Effects of Oral Alpha-Lipoic Acid Treatment on Diabetic Polyneuropathy: A Meta-Analysis and Systematic Review
Abstract
1. Introduction
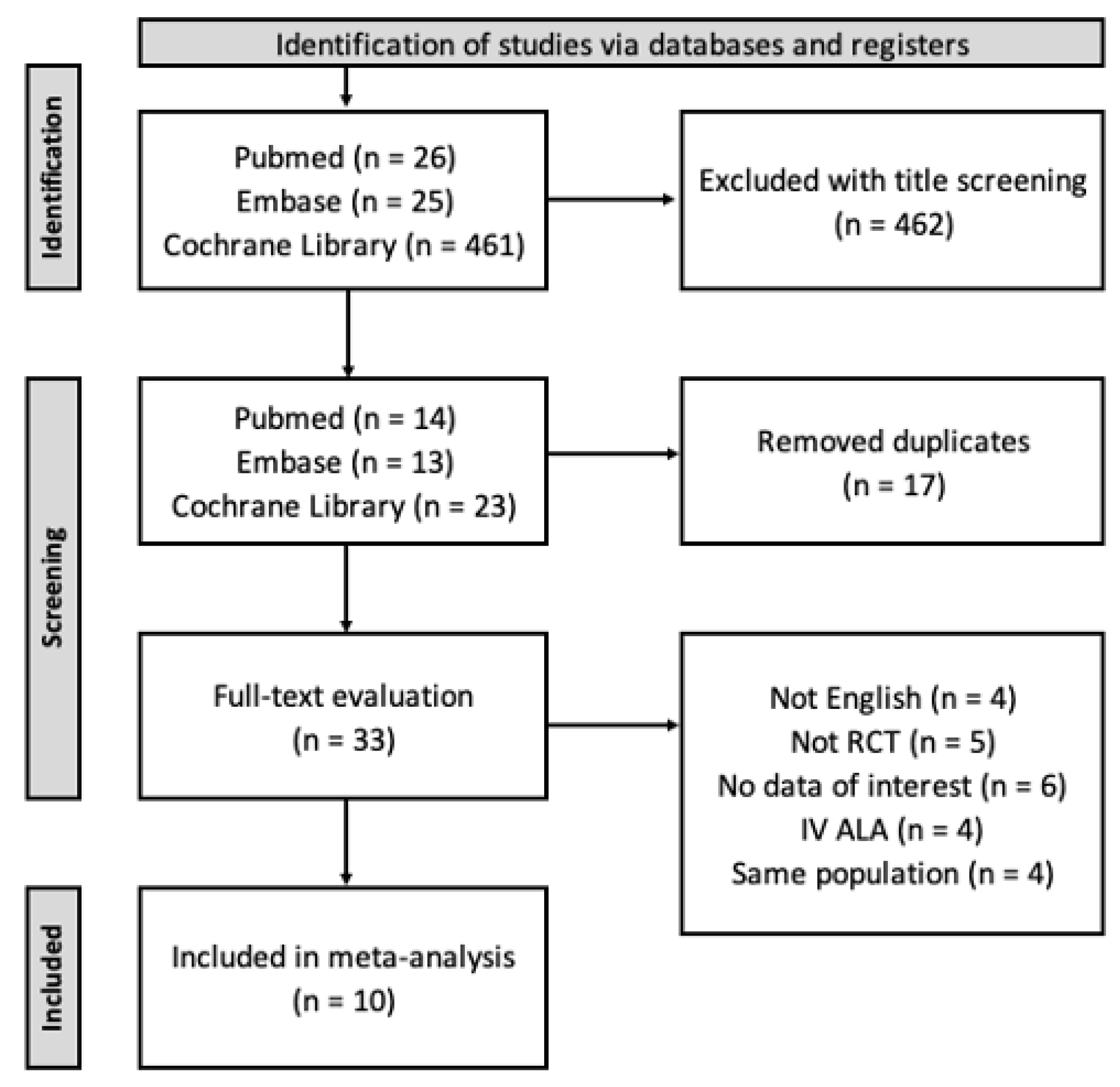
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Neuropathy Assessment
2.3.1. Primary Outcome: TSS
2.3.2. Secondary Outcome: NDS
2.3.3. Secondary Outcome: NIS and NIS-LL
2.3.4. Secondary Outcome: VPT
2.3.5. Secondary Outcome: NCS
2.3.6. Secondary Outcome: Global Satisfaction Score
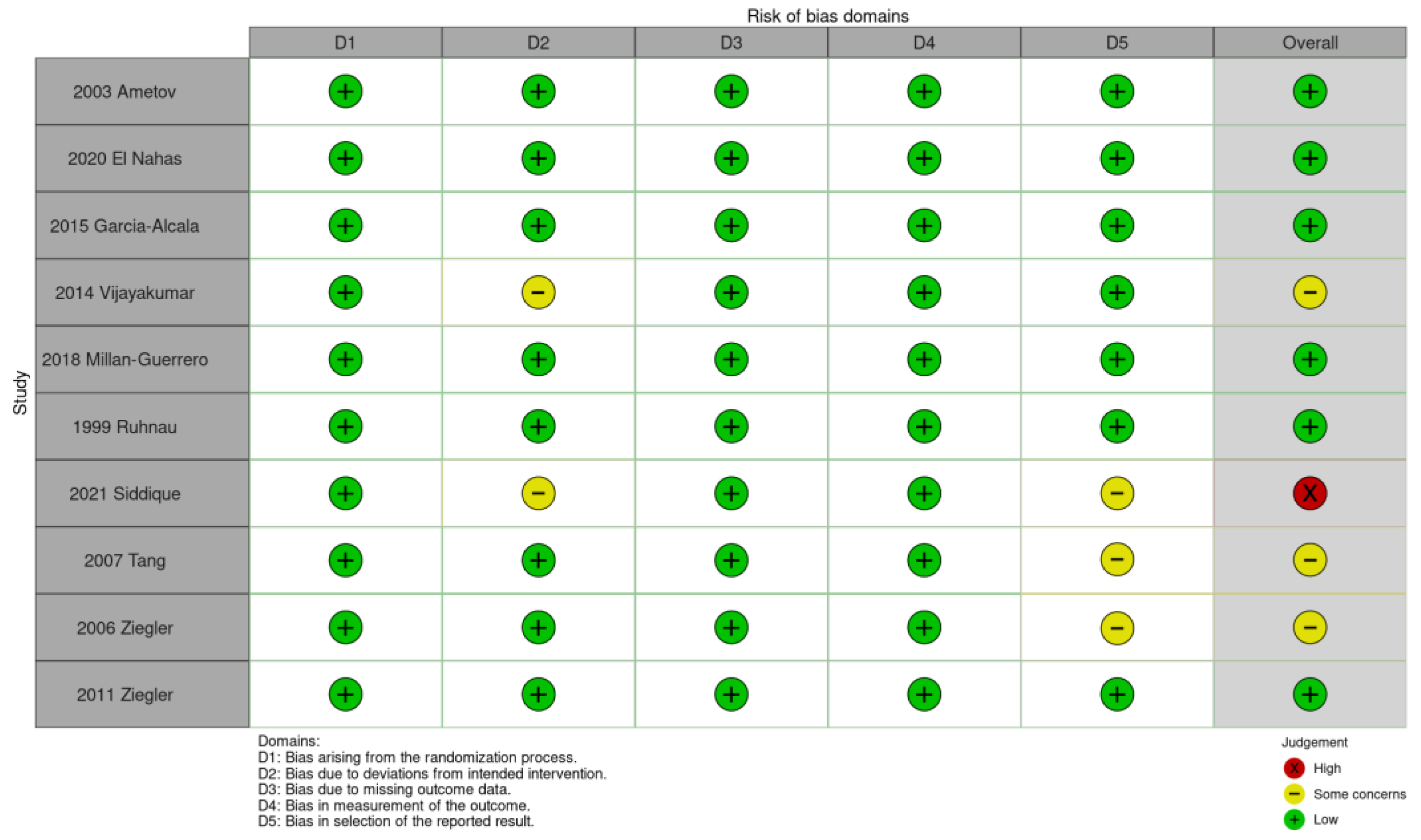
2.4. Data Extraction and Quality Assessment
2.5. Statistical Analysis
3. Results
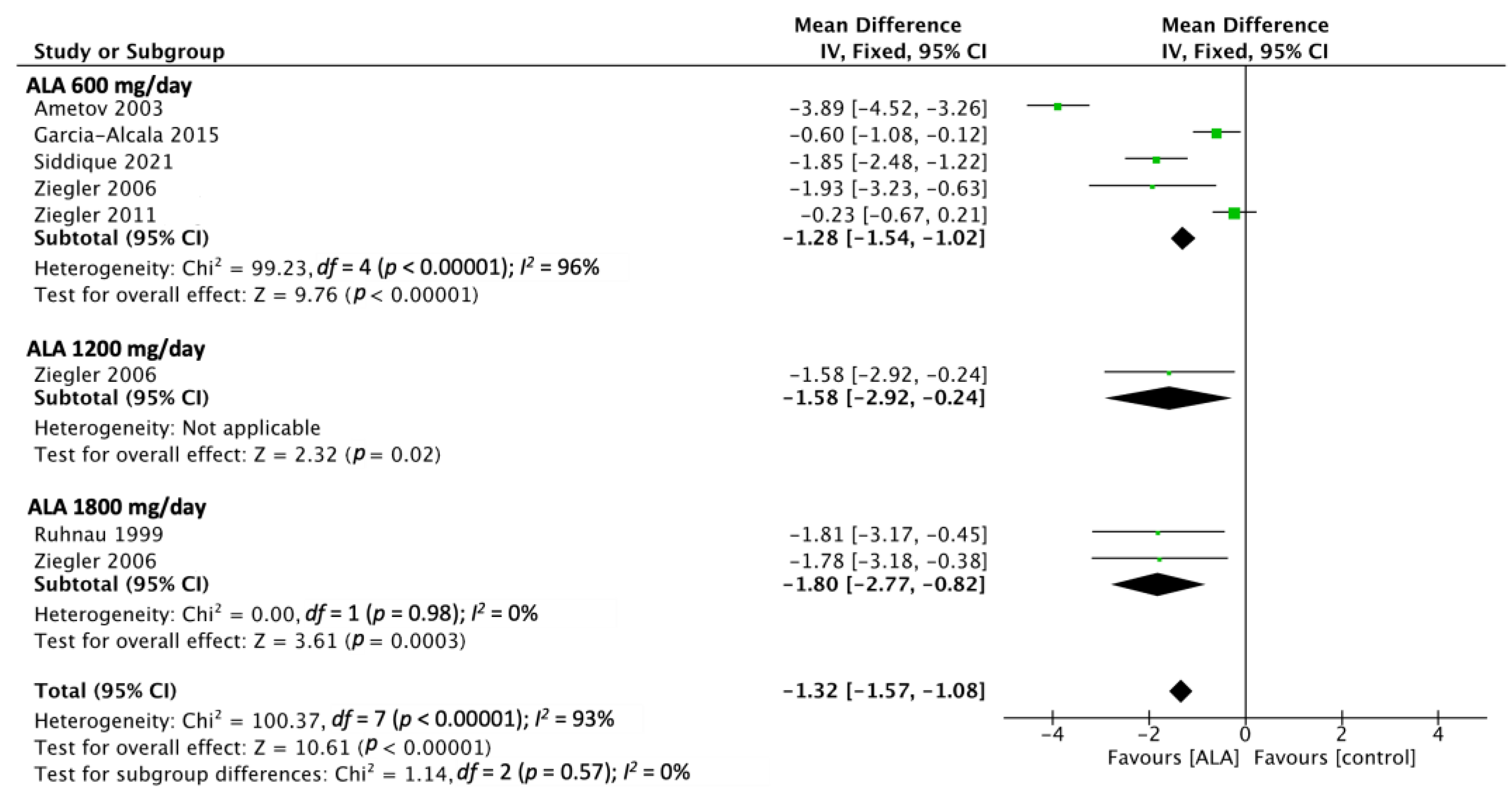
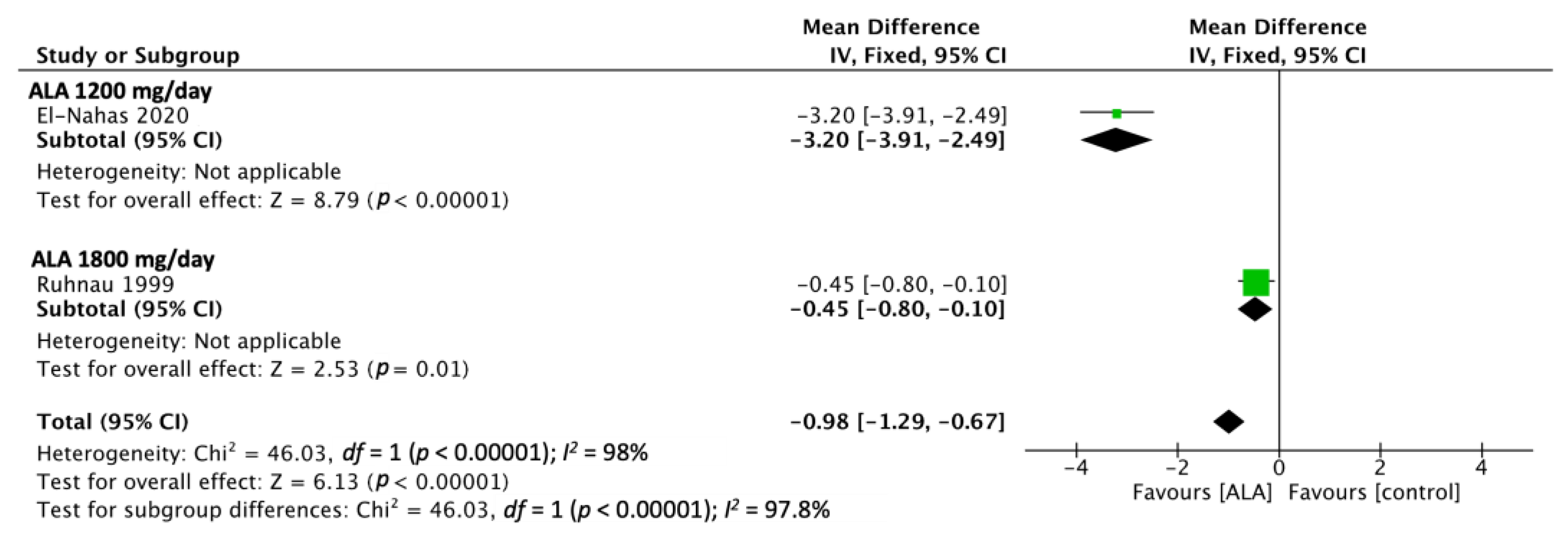
3.1. TSS
3.2. NDS
3.3. NIS
3.4. Global Satisfaction
3.5. Parameters for Which Nonsignificant Results Were Reported
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sloan, G.; Selvarajah, D.; Tesfaye, S. Pathogenesis, diagnosis and clinical management of diabetic sensorimotor peripheral neuropathy. Nat. Rev. Endocrinol. 2021, 17, 400–420. [Google Scholar] [CrossRef] [PubMed]
- Malik, R.A.; Andag-Silva, A.; Dejthevaporn, C.; Hakim, M.; Koh, J.S.; Pinzon, R.; Sukor, N.; Wong, K.S. Diagnosing peripheral neuropathy in South-East Asia: A focus on diabetic neuropathy. J. Diabetes Investig. 2020, 11, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, D.; Papanas, N.; Vinik, A.I.; Shaw, J.E. Epidemiology of polyneuropathy in diabetes and prediabetes. Handb. Clin. Neurol. 2014, 126, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Braffett, B.H.; Gubitosi-Klug, R.A.; Albers, J.W.; Feldman, E.L.; Martin, C.L.; White, N.H.; Orchard, T.J.; Lopes-Virella, M.; Lachin, J.M.; Pop-Busui, R.; et al. Risk Factors for Diabetic Peripheral Neuropathy and Cardiovascular Autonomic Neuropathy in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study. Diabetes 2020, 69, 1000–1010. [Google Scholar] [CrossRef] [PubMed]
