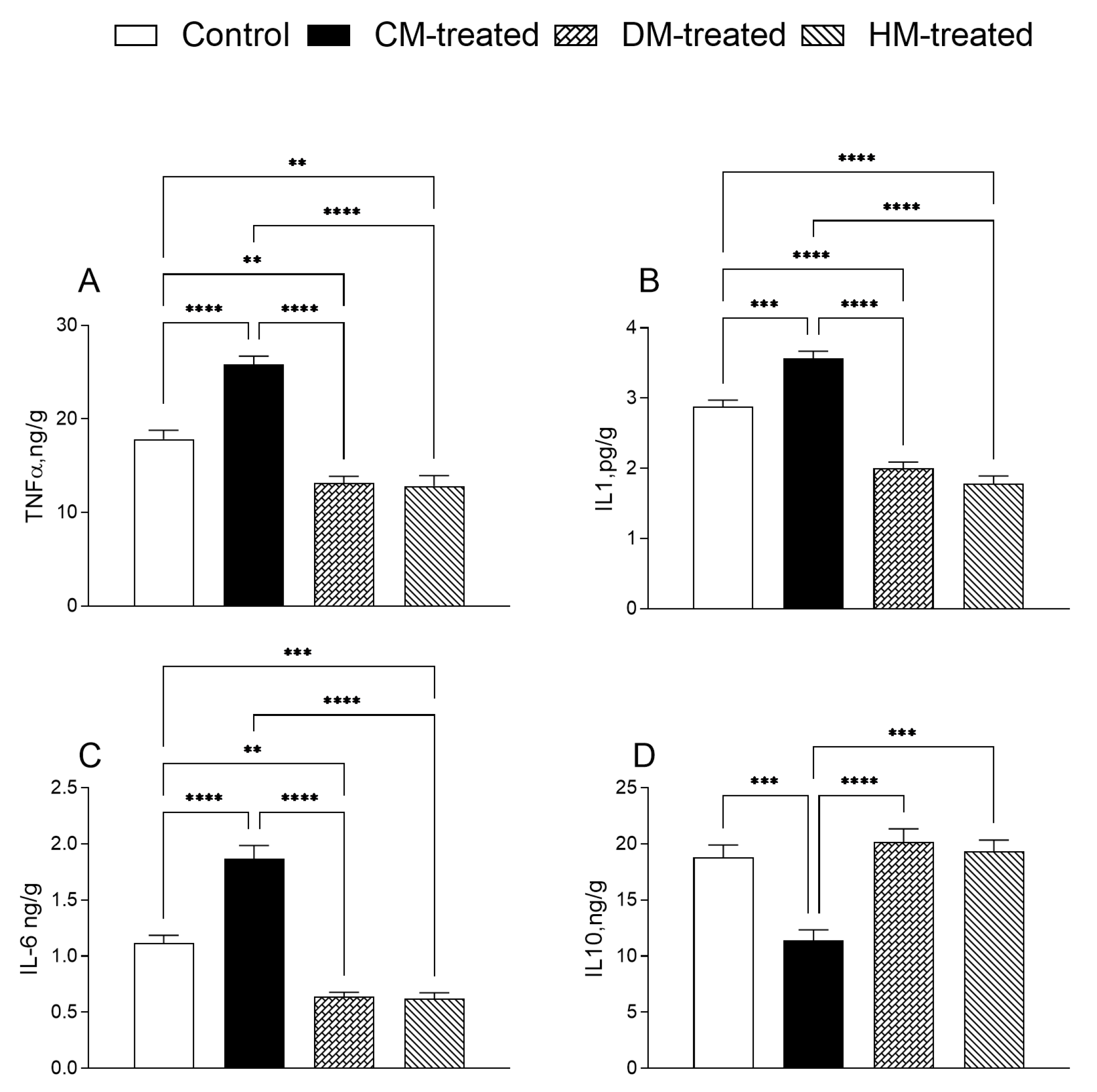
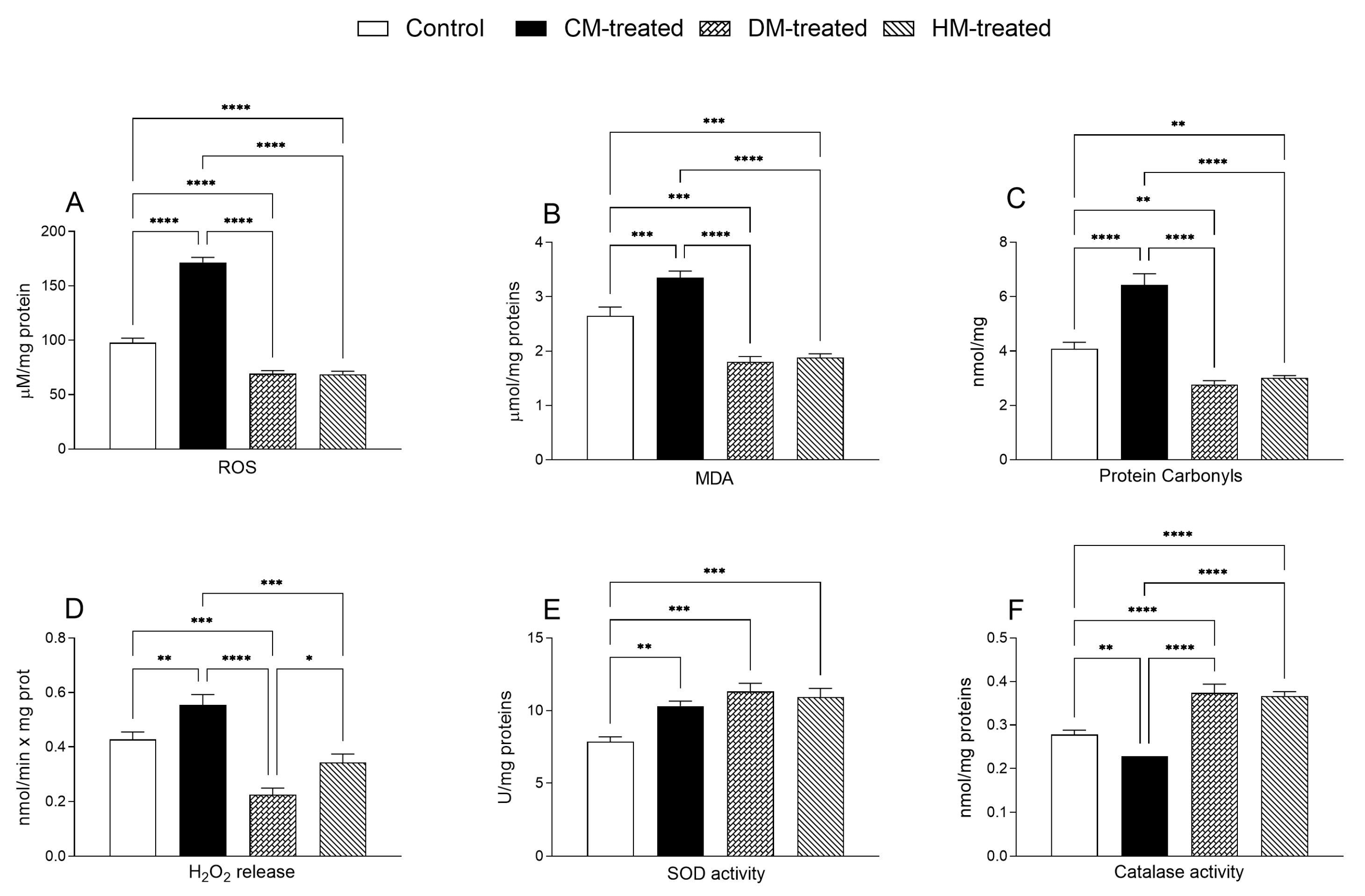
3.3. Hepatic Inflammatory and Oxidative States
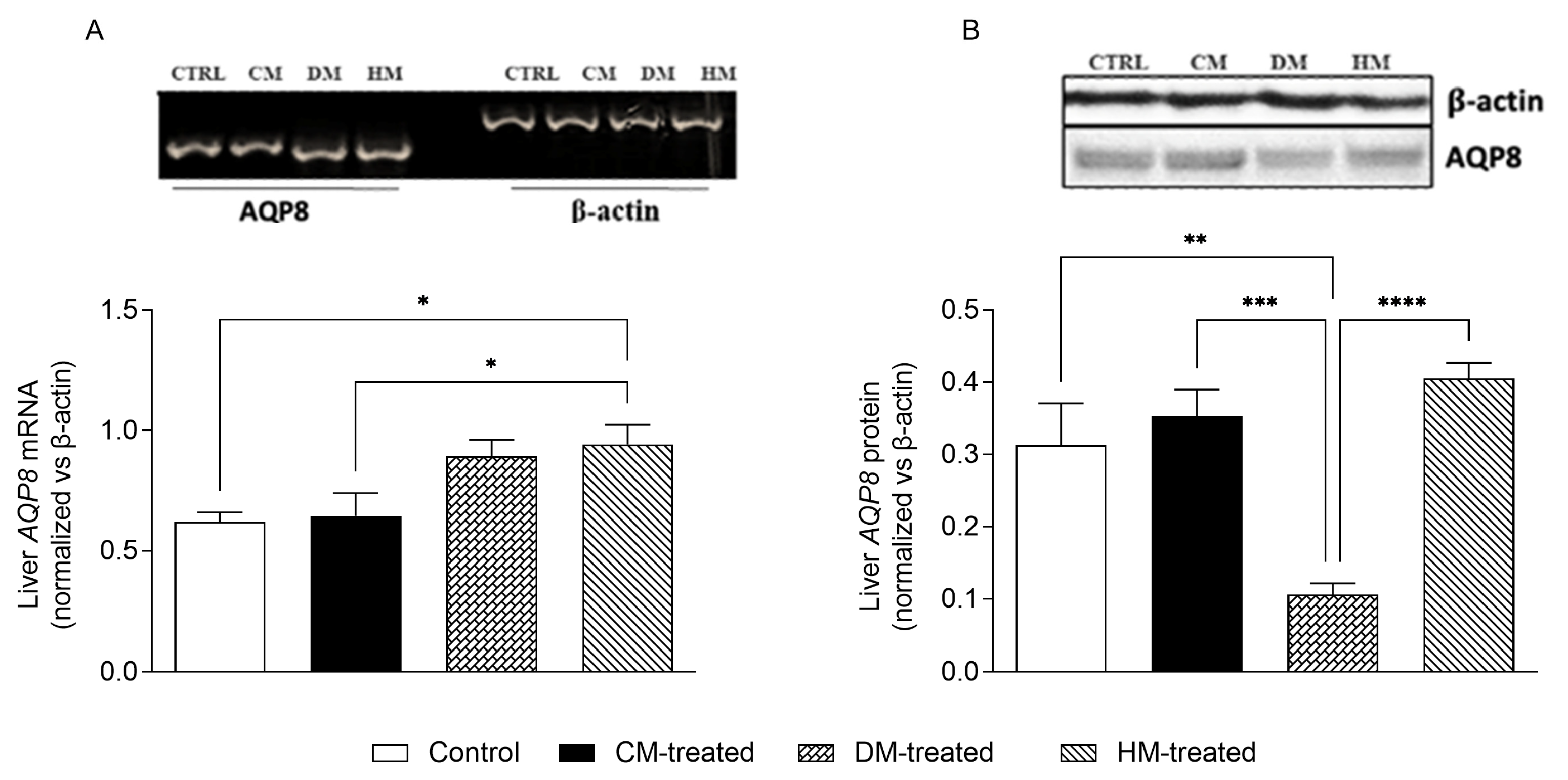
3.4. Mitochondrial AQP8 Is Significantly Reduced in DM-Fed Rats
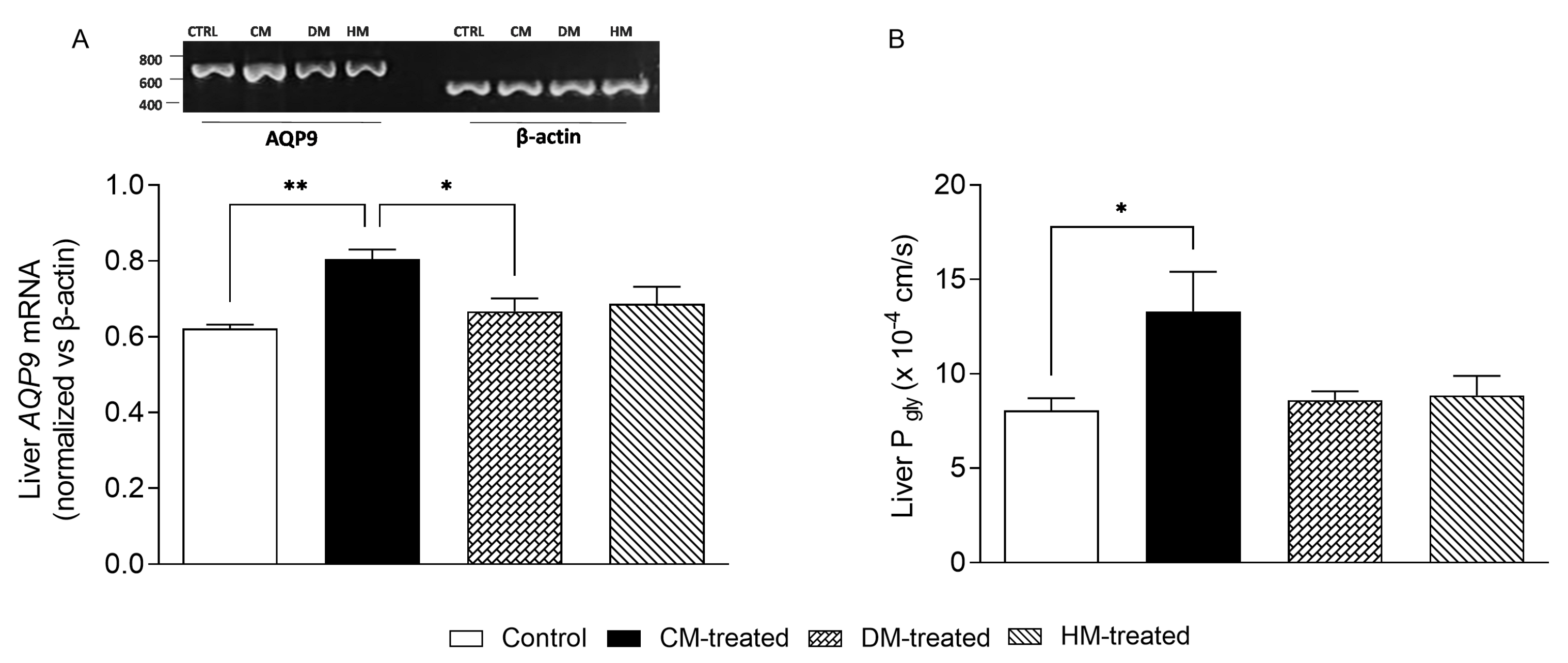
3.5. Liver AQP9 and Glycerol Permeability Are Increased in Rats Supplemented with Cow Milk
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shao, T.; Verma, H.K.; Pande, B.; Costanzo, V.; Ye, W.; Cai, Y.; Bhaskar, L.V.K.S. Physical Activity and Nutritional Influence on Immune Function: An Important Strategy to Improve Immunity and Health Status. Front. Physiol. 2021, 12, 751374. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Michalak, M.; Agellon, L.B. Importance of Nutrients and Nutrient Metabolism on Human Health. Yale J. Biol. Med. 2018, 91, 95–103. [Google Scholar] [PubMed]
- Gropper, S.S. The Role of Nutrition in Chronic Disease. Nutrients 2023, 15, 664. [Google Scholar] [CrossRef]
- Anderson, E.; Durstine, J.L. Physical activity, exercise, and chronic diseases: A brief review. Sports Med. Health Sci. 2019, 1, 3–10. [Google Scholar] [CrossRef]
- Rouhani, P.; Mirzaei, S.; Asadi, A.; Akhlaghi, M.; Saneei, P. Nutrient patterns in relation to metabolic health status in overweight and obese adolescents. Sci. Rep. 2023, 13, 119. [Google Scholar] [CrossRef]
- Neuhouser, M.L. The importance of healthy dietary patterns in chronic disease prevention. Nutr. Res. 2019, 70, 3–6. [Google Scholar] [CrossRef]
- Li, M.; Chi, X.; Wang, Y.; Setrerrahmane, S.; Xie, W.; Xu, H. Trends in insulin resistance: Insights into mechanisms and therapeutic strategy. Signal Transduct. Target. Ther. 2022, 7, 216. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Huang, Z.; Sang, D.; Gao, Q.; Li, Q. The Role of Nutrition in the Prevention and Intervention of Type 2 Diabetes. Front. Bioeng. Biotechnol. 2020, 8, 575442. [Google Scholar] [CrossRef]
- Michalski, M.C.; Genot, C.; Gayet, C.; Lopez, C.; Fine, F.; Joffre, F.; Vendeuvre, J.L.; Bouvier, J.; Chardigny, J.M.; Raynal-Ljutovac, K. Multiscale structures of lipids in foods as parameters affecting fatty acid bioavailability and lipid metabolism. Prog. Lipid Res. 2013, 52, 354–373. [Google Scholar] [CrossRef] [PubMed]
- Saghafi-Asl, M.; Mirmajidi, S.; Jafarabadi, M.A.; Vahid, F.; Shivappa, N.; Hébert, J.R.; Attari, V.E. The association of dietary patterns with dietary inflammatory index, systemic inflammation, and insulin resistance, in apparently healthy individuals with obesity. Sci. Rep. 2021, 11, 7515. [Google Scholar] [CrossRef]
- Hernández-Aguilera, A.; Rull, A.; Rodríguez-Gallego, E.; Riera-Borrull, M.; Luciano-Mateo, F.; Camps, J.; Menéndez, J.A.; Joven, J. Mitochondrial Dysfunction: A Basic Mechanism in Inflammation-Related Non-Communicable Diseases and Therapeutic Opportunities. Mediat. Inflamm. 2013, 2013, 135698. [Google Scholar] [CrossRef] [PubMed]
