Vitamin D Enhances Immune Effector Pathways of NK Cells Thus Providing a Mechanistic Explanation for the Increased Effectiveness of Therapeutic Monoclonal Antibodies
Abstract
1. Introduction
2. Materials and Methods
2.1. Treatment
2.2. Isolation of NK Cells
2.3. RNA Extraction
2.4. Expression Analysis per Microarray
2.5. Validation
2.6. Statistical Analysis
3. Results
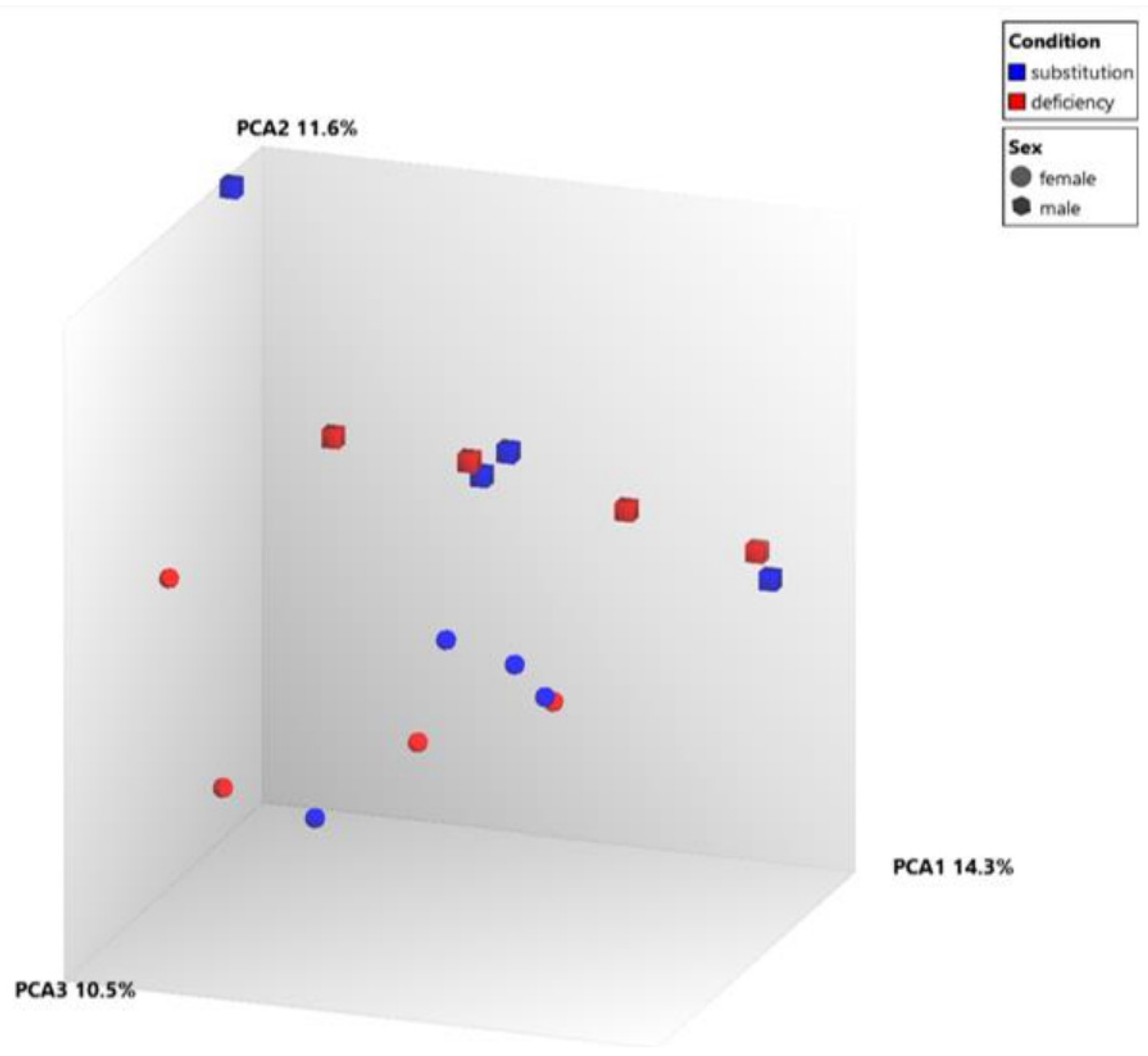
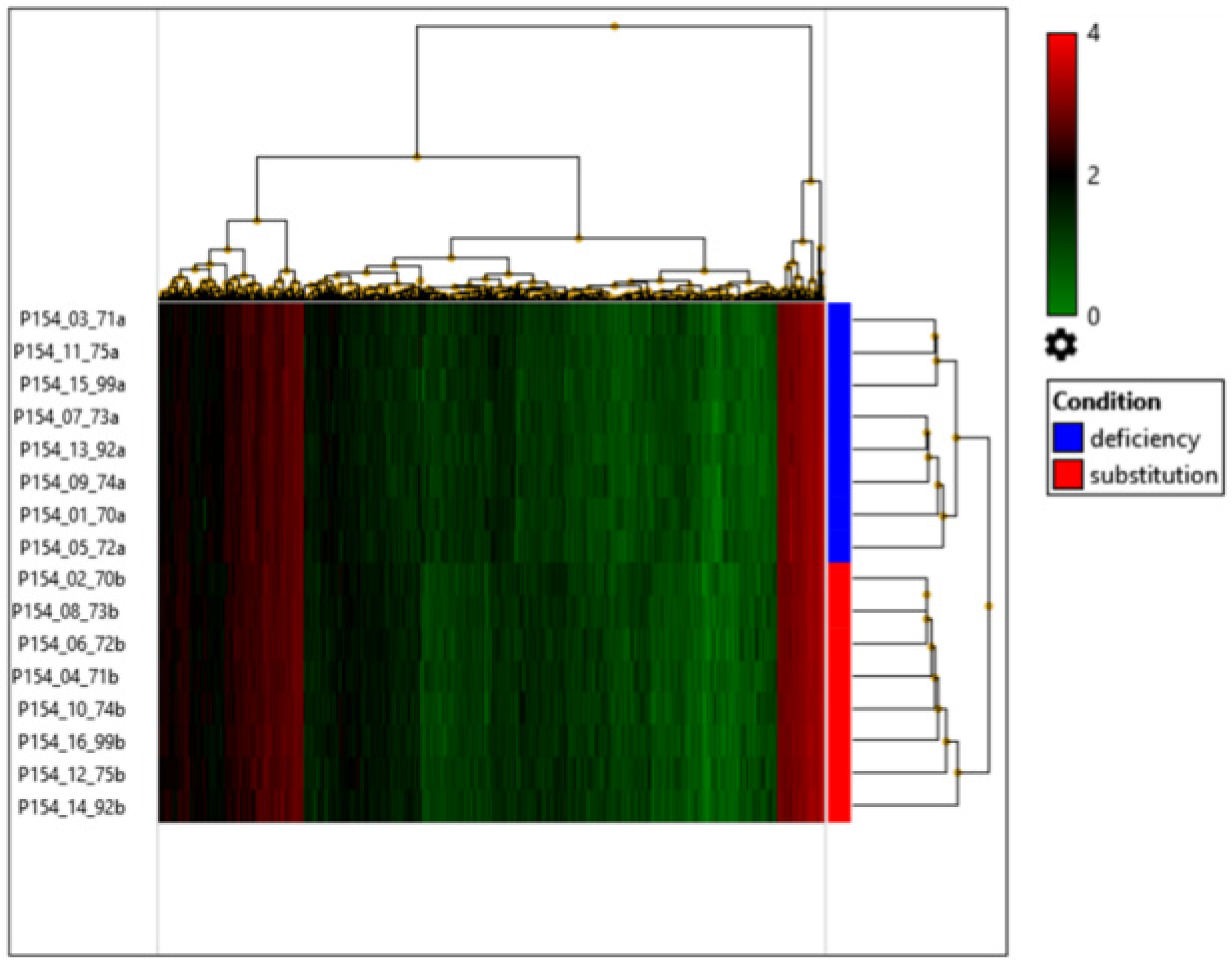
3.1. Gene Expression Changes in Vitamin-D-Supplemented Volunteers
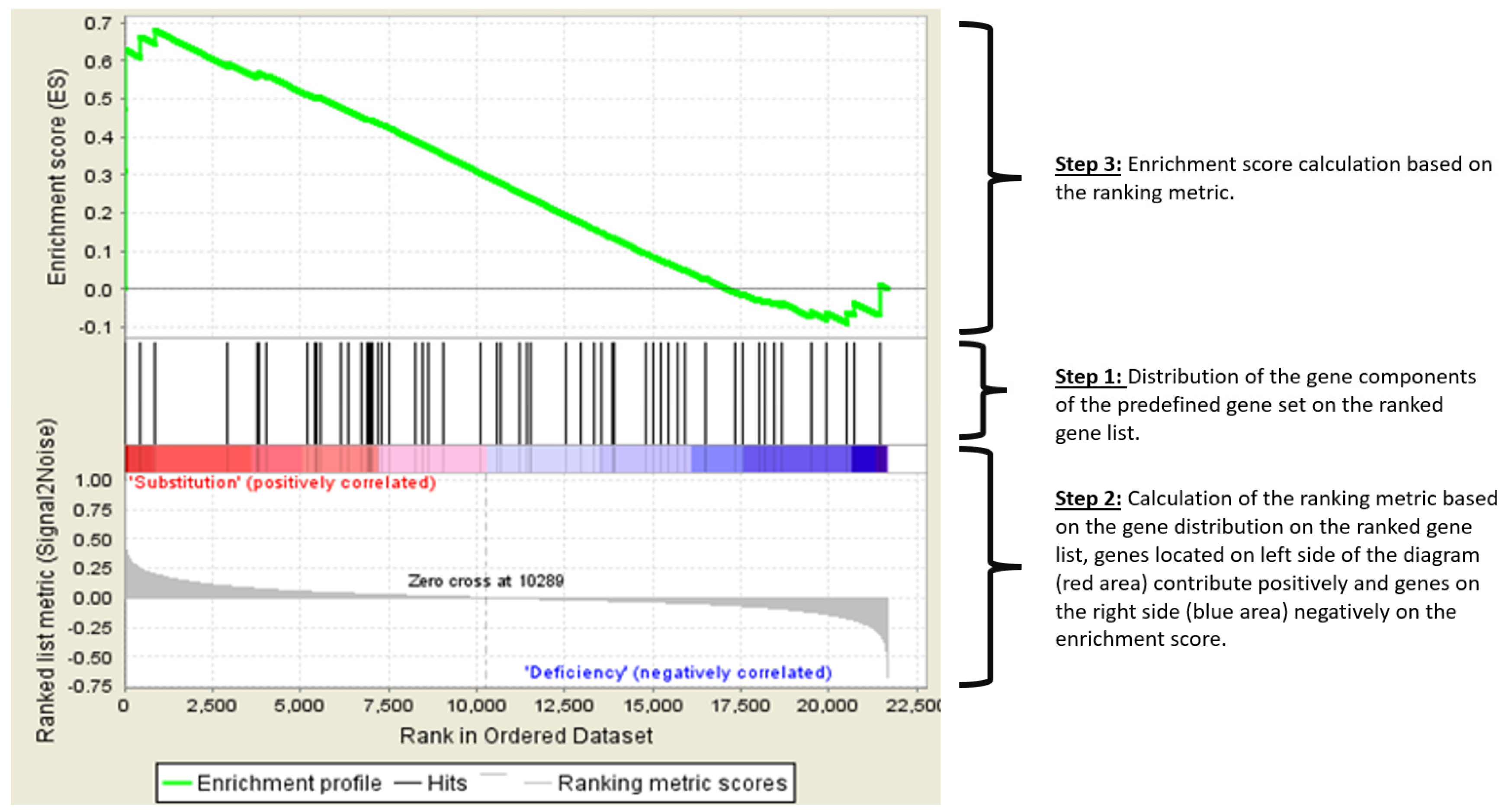
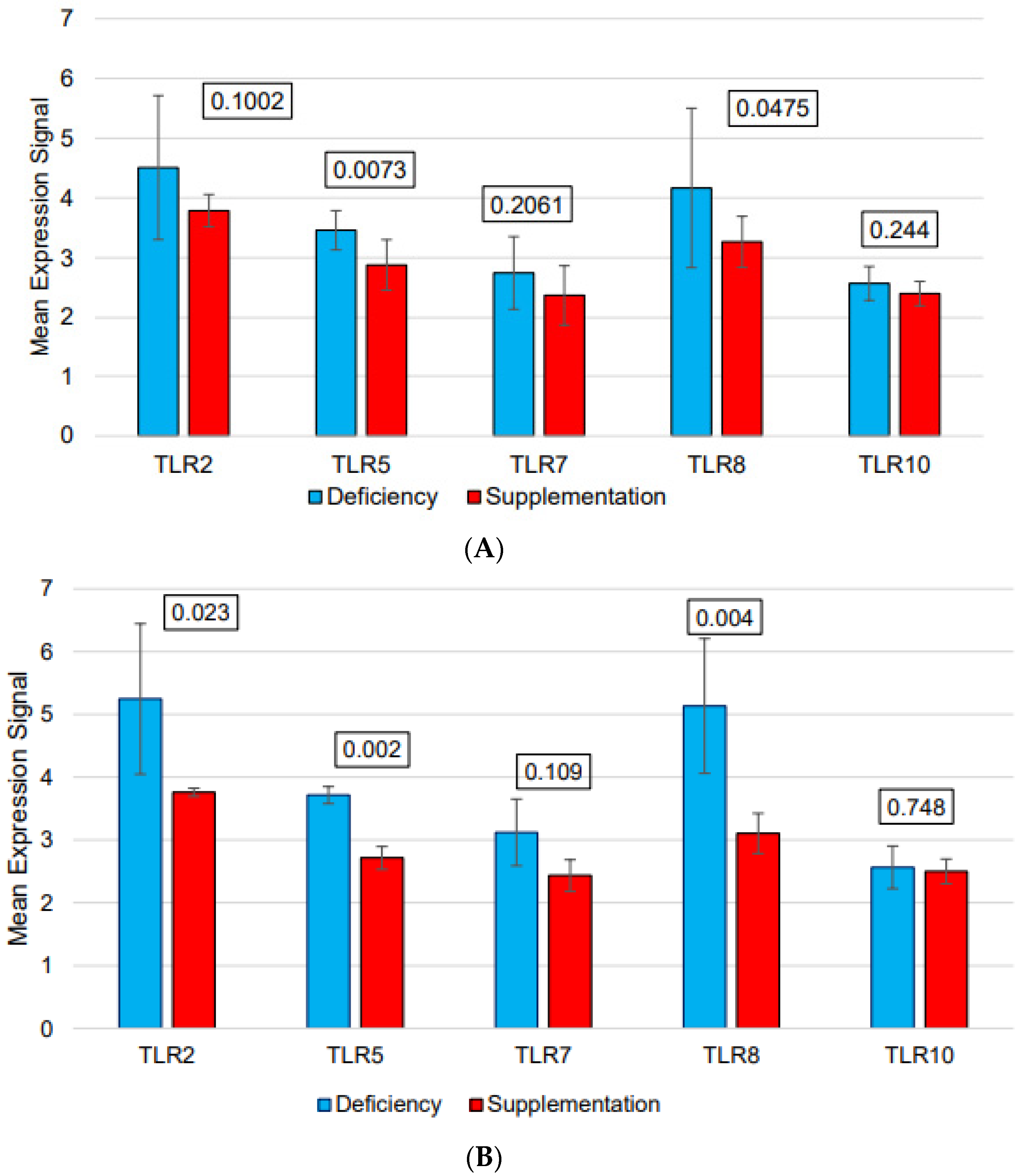
3.2. Pathway Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADCC | Antibody-dependent cellular cytotoxicity |
| ANOVA | Analysis of variance |
| FcyIIIA | low affinity IgG receptor |
| FcεR1 | High affinity IgE receptor |
| GO | Gene ontology |
| GSEA | Gene set enrichment analysis |
| HER2 | Human epidermal growth factor receptor 2 |
| IFN | Interferon |
| KEGG | Kyoto encyclopedia of genes and genomes |
| mAb | Monoclonal antibody |
| NK | Natural killer |
| PBS | phosphate-buffered saline |
| PBMC | peripheral blood mononucleated cell |
| TLR | toll-like receptor |
| qRT-PCR | quantitative real time polymerase chain reaction |
References
- Holick, M.F. Vitamin D Deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Slominski, A.T.; Chaiprasongsuk, A.; Janjetovic, Z.; Kim, T.K.; Stefan, J.; Slominski, R.M.; Hanumanthu, V.S.; Raman, C.; Qayyum, S.; Song, Y.; et al. Photoprotective Properties of Vitamin D and Lumisterol Hydroxyderivatives. Cell Biochem. Biophys. 2020, 78, 165–180. Available online: https://europepmc.org/articles/PMC7347247 (accessed on 26 July 2023). [CrossRef]