- Yagihashi, S.; Mizukami, H.; Sugimoto, K. Mechanism of diabetic neuropathy: Where are we now and where to go? J. Diabetes Investig. 2011, 2, 18–32. [Google Scholar] [CrossRef]
- Malik, R.A.; Newrick, P.G.; Sharma, A.K.; Jennings, A.; Ah-See, A.K.; Mayhew, T.M.; Jakubowski, J.; Boulton, A.J.M.; Ward, J.D. Microangiopathy in human diabetic neuropathy: Relationship between capillary abnormalities and the severity of neuropathy. Diabetologia 1989, 32, 92–102. [Google Scholar] [CrossRef]
- Vallianou, N.; Evangelopoulos, A.; Koutalas, P. Alpha-lipoic acid and diabetic neuropathy. Rev. Diabet. Stud. 2009, 6, 230–236. [Google Scholar] [CrossRef]
- Mitsui, Y.; Schmelzer, J.D.; Zollman, P.J.; Mitsui, M.; Tritschler, H.J.; Low, P.A. Alpha-lipoic acid provides neuroprotection from ischemia-reperfusion injury of peripheral nerve. J. Neurol. Sci. 1999, 163, 11–16. [Google Scholar] [CrossRef]
- Khan, H.; Singh, T.G.; Dahiya, R.S.; Abdel-Daim, M.M. α-Lipoic Acid, an Organosulfur Biomolecule a Novel Therapeutic Agent for Neurodegenerative Disorders: An Mechanistic Perspective. Neurochem. Res. 2022, 47, 1853–1864. [Google Scholar] [CrossRef]
- Ziegler, D.; Nowak, H.; Kempler, P.; Vargha, P.; Low, P.A.; Ziegler, D. Treatment of symptomatic diabetic polyneuropathy with the antioxidant α-lipoic acid: A meta-analysis. Diabet. Med. 2004, 21, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Bai, J.; Liu, W.; Hu, Y. A systematic review and meta-analysis of α-lipoic acid in the treatment of diabetic peripheral neuropathy. Eur. J. Endocrinol. 2012, 167, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Pan, J.; Yu, J.; Liu, X.; Liu, L.; Zuo, X.; Wu, P.; Deng, H.; Zhang, J.; Ji, A. Meta-analysis of methylcobalamin alone and in combination with lipoic acid in patients with diabetic peripheral neuropathy. Diabetes Res. Clin. Pract. 2013, 101, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Abubaker, S.A.; Alonazy, A.M.; Abdulrahman, A. Effect of Alpha-Lipoic Acid in the Treatment of Diabetic Neuropathy: A Systematic Review. Cureus 2022, 14, e25750. [Google Scholar] [CrossRef]