- Zlotorynski, E. Regulation of mitochondrial function by diet. Nat. Rev. Mol. Cell Biol. 2015, 16, 515. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, F.; Catapano, A.; Trinchese, G.; Cavaliere, G.; Culurciello, R.; Fogliano, C.; Penna, E.; Lucci, V.; Crispino, M.; Avallone, B.; et al. Dietary Micronutrient Management to Treat Mitochondrial Dysfunction in Diet-Induced Obese Mice. Int. J. Mol. Sci. 2021, 22, 2862. [Google Scholar] [CrossRef]
- Zhong, X.; Guo, L.; Zhang, L.; Li, Y.; He, R.; Cheng, G. Inflammatory potential of diet and risk of cardiovascular disease or mortality: A meta-analysis. Sci. Rep. 2017, 7, 6367. [Google Scholar] [CrossRef]
- Rungratanawanich, W.; Memo, M.; Uberti, D. Redox Homeostasis and Natural Dietary Compounds: Focusing on Antioxidants of Rice (Oryza sativa L.). Nutrients 2018, 10, 1605. [Google Scholar] [CrossRef]
- Cimmino, F.; Catapano, A.; Villano, I.; Maio, G.D.; Petrella, L.; Traina, G.; Pizzella, A.; Tudisco, R.; Cavaliere, G. Invited review: Human, cow, and donkey milk comparison: Focus on metabolic effects. J. Dairy Sci. 2023, 106, 3072–3085. [Google Scholar] [CrossRef]
- Mollica, M.; Trinchese, G.; Cimmino, F.; Penna, E.; Cavaliere, G.; Tudisco, R.; Musco, N.; Manca, C.; Catapano, A.; Monda, M.; et al. Milk Fatty Acid Profiles in Different Animal Species: Focus on the Potential Effect of Selected PUFAs on Metabolism and Brain Functions. Nutrients 2021, 13, 1111. [Google Scholar] [CrossRef] [PubMed]
- Cavaliere, G.; Trinchese, G.; Musco, N.; Infascelli, F.; De Filippo, C.; Mastellone, V.; Morittu, V.M.; Lombardi, P.; Tudisco, R.; Grossi, M.; et al. Milk from cows fed a diet with a high forage:concentrate ratio improves inflammatory state, oxidative stress, and mitochondrial function in rats. J. Dairy Sci. 2018, 101, 1843–1851. [Google Scholar] [CrossRef] [PubMed]
- Lionetti, L.; Cavaliere, G.; Bergamo, P.; Trinchese, G.; De Filippo, C.; Gifuni, G.; Gaita, M.; Pignalosa, A.; Donizzetti, I.; Putti, R.; et al. Diet supplementation with donkey milk upregulates liver mitochondrial uncoupling, reduces energy efficiency and improves antioxidant and antiinflammatory defences in rats. Mol. Nutr. Food Res. 2012, 56, 1596–1600. [Google Scholar] [CrossRef]
- Gaucheron, F. Milk and Dairy Products: A Unique Micronutrient Combination. J. Am. Coll. Nutr. 2011, 30, 400S–409S. [Google Scholar] [CrossRef]
- Knowles, T.A.; Hosfield, B.D.; Pecoraro, A.R.; Li, H.; Shelley, W.C.; Markel, T.A. It’s all in the milk: Chondroitin sulfate as potential preventative therapy for necrotizing enterocolitis. Pediatr. Res. 2021, 89, 1373–1379. [Google Scholar] [CrossRef]
- Pereira, P.C. Milk nutritional composition and its role in human health. Nutrition 2014, 30, 619–627. [Google Scholar] [CrossRef]
