One Season in Professional Cycling Is Enough to Negatively Affect Bone Health
Abstract
1. Introduction
2. Methodology
2.1. Subjects of Study
2.2. Study Protocol
2.3. Dual-Energy X-ray Absorptiometry (DXA)
2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klomsten Andersen, O.; Clarsen, B.; Garthe, I.; Mørland, M.; Stensrud, T. Bone health in elite Norwegian endurance cyclists and runners: A cross-sectional study. BMJ Open Sport Exerc. Med. 2018, 4, e000449. [Google Scholar] [CrossRef]
- Martínez-Noguera, F.J.; Alcaraz, P.E.; Ortolano-Ríos, R.; Dufour, S.; Marín-Pagán, C. Professional cyclists have lower levels of bone markers than amateurs. Is there a risk of osteoporosis in cyclist? Bone 2021, 153, 116102. [Google Scholar] [CrossRef]
- Scofield, K.L.; Hecht, S. Bone health in endurance athletes: Runners, cyclists, and swimmers. Curr. Sports Med. Rep. 2012, 11, 328–334. [Google Scholar] [CrossRef]
- Ubago-Guisado, E.; Vlachopoulos, D.; Fatouros, I.G.; Deli, C.K.; Leontsini, D.; Moreno, L.A.; Courteix, D.; Gracia-Marco, L. Longitudinal determinants of 12-month changes on bone health in adolescent male athletes. Arch. Osteoporos. 2018, 13, 106. [Google Scholar] [CrossRef]
- Vlachopoulos, D.; Barker, A.R.; Ubago-Guisado, E.; Fatouros, I.G.; Knapp, K.M.; Williams, C.A.; Gracia-Marco, L. Longitudinal Adaptations of Bone Mass, Geometry, and Metabolism in Adolescent Male Athletes: The PRO-BONE Study. J. Bone Miner. Res. 2017, 32, 2269–2277. [Google Scholar] [CrossRef]
- Vlachopoulos, D.; Barker, A.R.; Williams, C.A.; ArngríMsson, S.A.; Knapp, K.M.; Metcalf, B.S.; Fatouros, I.G.; Moreno, L.A.; Gracia-Marco, L. The Impact of Sport Participation on Bone Mass and Geometry in Male Adolescents. Med. Sci. Sports Exerc. 2017, 49, 317–326. [Google Scholar] [CrossRef]
- Vlachopoulos, D.; Ubago-Guisado, E.; Barker, A.R.; Metcalf, B.S.; Fatouros, I.G.; Avloniti, A.; Knapp, K.M.; Moreno, L.A.; Williams, C.A.; Gracia-Marco, L. Determinants of Bone Outcomes in Adolescent Athletes at Baseline: The PRO-BONE Study. Med. Sci. Sports Exerc. 2017, 49, 1389–1396. [Google Scholar] [CrossRef]
- Kohrt, W.M.; Barry, D.W.; Schwartz, R.S. Muscle forces or gravity: What predominates mechanical loading on bone? Med. Sci. Sports Exerc. 2009, 41, 2050–2055. [Google Scholar] [CrossRef]
- Loucks, A.B.; Kiens, B.; Wright, H.H. Energy availability in athletes. J. Sports Sci. 2011, 29, S7–S15. [Google Scholar] [CrossRef]