- The SYDNEY Trial Authors, for the SYDNEY Trial Study Group; Ametov, A.S.; Barinov, A.; Dyck, P.J.; Hermann, R.; Kozlova, N.; Litchy, W.J.; Low, P.A.; Nehrdich, D.; Novosadova, M.; et al. The Sensory Symptoms of Diabetic Polyneuropathy Are Improved With-Lipoic Acid. Diabetes Care 2003, 26, 770–776. Available online: http://diabetesjournals.org/care/article-pdf/26/3/770/665565/dc0303000770.pdf (accessed on 7 December 2022).
- Yang, Z.; Chen, R.; Zhang, Y.; Huang, Y.; Hong, T.; Sun, F.; Ji, L.; Zhan, S. Scoring systems to screen for diabetic peripheral neuropathy. Cochrane Database Syst. Rev. 2018, 2018, CD010974. [Google Scholar] [CrossRef]
- Dyck, P.J.B.; González-Duarte, A.; Obici, L.; Polydefkis, M.; Wiesman, J.F.; Antonino, I.; Litchy, W.J.; Dyck, P.J. Development of measures of polyneuropathy impairment in hATTR amyloidosis: From NIS to mNIS + 7. J. Neurol. Sci. 2019, 405, 116424. [Google Scholar] [CrossRef]
- Coelho, T.; Maia, L.F.; da Silva, A.M.; Cruz, M.W.; Planté-Bordeneuve, V.; Lozeron, P.; Suhr, O.B.; Campistol, J.M.; Conceição, I.M.; Schmidt, H.H.-J.; et al. Tafamidis for transthyretin familial amyloid polyneuropathy. Neurology 2012, 79, 785–792. [Google Scholar] [CrossRef]
- Garcia-Alcala, H.; Santos Vichido, C.I.; Islas Macedo, S.; Genestier-Tamborero, C.N.; Minutti-Palacios, M.; Hirales Tamez, O.; García, C.; Ziegler, D. Treatment with α-lipoic acid over 16 weeks in type 2 diabetic patients with symptomatic polyneuropathy who responded to initial 4-week high-dose loading. J. Diabetes Res. 2015, 2015, 189857. [Google Scholar] [CrossRef] [PubMed]