- Trinchese, G.; Cavaliere, G.; Filippo, C.D.; Aceto, S.; Prisco, M.; Chun, J.T.; Penna, E.; Negri, R.; Muredda, L.; Demurtas, A.; et al. Human Milk and Donkey Milk, Compared to Cow Milk, Reduce Inflammatory Mediators and Modulate Glucose and Lipid Metabolism, Acting on Mitochondrial Function and Oleylethanolamide Levels in Rat Skeletal Muscle. Front. Physiol. 2018, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Trinchese, G.; Cimmino, F.; Cavaliere, G.; Rosati, L.; Catapano, A.; Sorriento, D.; Murru, E.; Bernardo, L.; Pagani, L.; Bergamo, P.; et al. Heart Mitochondrial Metabolic Flexibility and Redox Status Are Improved by Donkey and Human Milk Intake. Antioxidants 2021, 10, 1807. [Google Scholar] [CrossRef]
- Trinchese, G.; Cavaliere, G.; Canani, R.B.; Matamoros, S.; Bergamo, P.; Filippo, C.D.; Aceto, S.; Gaita, M.; Cerino, P.; Negri, R.; et al. Human, donkey and cow milk differently affects energy efficiency and inflammatory state by modulating mitochondrial function and gut microbiota. J. Nutr. Biochem. 2015, 26, 1136–1146. [Google Scholar] [CrossRef]
- Corbin, K.D.; Carnero, E.A.; Dirks, B.; Igudesman, D.; Yi, F.; Marcus, A.; Davis, T.L.; Pratley, R.E.; Rittmann, B.E.; Krajmalnik-Brown, R.; et al. Host-diet-gut microbiome interactions influence human energy balance: A randomized clinical trial. Nat. Commun. 2023, 14, 3161. [Google Scholar] [CrossRef] [PubMed]
- Tarasenko, T.N.; McGuire, P.J. The liver is a metabolic and immunologic organ: A reconsideration of metabolic decompensation due to infection in inborn errors of metabolism (IEM). Mol. Genet. Metab. 2017, 121, 283–288. [Google Scholar] [CrossRef]
- Smith, R.L.; Soeters, M.R.; Wüst, R.C.I.; Houtkooper, R.H. Metabolic Flexibility as an Adaptation to Energy Resources and Requirements in Health and Disease. Endocr. Rev. 2018, 39, 489–517. [Google Scholar] [CrossRef]
- Parlakgül, G.; Arruda, A.P.; Pang, S.; Cagampan, E.; Min, N.; Güney, E.; Lee, G.Y.; Inouye, K.; Hess, H.F.; Xu, C.S.; et al. Regulation of liver subcellular architecture controls metabolic homeostasis. Nature 2022, 603, 736–742. [Google Scholar] [CrossRef]
- Geisler, C.E.; Hepler, C.; Higgins, M.R.; Renquist, B.J. Hepatic adaptations to maintain metabolic homeostasis in response to fasting and refeeding in mice. Nutr. Metab. 2016, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Debelius, J.; Brenner, D.A.; Karin, M.; Loomba, R.; Schnabl, B.; Knight, R. The gut–liver axis and the intersection with the microbiome. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Trefts, E.; Gannon, M.; Wasserman, D.H. The liver. Curr. Biol. 2017, 27, R1147–R1151. [Google Scholar] [CrossRef]
- Bellanti, F.; Lo Buglio, A.; Vendemiale, G. Hepatic Mitochondria-Gut Microbiota Interactions in Metabolism-Associated Fatty Liver Disease. Metabolites 2023, 13, 322. [Google Scholar] [CrossRef] [PubMed]
- Ipsen, D.H.; Lykkesfeldt, J.; Tveden-Nyborg, P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell. Mol. Life Sci. 2018, 75, 3313–3327. [Google Scholar] [CrossRef]