- Baeke, F.; Takiishi, T.; Korf, H.; Gysemans, C.; Mathieu, C. Vitamin D: Modulator of the immune system. Curr. Opin. Pharmacol. 2010, 10, 482–496. [Google Scholar] [CrossRef]
- Hossein-Nezhad, A.; Spira, A.; Holick, M.F. Influence of Vitamin D Status and Vitamin D3Supplementation on Genome Wide Expression of White Blood Cells: A Randomized Double-Blind Clinical Trial. PLoS ONE 2013, 8, e58725. [Google Scholar] [CrossRef]
- Yu, S.; Cantorna, M.T. The vitamin D receptor is required for iNKT cell development. Proc. Natl. Acad. Sci. USA 2008, 105, 5207–5212. [Google Scholar] [CrossRef]
- Mariani, E.; Ravaglia, G.; Forti, P.; Meneghetti, A.; Tarozzi, A.; Maioli, F.; Boschi, F.; Pratelli, L.; Pizzoferrato, A.; Piras, F.; et al. Vitamin D, thyroid hormones and muscle mass influence natural killer (NK) innate immunity in healthy nonagenarians and centenarians. Clin. Exp. Immunol. 1999, 116, 19–27. [Google Scholar] [CrossRef]
- Quesada, J.; Solana, R.; Martin, A.; Santamaria, M.; Serrano, I.; Martinez, M.; Aljama, P.; Peña, J. The effect of calcitriol on natural killer cell activity in hemodialyzed patients. J. Steroid. Biochem. 1989, 34, 423–425. Available online: https://www.sciencedirect.com/science/article/pii/0022473189901209 (accessed on 23 March 2023). [CrossRef]
- Bruns, H.; Büttner, M.; Fabri, M.; Mougiakakos, D.; Bittenbring, J.T.; Hoffmann, M.H.; Beier, F.; Pasemann, S.; Jitschin, R.; Hofmann, A.D.; et al. Vitamin D-dependent induction of cathelicidin in human macrophages results in cytotoxicity against high-grade B cell lymphoma. Sci. Transl. Med. 2015, 7, 282ra47. [Google Scholar] [CrossRef]