- El-Nahas, M.R.; Elkannishy, G.; Abdelhafez, H.; Elkhamisy, E.T.; El-Sehrawy, A.A. Oral Alpha Lipoic Acid Treatment for Symptomatic Diabetic Peripheral Neuropathy: A Randomized Double-Blinded Placebo-Controlled Study. Endocr. Metab. Immune Disord.-Drug Targets 2020, 20, 1531–1534. [Google Scholar] [CrossRef]
- Ziegler, D.; Low, P.A.; Litchy, W.J.; Boulton, A.J.; Vinik, A.I.; Freeman, R.; Samigullin, R.; Tritschler, H.; Munzel, U.; Maus, J.; et al. Efficacy and safety of antioxidant treatment with α-lipoic acid over 4 years in diabetic polyneuropathy: The NATHAN 1 trial. Diabetes Care 2011, 34, 2054–2060. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. Br. Med. J. 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef]
- Chi, K.Y.; Li, M.Y.; Chen, C.; Kang, E. Ten circumstances and solutions for finding the sample mean and standard deviation for meta-analysis. Syst. Rev. 2023, 12, 62. [Google Scholar] [CrossRef] [PubMed]
- Ruhnau, K.-J.; Meissner, H.P.; Finn, J.R.; Reljanovic, M.; Lobisch, M.; Schütte, K.; Nehrdich, D.; Tritschler, H.J.; Mehnert, H.; Ziegler, D.; et al. Effects of 3-week oral treatment with the antioxidant thioctic acid (α-lipoic acid) in symptomatic diabetic polyneuropathy. Diabet. Med. 1999, 16, 1040–1043. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, P.R.A.; Kalshetti, S.; Bhatt, J. Supplementation of α-lipoic acid in diabetic peripheral neuropathy: A prospective open label randomized controlled trial. Int. J. Pharm. Pharm. Sci. 2014, 6, 90–93. [Google Scholar]
- Millan-Guerrero, R.O.; García-Ramírez, A.; González-Pérez, O.; Trujillo-Hernández, B.; Isais-Millán, S.; Castillo-Varela, F.J. Efficacy of α-lipoic acid in the treatment of diabetic polyneuropathy. A Randomized, Double-Blind Study. Glob. Adv. Res. J. Med. Med. Sci. 2018, 7, 214–220. Available online: http://garj.org/garjmms (accessed on 7 December 2022). [CrossRef]