- Moro, T.; Tinsley, G.; Longo, G.; Grigoletto, D.; Bianco, A.; Ferraris, C.; Guglielmetti, M.; Veneto, A.; Tagliabue, A.; Marcolin, G.; et al. Time-restricted eating effects on performance, immune function, and body composition in elite cyclists: A randomized controlled trial. J. Int. Soc. Sports Nutr. 2020, 17, 65. [Google Scholar] [CrossRef]
- Mountjoy, M.; Sundgot-Borgen, J.; Burke, L.; Ackerman, K.E.; Blauwet, C.; Constantini, N.; Lebrun, C.; Lundy, B.; Melin, A.; Meyer, N.; et al. International Olympic Committee (IOC) Consensus Statement on Relative Energy Deficiency in Sport (RED-S): 2018 Update. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 316–331. [Google Scholar] [CrossRef]
- Burke, L.M.; Close, G.L.; Lundy, B.; Mooses, M.; Morton, J.P.; Tenforde, A.S. Relative Energy Deficiency in Sport in Male Athletes: A Commentary on Its Presentation Among Selected Groups of Male Athletes. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 364–374. [Google Scholar] [CrossRef]
- De Souza, M.J.; Koltun, K.J.; Williams, N.I. The Role of Energy Availability in Reproductive Function in the Female Athlete Triad and Extension of its Effects to Men: An Initial Working Model of a Similar Syndrome in Male Athletes. Sports Med. 2019, 49, 125–137. [Google Scholar] [CrossRef]
- Barry, D.W.; Hansen, K.C.; van Pelt, R.E.; Witten, M.; Wolfe, P.; Kohrt, W.M. Acute calcium ingestion attenuates exercise-induced disruption of calcium homeostasis. Med. Sci. Sports Exerc. 2011, 43, 617–623. [Google Scholar] [CrossRef]
- Hilkens, L.; Knuiman, P.; Heijboer, M.; Kempers, R.; Jeukendrup, A.E.; van Loon, L.J.C.; van Dijk, J.W. Fragile bones of elite cyclists: To treat or not to treat? J. Appl. Physiol. 2021, 131, 26–28. [Google Scholar] [CrossRef]
- Barry, D.W.; Kohrt, W.M. BMD decreases over the course of a year in competitive male cyclists. J. Bone Miner. Res. 2008, 23, 484–491. [Google Scholar] [CrossRef]
- Kohrt, W.M.; Wolfe, P.; Sherk, V.D.; Wherry, S.J.; Wellington, T.; Melanson, E.L.; Swanson, C.M.; Weaver, C.M.; Boxer, R.S. Dermal Calcium Loss Is Not the Primary Determinant of Parathyroid Hormone Secretion during Exercise. Med. Sci. Sports Exerc. 2019, 51, 2117–2124. [Google Scholar] [CrossRef]