- Pfreundschuh, M.; Trümper, L.; Osterborg, A.; Pettengell, R.; Trneny, M.; Imrie, K.; Ma, D.; Gill, D.; Walewski, J.; Zinzani, P.-L.; et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: A randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006, 7, 379–391. [Google Scholar] [CrossRef]
- Goede, V.; Fischer, K.; Busch, R.; Engelke, A.; Eichhorst, B.; Wendtner, C.M.; Chagorova, T.; de la Serna, J.; Dilhuydy, M.-S.; Illmer, T.; et al. Obinutuzumab plus Chlorambucil in Patients with CLL and Coexisting Conditions. N. Engl. J. Med. 2014, 370, 1101–1110. [Google Scholar] [CrossRef]
- Sehn, L.H.; Martelli, M.; Trněný, M.; Liu, W.; Bolen, C.R.; Knapp, A.; Sahin, D.; Sellam, G.; Vitolo, U. A randomized, open-label, Phase III study of obinutuzumab or rituximab plus CHOP in patients with previously untreated diffuse large B-Cell lymphoma: Final analysis of GOYA. J. Hematol. Oncol. 2020, 13, 71. [Google Scholar] [CrossRef] [PubMed]
- Bittenbring, J.T.; Neumann, F.; Altmann, B.; Achenbach, M.; Reichrath, J.; Ziepert, M.; Geisel, J.; Regitz, E.; Held, G.; Pfreundschuh, M. Vitamin D deficiency impairs rituximab-mediated cellular cytotoxicity and outcome of patients with diffuse large B-cell lymphoma treated with but not without rituximab. J. Clin. Oncol. 2014, 32, 3242–3248. [Google Scholar] [CrossRef]
- Cheson, B.D.; Leonard, J.P. Monoclonal Antibody Therapy for B-Cell Non-Hodgkin’s Lymphoma. N. Engl. J. Med. 2008, 359, 613–626. [Google Scholar] [CrossRef]
- Neumann, F.; Acker, F.; Schormann, C.; Pfreundschuh, M.; Bittenbring, J.T. Determination of optimum vitamin D3 levels for NK cell-mediated rituximab- and obinutuzumab-dependent cellular cytotoxicity. Cancer Immunol. Immunother. 2018, 67, 1709–1718. [Google Scholar] [CrossRef]