- Morio, B.; Panthu, B.; Bassot, A.; Rieusset, J. Role of mitochondria in liver metabolic health and diseases. Cell Calcium. 2021, 94, 102336. [Google Scholar] [CrossRef]
- Azad, A.K.; Raihan, T.; Ahmed, J.; Hakim, A.; Emon, T.H.; Chowdhury, P.A. Human Aquaporins: Functional Diversity and Potential Roles in Infectious and Non-infectious Diseases. Front. Genet. 2021, 12, 654865. [Google Scholar] [CrossRef] [PubMed]
- Agre, P. Nobel Lecture. Aquaporin water channels. BioSci. Rep. 2004, 24, 127–163. [Google Scholar] [CrossRef]
- Ishibashi, K.; Hara, S.; Kondo, S. Aquaporin water channels in mammals. Clin. Exp. Nephrol. 2009, 13, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Bienert, G.P.; Chaumont, F. Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochim. Biophys. Acta 2014, 1840, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Lebeck, J. Metabolic impact of the glycerol channels AQP7 and AQP9 in adipose tissue and liver. J. Mol. Endocrinol. 2014, 52, R165–R178. [Google Scholar] [CrossRef] [PubMed]
- Calamita, G.; Delporte, C. Aquaporins in Glandular Secretion. Adv. Exp. Med. Biol. 2023, 1398, 225–249. [Google Scholar] [CrossRef]
- Huebert, R.C.; Splinter, P.L.; Garcia, F.; Marinelli, R.A.; LaRusso, N.F. Expression and localization of aquaporin water channels in rat hepatocytes. Evidence for a role in canalicular bile secretion. J. Biol. Chem. 2002, 277, 22710–22717. [Google Scholar] [CrossRef]
- Calamita, G.; Gena, P.; Meleleo, D.; Ferri, D.; Svelto, M. Water permeability of rat liver mitochondria: A biophysical study. Biochim. Biophys. Acta 2006, 1758, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Capiglioni, A.M.; Müller, G.L.; Marrone, J.; Alvarez, M.L.; Marinelli, R.A. Enhanced ammonia detoxification to urea in hepatocytes transduced with human aquaporin-8 gene. Biotechnol. Bioeng. 2021, 118, 4331–4337. [Google Scholar] [CrossRef] [PubMed]
- Marinelli, R.A.; Marchissio, M.J. Mitochondrial aquaporin-8: A functional peroxiporin? Antioxid. Redox Signal. 2013, 19, 896. [Google Scholar] [CrossRef]
- Wu, B.; Beitz, E. Aquaporins with selectivity for unconventional permeants. Cell Mol Life Sci. 2007, 64, 2413–2421. [Google Scholar] [CrossRef]
- Brisson, D.; Vohl, M.C.; St-Pierre, J.; Hudson, T.J.; Gaudet, D. Glycerol: A neglected variable in metabolic processes? Bioessays 2001, 23, 534–542. [Google Scholar] [CrossRef]
- Reshef, L.; Olswang, Y.; Cassuto, H.; Blum, B.; Croniger, C.M.; Kalhan, S.C.; Tilghman, S.M.; Hanson, R.W. Glyceroneogenesis and the triglyceride/fatty acid cycle. J. Biol. Chem. 2003, 278, 30413–30416. [Google Scholar] [CrossRef] [PubMed]
- Elkjaer, M.; Vajda, Z.; Nejsum, L.N.; Kwon, T.; Jensen, U.B.; Amiry-Moghaddam, M.; Frøkiaer, J.; Nielsen, S. Immunolocalization of AQP9 in liver, epididymis, testis, spleen, and brain. Biochem. Biophys. Res. Commun. 2000, 276, 1118–1128. [Google Scholar] [CrossRef]