- Mountjoy, M.; Sundgot-Borgen, J.K.; Burke, L.M.; Ackerman, K.E.; Blauwet, C.; Constantini, N.; Lebrun, C.; Lundy, B.; Melin, A.K.; Meyer, N.L.; et al. IOC consensus statement on relative energy deficiency in sport (RED-S): 2018 update. Br. J. Sports Med. 2018, 52, 687–697. [Google Scholar] [CrossRef]
- Nattiv, A.; Loucks, A.B.; Manore, M.M.; Sanborn, C.F.; Sundgot-Borgen, J.; Warren, M.P. American College of Sports Medicine position stand. The female athlete triad. Med. Sci. Sports Exerc. 2007, 39, 1867–1882. [Google Scholar] [CrossRef]
- Elliott-Sale, K.J.; Tenforde, A.S.; Parziale, A.L.; Holtzman, B.; Ackerman, K.E. Endocrine Effects of Relative Energy Deficiency in Sport. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 335–349. [Google Scholar] [CrossRef]
- Olmedillas, H.; González-Agüero, A.; Moreno, L.A.; Casajus, J.A.; Vicente-Rodríguez, G. Cycling and bone health: A systematic review. BMC Med. 2012, 10, 168. [Google Scholar] [CrossRef]
- Oja, P.; Titze, S.; Bauman, A.; de Geus, B.; Krenn, P.; Reger-Nash, B.; Kohlberger, T. Health benefits of cycling: A systematic review. Scand. J. Med. Sci. Sports 2011, 21, 496–509. [Google Scholar] [CrossRef]
- Nagle, K.B.; Brooks, M.A. A Systematic Review of Bone Health in Cyclists. Sports Health 2011, 3, 235–243. [Google Scholar] [CrossRef]
- Medelli, J.; Lounana, J.; Menuet, J.J.; Shabani, M.; Cordero-MacIntyre, Z. Is osteopenia a health risk in professional cyclists? J. Clin. Densitom. 2009, 12, 28–34. [Google Scholar] [CrossRef]
- Peck, W.A. Consensus development conference: Diagnosis, prophylaxis, and treatment of osteoporosis. Am. J. Med. 1993, 94, 646–650. [Google Scholar] [CrossRef]
- Baker, B.S.; Reiser, R.F., 2nd. Longitudinal Assessment of Bone Mineral Density and Body Composition in Competitive Cyclists. J. Strength Cond. Res. 2017, 31, 2969–2976. [Google Scholar] [CrossRef]
- De Helsinki, D. Principios éticos para las investigaciones médicas en seres humanos. Asoc. Médica Mund. 2013, 59. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: New York, NY, USA, 2013. [Google Scholar]
- González-Agüero, A.; Olmedillas, H.; Gómez-Cabello, A.; Casajús, J.A.; Vicente-Rodríguez, G. Bone Structure and Geometric Properties at the Radius and Tibia in Adolescent Endurance-Trained Cyclists. Clin. J. Sport Med. 2017, 27, 69–77. [Google Scholar] [CrossRef]
- Glaser, D.L.; Kaplan, F.S. Osteoporosis. Definition and clinical presentation. Spine 1997, 22, 12s–16s. [Google Scholar] [CrossRef]