- Siddique, N.; Awais, F.F.; Shakil, M.; Sarwar, S.; Kakar, N.H.; Ullah, M.I. Effect of anti-oxidant (α-lipoic acid) treatment on improvement of diabetic neuropathic pain. Rawal Med. J. 2021, 46, 33. Available online: https://www.researchgate.net/publication/350192982 (accessed on 7 December 2022).
- Tang, J.; Wingerchuk, D.M.; Crum, B.A.; Rubin, D.I.; Demaerschalk, B.M. Alpha-lipoic acid may improve symptomatic diabetic polyneuropathy. Neurologist 2007, 13, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, D.; Ametov, A.; Barinov, A.; Dyck, P.J.; Gurieva, I.; Low, P.A.; Munzel, U.; Yakhno, N.; Raz, I.; Novosadova, M.; et al. Oral treatment with α-lipoic acid improves symptomatic diabetic polyneuropathy. Diabetes Care 2006, 29, 2365–2370. [Google Scholar] [CrossRef]
- Weidinger, A.; Kozlov, A.V. Biological Activities of Reactive Oxygen and Nitrogen Species: Oxidative Stress versus Signal Transduction. Biomolecules 2015, 5, 472. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef] [PubMed]
- Kehrer, J.P. The Haber-Weiss reaction and mechanisms of toxicity. Toxicology 2000, 149, 43–50. [Google Scholar] [CrossRef]
- Viana, M.D.M.; Lauria, P.S.S.; de Lima, A.A.; Opretzka, L.C.F.; Marcelino, H.R.; Villarreal, C.F. Alpha-Lipoic Acid as an Antioxidant Strategy for Managing Neuropathic Pain. Antioxidants 2022, 11, 2420. [Google Scholar] [CrossRef]
- Sifuentes-Franco, S.; Pacheco-Moisés, F.P.; Rodríguez-Carrizalez, A.D.; Miranda-Díaz, A.G. The Role of Oxidative Stress, Mitochondrial Function, and Autophagy in Diabetic Polyneuropathy. J. Diabetes Res. 2017, 2017, 1673081. [Google Scholar] [CrossRef]
- Mijnhout, G.S.; Kollen, B.J.; Alkhalaf, A.; Kleefstra, N.; Bilo, H.J.G. Alpha lipoic acid for symptomatic peripheral neuropathy in patients with diabetes: A meta-analysis of randomized controlled trials. Int. J. Endocrinol. 2012, 2012, 456279. [Google Scholar] [CrossRef] [PubMed]