- Van Groningen, L.; Opdenoordt, S.; Van Sorge, A.; Telting, D.; Giesen, A.; De Boer, H. Cholecalciferol loading dose guideline for vitamin D-deficient adults. Eur. J. Endocrinol. 2010, 162, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Henney, C.S.; Kuribayashi, K.; Kern, D.E.; Gillis, S. Interleukin-2 augments natural killer cell activity. Nature 1981, 291, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Al Omar, S.; Flanagan, B.F.; Almehmadi, M.; Christmas, S.E. The effects of IL-17 upon human natural killer cells. Cytokine 2013, 62, 123–130. [Google Scholar] [CrossRef]
- Dou, Y.; Xing, J.; Kong, G.; Wang, G.; Lou, X.; Xiao, X.; Vivier, E.; Li, X.C.; Zhang, Z. Identification of the E3 Ligase TRIM29 as a Critical Checkpoint Regulator of NK Cell Functions. J. Immunol. 2019, 203, 873–880. [Google Scholar] [CrossRef]
- Matalon, O.; Barda-Saad, M. Cbl ubiquitin ligases mediate the inhibition of natural killer cell activity. Commun. Integr. Biol. 2016, 9, e1216739. [Google Scholar] [CrossRef]
- Thomas, M.; Wills, M.; Lehner, P.J. Natural killer cell evasion by an E3 ubiquitin ligase from Kaposi’s sarcoma-associated herpesvirus. Biochem. Soc. Trans. 2008, 36, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Carmody, R.J.; Ruan, Q.; Palmer, S.; Hilliard, B.; Chen, Y.H. Negative Regulation of Toll-Like Receptor Signaling by NF-κB p50 Ubiquitination Blockade. Science 2007, 317, 675–678. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.F.; Zhang, X.; Van Dams, H.; Ten Dijkes, P.; Huang, H.; Zhangs, L. Ubiquitin-specific protease 4 mitigates toll-like/interleukin-1 receptor signaling and regulates innate immune activation. J. Biol. Chem. 2012, 287, 11002–11010. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Sato, S.; Ishii, K.J.; Coban, C.; Hemmi, H.; Yamamoto, M.; Terai, K.; Matsuda, M.; Inoue, J.-I.; Uematsu, S.; et al. Interferon-α induction through Toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nat. Immunol. 2004, 5, 1061. [Google Scholar] [CrossRef]
- Yu, Y.; Hayward, G.S. The Ubiquitin E3 Ligase RAUL Negatively Regulates Type I Interferon through Ubiquitination of the Transcription Factors IRF7 and IRF3. Immunity 2010, 33, 863–877. [Google Scholar] [CrossRef]
- Aquino-López, A.; Senyukov, V.V.; Vlasic, Z.; Kleinerman, E.S.; Lee, D.A. Interferon gamma induces changes in Natural Killer (NK) cell ligand expression and alters NK cell-mediated lysis of pediatric cancer cell lines. Front. Immunol. 2017, 8, 391. [Google Scholar] [CrossRef]
- Tilly, H.; Morschhauser, F.; Bartlett, N.L.; Mehta, A.; Salles, G.; Haioun, C.; Munoz, J.; Chen, A.I.; Kolibaba, K.; Lu, D.; et al. Polatuzumab vedotin in combination with immunochemotherapy in patients with previously untreated diffuse large B-cell lymphoma: An open-label, non-randomised, phase 1b-2 study. Lancet Oncol. 2019, 20, 998–1010. [Google Scholar] [CrossRef]
| Sex | Age (Years) | Vitamin D Levels in Deficient Status (ng/mL) | Vitamin D Levels After Supplementation (ng/mL) | |
|---|---|---|---|---|
| male | 78 | 5.9 | 64.3 | |
| female | 71 | 23 | 68.2 | |
| male | 57 | 15.7 | 62.6 | |
| female | 78 | 4.6 | 68.8 | |
| male | 79 | 6.1 | 72.8 | |
| female | 79 | 9.7 | 68.5 | |
| male | 86 | 10.3 | 58.2 | |
| female | 42 | 8.8 | 61.5 | |
| Mean | 71.3 | 10.5 | 65.6 | |