- Calamita, G.; Gena, P.; Ferri, D.; Rosito, A.; Rojek, A.; Nielsen, S.; Marinelli, R.A.; Frühbeck, G.; Svelto, M. Biophysical assessment of aquaporin-9 as principal facilitative pathway in mouse liver import of glucogenetic glycerol. Biol. Cell 2012, 104, 342–351. [Google Scholar] [CrossRef]
- Jelen, S.; Wacker, S.; Aponte-Santamaría, C.; Skott, M.; Rojek, A.; Johanson, U.; Kjellbom, P.; Nielsen, S.; de Groot, B.L.; Rützler, M. Aquaporin-9 protein is the primary route of hepatocyte glycerol uptake for glycerol gluconeogenesis in mice. J. Biol. Chem. 2011, 286, 44319–44325. [Google Scholar] [CrossRef]
- Gena, P.; Buono, N.D.; D’Abbicco, M.; Mastrodonato, M.; Berardi, M.; Svelto, M.; Lopez, L.; Calamita, G. Dynamical modeling of liver Aquaporin-9 expression and glycerol permeability in hepatic glucose metabolism. Eur. J. Cell Biol. 2017, 96, 61–69. [Google Scholar] [CrossRef]
- Da Silva, I.V.; Garra, S.; Calamita, G.; Soveral, G. The Multifaceted Role of Aquaporin-9 in Health and Its Potential as a Clinical Biomarker. Biomolecules 2022, 12, 897. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Catalán, V.; Gómez-Ambrosi, J.; García-Navarro, S.; Rotellar, F.; Valentí, V.; Silva, C.; Gil, M.J.; Salvador, J.; Burrell, M.A.; et al. Insulin- and leptin-mediated control of aquaglyceroporins in human adipocytes and hepatocytes is mediated via the PI3K/Akt/mTOR signaling cascade. J. Clin. Endocrinol. Metab. 2011, 96, E586–E597. [Google Scholar] [CrossRef]
- Rojek, A.M.; Skowronski, M.T.; Füchtbauer, E.-M.; Füchtbauer, A.C.; Fenton, R.A.; Agre, P.; Frøkiaer, J.; Nielsen, S. Defective glycerol metabolism in aquaporin 9 (AQP9) knockout mice. Proc. Natl. Acad. Sci. USA 2007, 104, 3609–3614. [Google Scholar] [CrossRef] [PubMed]
- Kuriyama, H.; Shimomura, I.; Kishida, K.; Kondo, H.; Furuyama, N.; Nishizawa, H.; Maeda, N.; Matsuda, M.; Nagaretani, H.; Kihara, S.; et al. Coordinated regulation of fat-specific and liver-specific glycerol channels, aquaporin adipose and aquaporin 9. Diabetes 2002, 51, 2915–2921. [Google Scholar] [CrossRef] [PubMed]
- Gena, P.; Mastrodonato, M.; Portincasa, P.; Fanelli, E.; Mentino, D.; Rodríguez, A.; Marinelli, R.A.; Brenner, C.; Frühbeck, G.; Svelto, M.; et al. Liver glycerol permeability and aquaporin-9 are dysregulated in a murine model of Non-Alcoholic Fatty Liver Disease. PLoS ONE 2013, 8, e78139. [Google Scholar] [CrossRef]
- Rodríguez, A.; Gena, P.; Méndez-Giménez, L.; Rosito, A.; Valentí, V.; Rotellar, F.; Sola, I.; Moncada, R.; Silva, C.; Svelto, M.; et al. Reduced hepatic aquaporin-9 and glycerol permeability are related to insulin resistance in non-alcoholic fatty liver disease. Int. J. Obes. 2014, 38, 1213–1220. [Google Scholar] [CrossRef]
- Rodríguez, A.; Marinelli, R.A.; Tesse, A.; Frühbeck, G.; Calamita, G. Sexual Dimorphism of Adipose and Hepatic Aquaglyceroporins in Health and Metabolic Disorders. Front. Endocrinol. 2015, 6, 171. [Google Scholar] [CrossRef]