- Florenzo, M.; Cinzia, N.; Giorgio, G.; Valerio, N.; Piera, D.M. Human Bioavailability and Pharmacokinetic Profile of Different Formulations Delivering Alpha Lipoic Acid. Open Access Sci. Rep. 2012, 1, 1–6. [Google Scholar] [CrossRef]
- Backonja, M.; Attal, N.; Baron, R.; Bouhassira, D.; Drangsholt, M.; Dyck, P.J.; Edwards, R.R.; Freeman, R.; Gracely, R.; Haanpaa, M.L.; et al. Value of quantitative sensory testing in neurological and pain disorders: NeuPSIG consensus. Pain 2013, 154, 1807–1819. [Google Scholar] [CrossRef] [PubMed]
- Lauria, G.; Lombardi, R. Recent Advances: Skin biopsy: A new tool for diagnosing peripheral neuropathy. Br. Med. J. 2007, 334, 1159. [Google Scholar] [CrossRef]
- Petropoulos, I.N.; Ponirakis, G.; Ferdousi, M.; Azmi, S.; Kalteniece, A.; Khan, A.; Gad, H.; Bashir, B.; Marshall, A.; Boulton, A.J.; et al. Corneal Confocal Microscopy: A Biomarker for Diabetic Peripheral Neuropathy. Clin. Ther. 2021, 43, 1457–1475. [Google Scholar] [CrossRef]
- Tesfaye, S.; Boulton, A.J.; Dyck, P.J.; Freeman, R.; Horowitz, M.; Kempler, P.; Lauria, G.; Malik, R.A.; Spallone, V.; Vinik, A.; et al. Diabetic neuropathies: Update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010, 33, 2285–2293. [Google Scholar] [CrossRef]
- Ahmed, A.; Bril, V.; Orszag, A.; Paulson, J.; Yeung, E.; Ngo, M.; Orlov, S.; Perkins, B.A. Detection of diabetic sensorimotor polyneuropathy by corneal confocal microscopy in type 1 diabetes: A concurrent validity study. Diabetes Care 2012, 35, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.J.H.; Lovblom, L.E.; Ferdousi, M.; Halpern, E.M.; Jeziorska, M.; Pacaud, D.; Pritchard, N.; Dehghani, C.; Edwards, K.; Srinivasan, S.; et al. Rapid corneal nerve fiber loss: A marker of diabetic neuropathy onset and progression. Diabetes Care 2020, 43, 1829–1835. [Google Scholar] [CrossRef]