| Standard deviation | 14.6 | 6.1 | 4.8 | |
| Median | 78 | 9.25 | 66.25 | |
| Range | 42–86 | 4.6–23 | 58.2–72.8 | |
| Upregulated Genes | Downregulated Genes | ||||
|---|---|---|---|---|---|
| CDH19 | MAP4K1 | THOP1 | ADORA2B | KIF15 | UGT2B17 |
| CELSR3-AS1 | MED24 | TOX2 | AFF4 | KIF1B | UPRT |
| CHST15 | MIR1244-1 | TP53I13 | ANAPC4 | KRTAP4-2 | VWC2L-IT1 |
| COQ3 | MIR548T | TRAV8-4 | ANP32B | LRRC1 | ZEB2 |
| CRELD2 | MIR570 | TRIM51HP | ANXA8L1 | LRRFIP2 | ZNF709 |
| CRSP8P | MT1B | TTTY13 | ASIP | MAF | ZNF839 |
| CTDNEP1 | MTRNR2L5 | UCHL1 | ATP11C | MBTD1 | |
| CUEDC2 | MYBL2 | UPK3B | BBS5 | METTL14 | |
| CYP21A1P | MYO7A | UROS | BLOC1S6 | MGC16025 | |
| DDX39A | NAV2-AS5 | USH2A | C15orf57 | MIEF1 | |
| DENND6B | NRG4 | VAMP2 | C16orf46 | MIR1-1 | |
| DMRTA1 | OR52W1 | VIP | CAMTA1 | MIR140 | |
| DRD2 | P4HA2 | WBSCR16 | CCDC81 | MIR3175 | |
| EEF1A2 | PCBP4 | YKT6 | CCR10 | MIR330 | |
| EIF5AL1 | PERP | ZNF324 | CCSAP | MIR4496 | |
| ELFN1 | PI4KA | ZNF692 | CCT2 | MIR933 | |
| EPB41L1 | PI4KAP1 | ZNF728 | CDC14C | NFATC4 | |
| EXOC6B | PKD2L1 | ZNF733P | CDH13 | NKAIN2 | |
| F9 | PLXDC1 | ZNF788 | CDKL5 | OPCML | |
| FAM189B | POLR2L | CEP57L1 | OR6C70 | ||
| FAM217A | PPM1M | CHORDC1 | PADI4 | ||
| FAM219A | PPP1R15B | CLIP4 | PGRMC2 | ||
| FAM25C | PRAMEF2 | CMSS1 | PKN2 | ||
| FAM53A | PRPF31 | CTRB1 | PLA2G4A | ||
| FBL | PRR23D2 | CYTIP | PLEKHH2 | ||
| FBLN7 | PRRT3 | DEFB124 | PRKCH | ||
| FBXL6 | PTPRCAP | DENND5B-AS1 | PRR3 | ||
| FN1 | PXMP2 | DHX15 | PSMC6 | ||
| GPR146 | RARRES3 | DICER1 | PTGER4 | ||
| HIST1H4F | RASA4B | DISP2 | RAD51AP2 | ||
| HLA-C | RASL10B | DNAJA4 | RBM44 | ||
| HPSE2 | RETN | DOCK5 | RGPD1 | ||
| HULC | RHBDF1 | DSCAM-IT1 | RHOB | ||