- Elnegaard, J.J.; Iena, F.M.; Herold, J.; Lebeck, J. Sex-specific effect of AQP9 deficiency on hepatic triglyceride metabolism in mice with diet-induced obesity. J. Physiol. 2023. ahead of print. [Google Scholar] [CrossRef]
- Cacho, J.; Sevillano, J.; de Castro, J.; Herrera, E.; Ramos, M.P. Validation of simple indexes to assess insulin sensitivity during pregnancy in Wistar and Sprague-Dawley rats. Am. J. Physiol.-Endocrinol. Metab. 2008, 295, E1269–E1276. [Google Scholar] [CrossRef] [PubMed]
- Barja, G. Mitochondrial Free Radical Production and Aging in Mammals and Birdsa. Ann. N. York Acad. Sci. 1998, 854, 224–238. [Google Scholar] [CrossRef] [PubMed]
- Iossa, S.; Lionetti, L.; Mollica, M.P.; Crescenzo, R.; Barletta, A.; Liverini, G. Effect of long-term high-fat feeding on energy balance and liver oxidative activity in rats. Br. J. Nutr. 2000, 84, 377–385. [Google Scholar] [CrossRef]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.G.; Ahn, B.W.; Shaltiel, S.; Stadtman, E.R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzym. 1990, 186, 464–478. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzym. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Montoliu, C.; Vallés, S.; Renau-Piqueras, J.; Guerri, C. Ethanol-Induced Oxygen Radical Formation and Lipid Peroxidation in Rat Brain: Effect of Chronic Alcohol Consumption. J. Neurochem. 2002, 63, 1855–1862. [Google Scholar] [CrossRef]
- Marinelli, R.A.; Tietz, P.S.; Caride, A.J.; Huang, B.Q.; LaRusso, N.F. Water transporting properties of hepatocyte basolateral and canalicular plasma membrane domains. J. Biol. Chem. 2003, 278, 43157–43162. [Google Scholar] [CrossRef]
- Avallone, B.; Agnisola, C.; Cerciello, R.; Panzuto, R.; Simoniello, P.; Cretì, P.; Motta, C.M. Structural and functional changes in the zebrafish (Danio rerio) skeletal muscle after cadmium exposure. Cell Biol. Toxicol. 2015, 31, 273–283. [Google Scholar] [CrossRef]
- Sodano, F.; Avallone, B.; Tizzano, M.; Fogliano, C.; Rolando, B.; Gazzano, E.; Riganti, C.; Magliocca, S.; Cuozzo, M.; Albrizio, S.; et al. Ketogal Safety Profile in Human Primary Colonic Epithelial Cells and in Mice. Pharmaceuticals 2021, 14, 1149. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, R.M.; Almalki, W.H.; Farouk, E.; Kamel, H.F.M. Assessment of Hepatotoxicity of Obesity in Female Patients’ through Histopathological, Biochemical and Immunohistochemical Study. Am. J. Med. Med. Sci. 2013, 3, 126–132. [Google Scholar]
- Tamma, G.; Valenti, G.; Grossini, E.; Donnini, S.; Marino, A.; Marinelli, R.A.; Calamita, G. Aquaporin Membrane Channels in Oxidative Stress, Cell Signaling, and Aging: Recent Advances and Research Trends. Oxidative Med. Cell. Longev. 2018, 2018, 1501847. [Google Scholar] [CrossRef] [PubMed]