- Doyle, F.; Brown, J.; Lachance, C. Relation between bone mass and muscle weight. Lancet 1970, 1, 391–393. [Google Scholar] [CrossRef]
- Lane, H.W. Nutrition in space: Evidence from the U.S. and the U.S.S.R. Nutr. Rev. 1992, 50, 3–6. [Google Scholar] [CrossRef]
- May, H.; Murphy, S.; Khaw, K.T. Age-associated bone loss in men and women and its relationship to weight. Age Ageing 1994, 23, 235–240. [Google Scholar] [CrossRef]
- Leib, E.S.; Lewiecki, E.M.; Binkley, N.; Hamdy, R.C. Official positions of the International Society for Clinical Densitometry. J. Clin. Endocrinol. Metab. 2004, 7, 3651–3655. [Google Scholar] [CrossRef]
- Kanis, J.A.; Delmas, P.; Burckhardt, P.; Cooper, C.; Torgerson, D. Guidelines for diagnosis and management of osteoporosis. The European Foundation for Osteoporosis and Bone Disease. Osteoporos. Int. 1997, 7, 390–406. [Google Scholar] [CrossRef]
- Nichols, J.F.; Palmer, J.E.; Levy, S.S. Low bone mineral density in highly trained male master cyclists. Osteoporos. Int. 2003, 14, 644–649. [Google Scholar] [CrossRef]
- Smathers, A.M.; Bemben, M.G.; Bemben, D.A. Bone density comparisons in male competitive road cyclists and untrained controls. Med. Sci. Sports Exerc. 2009, 41, 290–296. [Google Scholar] [CrossRef]
- Judex, S.; Gupta, S.; Rubin, C. Regulation of mechanical signals in bone. Orthod. Craniofacial Res. 2009, 12, 94–104. [Google Scholar] [CrossRef]
- Milgrom, C.; Finestone, A.; Simkin, A.; Ekenman, I.; Mendelson, S.; Millgram, M.; Nyska, M.; Larsson, E.; Burr, D. In-vivo strain measurements to evaluate the strengthening potential of exercises on the tibial bone. J. Bone Jt. Surg. Br. Vol. 2000, 82, 591–594. [Google Scholar] [CrossRef]
- Weaver, C.M.; Gordon, C.M.; Janz, K.F.; Kalkwarf, H.J.; Lappe, J.M.; Lewis, R.; O’Karma, M.; Wallace, T.C.; Zemel, B.S. The National Osteoporosis Foundation’s position statement on peak bone mass development and lifestyle factors: A systematic review and implementation recommendations. Osteoporos. Int. 2016, 27, 1281–1386. [Google Scholar] [CrossRef]
- Santos, L.; Elliott-Sale, K.J.; Sale, C. Exercise and bone health across the lifespan. Biogerontology 2017, 18, 931–946. [Google Scholar] [CrossRef]