| IFITM5 | RNU5D-1 | EFCAB10 | RIOK3 | ||
| IFNL3 | RPH3AL | EHHADH | RPH3A | ||
| IL17RE | RPL21P28 | EMC2 | RSPH10B | ||
| IL2RB | RPL23AP87 | EXOG | SAYSD1 | ||
| INO80E | RPS27 | FAM208B | SCGB2B3P | ||
| ITGAM | SEC11A | FBXW9 | SCUBE1 | ||
| ITGAX | SH3GLB2 | FEM1C | SELK | ||
| IVL | SLPI | FETUB | SENP2 | ||
| KDM8 | SMARCB1 | FGD5P1 | SENP5 | ||
| KIF25 | SNAR-I | GABPB1-AS1 | SERBP1 | ||
| KRT17 | SNORA35 | GACAT2 | SLMAP | ||
| KRTAP22-2 | SNORD116-14 | GCOM1 | SMCHD1 | ||
| KRTAP5-1 | SNORD20 | GPATCH2L | SMNDC1 | ||
| LIN7A | SNORD32B | GS1-279B7.1 | SNORD114-3 | ||
| LINC00619 | SOX13 | HACD1 | SPINT1 | ||
| LINC00881 | SPATA20 | HOXD12 | SRPK1 | ||
| LINC01144 | SPINK1 | HYAL2 | TAOK1 | ||
| LINC01456 | SSH3 | IMMP1L | TPI1P3 | ||
| LINC01624 | STARD4-AS1 | IP6K2 | TRBV5-4 | ||
| LRRC42 | STARD9 | IPO11 | TSFM | ||
| LRRC74A | TAAR3 | KCNE1 | TTC39B | ||
| Gene Symbol | Gene Name | Fold Change | p-Value |
|---|---|---|---|
| TRAV8–4 | T cell receptor alpha variable 8–4 | −1.54 | 0.00761 |
| MAP4K1 | mitogen-activated protein kinase kinase kinase kinase 1 | −1.34 | 0.00388 |
| IFNL3 | interferon, lambda 3 | −1.30 | 0.00671 |
| IL17RE | interleukin 17 receptor E | −1.24 | 0.00443 |
| POLR2L | polymerase (RNA) II (DNA directed) polypeptide L, 7.6kDa | −1.23 | 0.00904 |
| VIP | vasoactive intestinal peptide | −1.22 | 0.00146 |
| C1orf147 | chromosome 1 open reading frame 147 | −1.21 | 0.00525 |
| IFITM5 | interferon induced transmembrane protein 5 | −1.19 | 0.00350 |
| SLPI | secretory leukocyte peptidase inhibitor | −1.19 | 0.00999 |
| DRD2 | dopamine receptor D2 | −1.18 | 0.00661 |