- Hoogerland, J.A.; Staels, B.; Dombrowicz, D. Immune–metabolic interactions in homeostasis and the progression to NASH. Trends Endocrinol. Metab. 2022, 33, 690–709. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.W.; Harmon, C.; O’Farrelly, C. Liver immunology and its role in inflammation and homeostasis. Cell. Mol. Immunol. 2016, 13, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Han, H.-S.; Kang, G.; Kim, J.S.; Choi, B.H.; Koo, S.-H. Regulation of glucose metabolism from a liver-centric perspective. Exp. Mol. Med. 2016, 48, e218. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhang, S.; Ma, J.; Liu, S.; Chen, S. In Search of Zonation Markers to Identify Liver Functional Disorders. Oxidative Med. Cell. Longev. 2020, 2020, 9374896. [Google Scholar] [CrossRef]
- Adeva-Andany, M.M.; González-Lucán, M.; Donapetry-García, C.; Fernández-Fernández, C.; Ameneiros-Rodríguez, E. Glycogen metabolism in humans. BBA Clin. 2016, 5, 85–100. [Google Scholar] [CrossRef]
- Li, L.; Liu, X.; Guo, H. The nutritional ingredients and antioxidant activity of donkey milk and donkey milk powder. Food Sci. Biotechnol. 2017, 27, 393–400. [Google Scholar] [CrossRef]
- Barosa, C.; Silva, C.; Fagulha, A.; Barros, L.; Caldeira, M.M.; Carvalheiro, M.; Jones, J.G. Sources of hepatic glycogen synthesis following a milk-containing breakfast meal in healthy subjects. Metabolism 2012, 61, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Stahel, P.; Kim, J.J.; Xiao, C.; Cant, J.P. Of the milk sugars, galactose, but not prebiotic galacto-oligosaccharide, improves insulin sensitivity in male Sprague-Dawley rats. PLoS ONE 2017, 12, e0172260. [Google Scholar] [CrossRef]
- Gunnerud, U.J.; Heinzle, C.; Holst, J.J.; Östman, E.M.; Björck, I.M.E. Effects of Pre-Meal Drinks with Protein and Amino Acids on Glycemic and Metabolic Responses at a Subsequent Composite Meal. PLoS ONE 2012, 7, e44731. [Google Scholar] [CrossRef] [PubMed]
- Patti, M.-E.; Corvera, S. The Role of Mitochondria in the Pathogenesis of Type 2 Diabetes. Endocr. Rev. 2010, 31, 364–395. [Google Scholar] [CrossRef]
- Nambiar, S.M.; Lee, J.; Yanum, J.A.; Garcia, V.; Jiang, H.; Dai, G. Maternal hepatocytes heterogeneously and dynamically exhibit developmental phenotypes partially via yes-associated protein 1 during pregnancy. Am. J. Physiol.-Gastrointest. Liver Physiol. 2023, 324, G38–G50. [Google Scholar] [CrossRef] [PubMed]
- Mayoral Monibas, R.; Johnson, A.M.F.; Osborn, O.; Traves, P.G.; Mahata, S.K. Distinct Hepatic Macrophage Populations in Lean and Obese Mice. Front. Endocrinol. 2016, 7, 152. [Google Scholar] [CrossRef]
- Zhang, C.-Y.; Yuan, W.-G.; He, P.; Lei, J.-H.; Wang, C.-X. Liver fibrosis and hepatic stellate cells: Etiology, pathological hallmarks and therapeutic targets. World J. Gastroenterol. 2016, 22, 10512. [Google Scholar] [CrossRef]
- Danielli, M.; Marrone, J.; Capiglioni, A.M.; Marinelli, R.A. Data of H2O2 release from AQP8-knockdown rat hepatocyte mitochondria. Data Brief 2019, 23, 103722. [Google Scholar] [CrossRef] [PubMed]