- Viner, R.T.; Harris, M.; Berning, J.R.; Meyer, N.L. Energy Availability and Dietary Patterns of Adult Male and Female Competitive Cyclists With Lower Than Expected Bone Mineral Density. Int. J. Sports Nutr. Exerc. Metab. 2015, 25, 594–602. [Google Scholar] [CrossRef]
- Ducher, G.; Turner, A.I.; Kukuljan, S.; Pantano, K.J.; Carlson, J.L.; Williams, N.I.; De Souza, M.J. Obstacles in the optimization of bone health outcomes in the female athlete triad. Sports Med. 2011, 41, 587–607. [Google Scholar] [CrossRef]
- Loucks, A.B. Low energy availability in the marathon and other endurance sports. Sports Med. 2007, 37, 348–352. [Google Scholar] [CrossRef]
- Lloyd, T.; Triantafyllou, S.J.; Baker, E.R.; Houts, P.S.; Whiteside, J.A.; Kalenak, A.; Stumpf, P.G. Women athletes with menstrual irregularity have increased musculoskeletal injuries. Med. Sci. Sports Exerc. 1986, 18, 374–379. [Google Scholar] [CrossRef]
- Huhmann, K. Menses Requires Energy: A Review of How Disordered Eating, Excessive Exercise, and High Stress Lead to Menstrual Irregularities. Clin. Ther. 2020, 42, 401–407. [Google Scholar] [CrossRef]
- Caronia, L.M.; Martin, C.; Welt, C.K.; Sykiotis, G.P.; Quinton, R.; Thambundit, A.; Avbelj, M.; Dhruvakumar, S.; Plummer, L.; Hughes, V.A.; et al. A genetic basis for functional hypothalamic amenorrhea. N. Engl. J. Med. 2011, 364, 215–225. [Google Scholar] [CrossRef]
- Hackney, A.C.; Fahrner, C.L.; Gulledge, T.P. Basal reproductive hormonal profiles are altered in endurance trained men. J. Sports Med. Phys. Fit. 1998, 38, 138–141. [Google Scholar]
- McColl, E.M.; Wheeler, G.D.; Gomes, P.; Bhambhani, Y.; Cumming, D.C. The effects of acute exercise on pulsatile LH release in high-mileage male runners. Clin. Endocrinol. 1989, 31, 617–621. [Google Scholar] [CrossRef]
- MacConnie, S.E.; Barkan, A.; Lampman, R.M.; Schork, M.A.; Beitins, I.Z. Decreased hypothalamic gonadotropin-releasing hormone secretion in male marathon runners. N. Engl. J. Med. 1986, 315, 411–417. [Google Scholar] [CrossRef]
- Hackney, A.C. Endurance training and testosterone levels. Sports Med. 1989, 8, 117–127. [Google Scholar] [CrossRef]
- Wheeler, G.D.; Wall, S.R.; Belcastro, A.N.; Cumming, D.C. Reduced serum testosterone and prolactin levels in male distance runners. Jama 1984, 252, 514–516. [Google Scholar] [CrossRef]
- Hackney, A.; Moore, A.; Brownlee, K. Testosterone and endurance exercise: Development of the âεœexercise-hypogonadal male conditionâε□. Acta Physiol. Hung. 2005, 92, 121–137. [Google Scholar] [CrossRef]