- Azmi, S.; Jeziorska, M.; Ferdousi, M.; Petropoulos, I.N.; Ponirakis, G.; Marshall, A.; Alam, U.; Asghar, O.; Atkinson, A.; Jones, W.; et al. Early nerve fibre regeneration in individuals with type 1 diabetes after simultaneous pancreas and kidney transplantation. Diabetologia 2019, 62, 1478–1487. [Google Scholar] [CrossRef]
- Bril, V.; Breiner, A.; Perkins, B.A.; Zochodne, D. 2018 Clinical Practice Guidelines Neuropathy Diabetes Canada Clinical Practice Guidelines Expert Committee. Can. J. Diabetes 2018, 42, S217–S221. [Google Scholar] [CrossRef] [PubMed]
- Feldman, E.L.; Callaghan, B.C.; Pop-Busui, R.; Zochodne, D.W.; Wright, D.E.; Bennett, D.L.; Bril, V.; Russell, J.W.; Viswanathan, V. Diabetic neuropathy. Nat. Rev. Dis. Primers 2019, 5, 41. [Google Scholar] [CrossRef] [PubMed]
- Price, R.; Smith, D.; Franklin, G.; Gronseth, G.; Pignone, M.; David, W.S.; Armon, C.; Perkins, B.A.; Bril, V.; Rae-Grant, A.; et al. Oral and Topical Treatment of Painful Diabetic Polyneuropathy: Practice Guideline Update Summary: Report of the AAN Guideline Subcommittee. Neurology 2022, 98, 31–43. [Google Scholar] [CrossRef] [PubMed]


| Frequency/Severity | Absent | Mild | Moderate | Severe |
|---|---|---|---|---|
| Occasionally | 0 | 1.00 | 2.00 | 3.00 |
| Often | 0 | 1.33 | 2.33 | 3.33 |
| Continuously | 0 | 1.66 | 2.66 | 3.66 |
| Exam | Score |
|---|---|
| Vibration (128-tuning fork) | 0 = present, 1 = reduce/absent |
| Temperature (cold-tuning fork) | 0 = present, 1 = reduce/absent |
| Pinprick | 0 = present, 1 = reduce/absent |
| Ankle reflex | 0 = normal, 1 = present with reinforcement, 2 = absent |
| Study | Female, % | Age (Mean, Year) | A1c (Mean, %) | DM Duration (Mean, Year) | ALA/Placebo, n/N | Dosage, mg | Length, Week | Measures |
|---|---|---|---|---|---|---|---|---|
| 2003 | 68% | 56.1 1 | - 2 | 14.55 | 60/60 | 600 | 14 | TSS, NIS, NIS-LL, global satisfaction, NCS |
| Ametov [14] | ||||||||