- Krüger, C.; Jörns, A.; Kaynert, J.; Waldeck-Weiermair, M.; Michel, T.; Elsner, M.; Lenzen, S. The importance of aquaporin-8 for cytokine-mediated toxicity in rat insulin-producing cells. Free Radic. Biol. Med. 2021, 174, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Krüger, C.; Waldeck-Weiermair, M.; Kaynert, J.; Pokrant, T.; Komaragiri, Y.; Otto, O.; Michel, T.; Elsner, M. AQP8 is a crucial H2O2 transporter in insulin-producing RINm5F cells. Redox. Biol. 2021, 43, 101962. [Google Scholar] [CrossRef] [PubMed]


| Diet Composition | Chow | CM | DM | HM | Chow + CM | Chow + DM | Chow + HM |
|---|---|---|---|---|---|---|---|
| Protein (%) | 29 | 30.7 | 18 | 12.5 | 29.2 | 27.5 | 26.7 |
| Lipid (%) | 10.6 | 34 | 4.7 | 35 | 13.9 | 9.8 | 14.1 |
| Carbohydrate (%) | 60 | 35.5 | 77.3 | 52.5 | 56.5 | 62.4 | 58.9 |
| Energy density (kJ/g) | 15.8 | 3.46 | 1.48 | 3.24 | 14.0 | 13.8 | 14.0 |
| Energy Intake | Control | CM-Treated | DM-Treated | HM-Treated |
|---|---|---|---|---|
| Total Energy (kJ) | 12,432 ± 176 | 14,071 ± 204 * | 14,288 ± 222 * | 14,095 ± 202 * |
| Chow (kJ) | 12,432 ± 164 | 12,064 ± 184 (85.74%) | 12,280 ± 203 (85.95%) | 12,091 ± 188 (85.78%) |
| Milk (kJ) | 2007 ± 14 (14.26%) | 2008 ± 18 (14.05%) | 2004 ± 14 (14.22%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trinchese, G.; Gena, P.; Cimmino, F.; Cavaliere, G.; Fogliano, C.; Garra, S.; Catapano, A.; Petrella, L.; Di Chio, S.; Avallone, B.; et al. Hepatocyte Aquaporins AQP8 and AQP9 Are Engaged in the Hepatic Lipid and Glucose Metabolism Modulating the Inflammatory and Redox State in Milk-Supplemented Rats. Nutrients 2023, 15, 3651. https://doi.org/10.3390/nu15163651
Trinchese G, Gena P, Cimmino F, Cavaliere G, Fogliano C, Garra S, Catapano A, Petrella L, Di Chio S, Avallone B, et al. Hepatocyte Aquaporins AQP8 and AQP9 Are Engaged in the Hepatic Lipid and Glucose Metabolism Modulating the Inflammatory and Redox State in Milk-Supplemented Rats. Nutrients. 2023; 15(16):3651. https://doi.org/10.3390/nu15163651
Chicago/Turabian StyleTrinchese, Giovanna, Patrizia Gena, Fabiano Cimmino, Gina Cavaliere, Chiara Fogliano, Sabino Garra, Angela Catapano, Lidia Petrella, Silvia Di Chio, Bice Avallone, and et al. 2023. "Hepatocyte Aquaporins AQP8 and AQP9 Are Engaged in the Hepatic Lipid and Glucose Metabolism Modulating the Inflammatory and Redox State in Milk-Supplemented Rats" Nutrients 15, no. 16: 3651. https://doi.org/10.3390/nu15163651
APA StyleTrinchese, G., Gena, P., Cimmino, F., Cavaliere, G., Fogliano, C., Garra, S., Catapano, A., Petrella, L., Di Chio, S., Avallone, B., Calamita, G., & Mollica, M. P. (2023). Hepatocyte Aquaporins AQP8 and AQP9 Are Engaged in the Hepatic Lipid and Glucose Metabolism Modulating the Inflammatory and Redox State in Milk-Supplemented Rats. Nutrients, 15(16), 3651. https://doi.org/10.3390/nu15163651