- Kraemer, W.J.; Fragala, M.S.; Watson, G.; Volek, J.S.; Rubin, M.; French, D.; Maresh, C.; Vingren, J.; Hatfield, D.; Spiering, B. Hormonal responses to a 160-km race across frozen Alaska. Br. J. Sports Med. 2008, 42, 116–120. [Google Scholar] [CrossRef]
- Kupchak, B.R.; Kraemer, W.J.; Hoffman, M.D.; Phinney, S.D.; Volek, J.S. The impact of an ultramarathon on hormonal and biochemical parameters in men. Wilderness Environ. Med. 2014, 25, 278–288. [Google Scholar] [CrossRef]
- Keay, N.; Francis, G.; Hind, K. Low energy availability assessed by a sport-specific questionnaire and clinical interview indicative of bone health, endocrine profile and cycling performance in competitive male cyclists. BMJ Open Sport Exerc. Med. 2018, 4, e000424. [Google Scholar] [CrossRef]
- Fensham, N.C.; Heikura, I.A.; McKay, A.K.A.; Tee, N.; Ackerman, K.E.; Burke, L.M. Short-term carbohydrate restriction impairs bone formation at rest and during prolonged exercise to a greater degree than low energy availability. J. Bone Miner. Res. 2022, 37, 1915–1925. [Google Scholar] [CrossRef]
- Lombardi, G.; Lanteri, P.; Graziani, R.; Colombini, A.; Banfi, G.; Corsetti, R. Bone and energy metabolism parameters in professional cyclists during the Giro d’Italia 3-weeks stage race. PLoS ONE 2012, 7, e42077. [Google Scholar] [CrossRef]
- Mouzopoulos, G.; Stamatakos, M.; Tzurbakis, M.; Tsembeli, A.; Manti, C.; Safioleas, M.; Skandalakis, P. Changes of bone turnover markers after marathon running over 245 km. Int. J. Sports Med. 2007, 28, 576–579. [Google Scholar] [CrossRef]
- Oosthuyse, T.; Badenhorst, M.; Avidon, I. Bone resorption is suppressed immediately after the third and fourth days of multiday cycling but persistently increased following overnight recovery. Appl. Physiol. Nutr. Metab. 2014, 39, 64–73. [Google Scholar] [CrossRef]
- Barry, D.W.; Kohrt, W.M. Acute effects of 2 hours of moderate-intensity cycling on serum parathyroid hormone and calcium. Calcif. Tissue Int. 2007, 80, 359–365. [Google Scholar] [CrossRef]
- Hutson, M.; Blagrove, R.; O’Donnell, E.; Brooke-Wavell, K. Commentaries on Viewpoint: Fragile bones of elite cyclists: To treat or not to treat? J. Appl. Physiol. 2021, 131, 29–33. [Google Scholar] [CrossRef]