| 2020 | 61% | 53.4 | 8.2 | 11.2 | 100/100 | 1200 | 24 | NDS, VPT, VAS |
| El Nahas [19] | ||||||||
| 2015 | 67% | 58.27 | 8.68 | 11.74 | 16/17 | 600 | 20 | TSS, VPT |
| Garcia-Alcala [18] | ||||||||
| 2014 | 25% | 55.2 | - | 12.5 | 10/10 | 600 | 12 | NCS |
| Vijayakumar [25] | ||||||||
| 2018 | 60% | 50.89 | - | 10.13 | 51/49 | 1200 | 4 | NCS |
| Millan-Guerrero [26] | ||||||||
| 1999 | 50% | 61.3 | 7.4 | 11.5 | 12/12 | 1800 | 3 | TSS, NDS |
| Ruhnau [24] | ||||||||
| 2021 | 49% | 46.88 | 8.45 | 10.64 | 55/55 | 600 | 24 | TSS |
| Siddique [27] | ||||||||
| 2007 | 60% | 57.78 | 7.7 | 14 | 138/43 | 600, 1200, 1800 | 5 | global satisfaction |
| Tang [28] | ||||||||
| 2006 | 60% | 57.78 | 7.7 | 14 | 138/43 | 600, 1200, 1800 | 5 | TSS, NSC, NIS, NIS-LL, global satisfaction |
| Ziegler [29] | ||||||||
| 2011 | 67% | 53.6 | 8.85 | 13.4 | 230/224 | 600 | 104 | TSS, NIS, NIS-LL, VPT, NCS |
| Ziegler [20] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, R.-Y.; Huang, I.-C.; Chen, C.; Sung, J.-Y. Effects of Oral Alpha-Lipoic Acid Treatment on Diabetic Polyneuropathy: A Meta-Analysis and Systematic Review. Nutrients 2023, 15, 3634. https://doi.org/10.3390/nu15163634
Hsieh R-Y, Huang I-C, Chen C, Sung J-Y. Effects of Oral Alpha-Lipoic Acid Treatment on Diabetic Polyneuropathy: A Meta-Analysis and Systematic Review. Nutrients. 2023; 15(16):3634. https://doi.org/10.3390/nu15163634
Chicago/Turabian StyleHsieh, Ruey-Yu, I-Chen Huang, Chiehfeng Chen, and Jia-Ying Sung. 2023. "Effects of Oral Alpha-Lipoic Acid Treatment on Diabetic Polyneuropathy: A Meta-Analysis and Systematic Review" Nutrients 15, no. 16: 3634. https://doi.org/10.3390/nu15163634
APA StyleHsieh, R.-Y., Huang, I.-C., Chen, C., & Sung, J.-Y. (2023). Effects of Oral Alpha-Lipoic Acid Treatment on Diabetic Polyneuropathy: A Meta-Analysis and Systematic Review. Nutrients, 15(16), 3634. https://doi.org/10.3390/nu15163634