- Hutson, M.J.; O’Donnell, E.; Brooke-Wavell, K.; Sale, C.; Blagrove, R.C. Effects of Low Energy Availability on Bone Health in Endurance Athletes and High-Impact Exercise as A Potential Countermeasure: A Narrative Review. Sports Med. 2021, 51, 391–403. [Google Scholar] [CrossRef]
- Myburgh, K.H.; Hutchins, J.; Fataar, A.B.; Hough, S.F.; Noakes, T.D. Low bone density is an etiologic factor for stress fractures in athletes. Ann. Intern. Med. 1990, 113, 754–759. [Google Scholar] [CrossRef]
- Sale, C.; Elliott-Sale, K.J. Nutrition and Athlete Bone Health. Sports Med. 2019, 49, 139–151. [Google Scholar] [CrossRef]
- Yao, P.; Bennett, D.; Mafham, M.; Lin, X.; Chen, Z.; Armitage, J.; Clarke, R. Vitamin D and Calcium for the Prevention of Fracture: A Systematic Review and Meta-analysis. JAMA Netw. Open 2019, 2, e1917789. [Google Scholar] [CrossRef]
- Jin, J. Vitamin D and Calcium Supplements for Preventing Fractures. JAMA 2018, 319, 1630. [Google Scholar] [CrossRef]
- König, D.; Oesser, S.; Scharla, S.; Zdzieblik, D.; Gollhofer, A. Specific Collagen Peptides Improve Bone Mineral Density and Bone Markers in Postmenopausal Women-A Randomized Controlled Study. Nutrients 2018, 10, 97. [Google Scholar] [CrossRef]
- Shaw, G.; Lee-Barthel, A.; Ross, M.L.; Wang, B.; Baar, K. Vitamin C-enriched gelatin supplementation before intermittent activity augments collagen synthesis. Am. J. Clin. Nutr. 2017, 105, 136–143. [Google Scholar] [CrossRef]




| Characteristics | |
|---|---|
| Age (years) | 27.3 (3.40) |
| Body mass (kg) | 72.7 (5.98) |
| Height (cm) | 180.0 (5.89) |
| FM (kg) | 5.01 (1.74) |
| FFM (kg) | 64.7 (4.88) |
| 2018 | 2019 | Time p-Value | ES | |
|---|---|---|---|---|
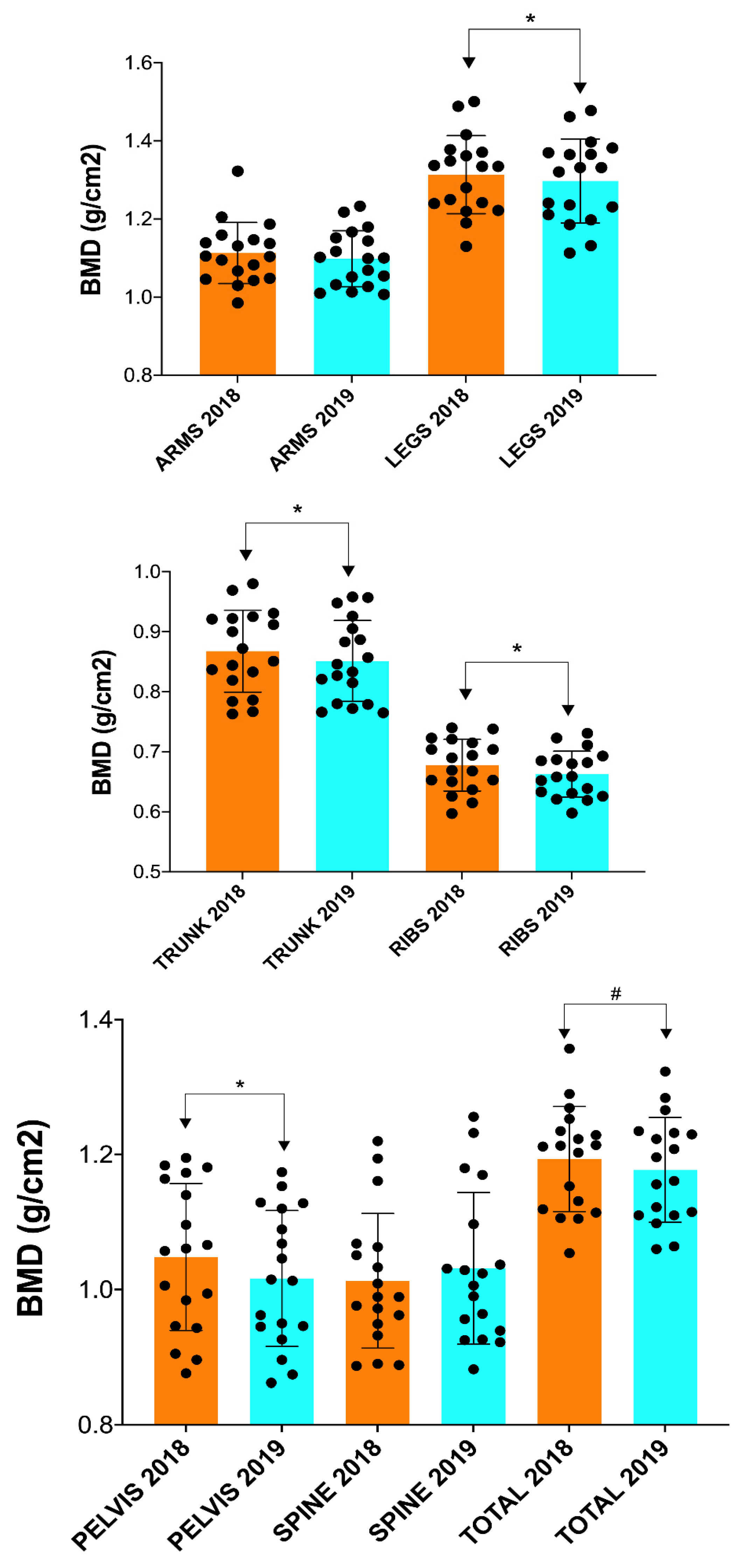
| BMD Arms | 1.11 (0.08) | 1.10 (0.07) | 0.102 | 0.407 |
| BMD Legs | 1.31 (0.10) | 1.30 (0.11) | 0.017 * | 0.504 |
| BMD Trunk | 0.868 (0.07) | 0.851 (0.07) | 0.012 * | 0.489 |
| BMD Ribs | 0.678 (0.04) | 0.663 (0.04) | 0.003 * | 0.814 |
| BMD Pelvis | 1.05 (0.11) | 1.02 (0.10) | 0.001 * | 0.811 |
| BMD Spine | 1.01 (0.10) | 1.03 (0.11) | 0.081 | 0.278 |
| BMD Total | 1.19 (0.08) | 1.18 (0.08) | 0.061 | 0.410 |
| BMC Arms | 434 (51.4) | 452 (66.7) | 0.033 * | 0.320 |
| BMC Legs | 1213 (106) | 1195 (107) | 0.663 | 0.235 |
| BMC Trunk | 722 (125) | 711 (130) | 0.260 | 0.275 |
| BMC Ribs | 262 (49.5) | 265 (62.9) | 0.499 | 0.092 |
| BMC Pelvis | 248 (52.2) | 244 (53.4) | 0.427 | 0.192 |
| BMC Spine | 212 (36.5) | 201 (38.5) | 0.003 * | 0.648 |
| BMC Total | 2879 (315) | 2869 (322) | 0.187 | 0.324 |
| BA Arms | 390 (34.0) | 400 (30.9) | 0.023 * | 0.587 |
| BA Legs | 923 (44.2) | 906 (134) | 0.257 | 0.139 |
| BA Trunk | 827 (87.3) | 832 (123.0) | 0.459 | 0.063 |
| BA Ribs | 385 (53.5) | 399 (87.5) | 0.528 | 0.242 |
| BA Pelvis | 235 (30.1) | 239 (40.7) | 0.794 | 0.176 |
| BA Spine | 208 (20.2) | 194 (24.1) | 0.003 * | 0.828 |
| BA Total | 2391 (147) | 2436 (190) | 0.138 | 0.396 |
| FM Arm left | 221 (76.0) | 232 (94.7) | 0.337 | 0.233 |
| FM Arm right | 241 (80.9) | 249 (99.8) | 0.446 | 0.184 |
| FM Trunk | 2151(741) | 2008 (657) | 0.459 | 0.291 |
| FM Leg left | 1025 (445) | 997 (422) | 0.865 | 0.120 |
| FM Leg right | 1041 (445) | 1021 (442) | 0.865 | 0.084 |
| Fat Mass Total | 5014 (1737) | 4835 (1715) | 0.640 | 0.177 |
| FFM Arm left | 3645 (417) | 3719 (380) | 0.097 | 0.414 |
| FFM Arm right | 3976 (498) | 4009 (392) | 0.535 | 0.149 |
| FFM Trunk | 27,394 (2355) | 27,290 (2267) | 0.638 | 0.113 |
| FFM Leg left | 12,463 (937) | 12,527 (895) | 0.470 | 0.174 |
| FFM Leg right | 12,696 (934) | 12,811 (865) | 0.159 | 0.348 |
| Fat-Free Mass Total | 64,722 (4883) | 64,880 (4636) | 0.629 | 0.116 |
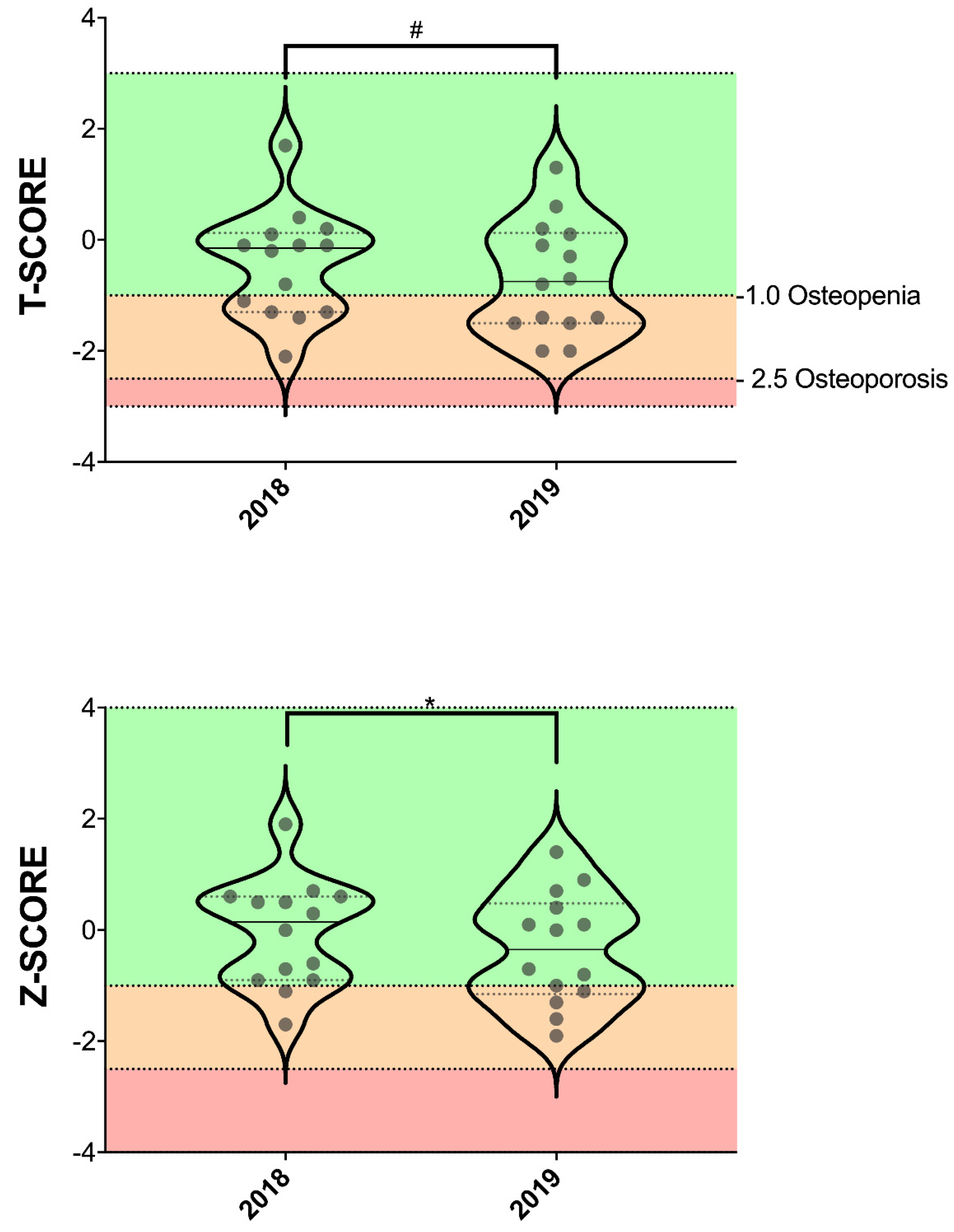
| T-score | −0.436 (0.965) | −0.679 (1.012) | 0.061 | 0.462 |
| Z-score | −0.057 (0.958) | −0.343 (1.000) | 0.021 * | 0.556 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Noguera, F.J.; Alcaraz, P.E.; Ortolano-Ríos, R.; Marín-Pagán, C. One Season in Professional Cycling Is Enough to Negatively Affect Bone Health. Nutrients 2023, 15, 3632. https://doi.org/10.3390/nu15163632
Martínez-Noguera FJ, Alcaraz PE, Ortolano-Ríos R, Marín-Pagán C. One Season in Professional Cycling Is Enough to Negatively Affect Bone Health. Nutrients. 2023; 15(16):3632. https://doi.org/10.3390/nu15163632
Chicago/Turabian StyleMartínez-Noguera, Francisco Javier, Pedro E. Alcaraz, Raquel Ortolano-Ríos, and Cristian Marín-Pagán. 2023. "One Season in Professional Cycling Is Enough to Negatively Affect Bone Health" Nutrients 15, no. 16: 3632. https://doi.org/10.3390/nu15163632
APA StyleMartínez-Noguera, F. J., Alcaraz, P. E., Ortolano-Ríos, R., & Marín-Pagán, C. (2023). One Season in Professional Cycling Is Enough to Negatively Affect Bone Health. Nutrients, 15(16), 3632. https://doi.org/10.3390/nu15163632