| ITGAM | integrin, alpha M (complement component 3 receptor 3 subunit) | −1.16 | 0.00232 |
| IL2RB | interleukin 2 receptor, beta | −1.13 | 0.00802 |
| VAMP2 | vesicle associated membrane protein 2 | −1.10 | 0.00815 |
| MED24 | mediator complex subunit 24 | −1.09 | 0.00743 |
| NRG4 | neuregulin 4 | −1.09 | 0.00871 |
| HLA-C | major histocompatibility complex, class I, C | −1.08 | 0.00426 |
| CCR10 | chemokine (C-C motif) receptor 10 | 1.12 | 0.00455 |
| PSMC6 | proteasome 26S subunit, ATPase 6 | 1.13 | 0.00591 |
| PTGER4 | prostaglandin E receptor 4 (subtype EP4) | 1.16 | 0.00388 |
| SRPK1 | SRSF protein kinase 1 | 1.18 | 0.00589 |
| NFATC4 | nuclear factor of activated T-cells, cytoplasmic, calcineurin-dependent 4 | 1.19 | 0.00520 |
| BLOC1S6 | biogenesis of lysosomal organelles complex-1, subunit 6, pallidin | 1.22 | 0.00058 |
| DEFB124 | defensin, beta 124 | 1.23 | 0.00058 |
| TRBV5-4 | T cell receptor beta variable 5-4 | 1.30 | 0.00337 |
| PADI4 | peptidyl arginine deiminase, type IV | 1.41 | 0.00820 |
| PLA2G4A | phospholipase A2, group IVA (cytosolic, calcium-dependent) | 1.67 | 0.00099 |
| Gene Symbol | Gene Name |
|---|---|
| IFNA10 | interferon, alpha 10 |
| IFNA6 | interferon, alpha 6 |
| IFNA4 | interferon, alpha 4 |
| IFNA2 | interferon, alpha 2 |
| PPP3R2 | protein phosphatase 3 (formerly 2B), regulatory subunit B, 19kDa, beta isoform (calcineurin B, type II) |
| RAC3 | ras-related C3 botulinum toxin substrate 3 (rho family, small GTP binding protein Rac3) |
| RAET1L | retinoic acid early transcript 1L |
| IFNA7 | interferon, alpha 7 |
| NCR2 | natural cytotoxicity triggering receptor 2 |
| IFNA13 | interferon, alpha 13 |
| SHC2 | SHC (Src homology 2 domain containing) transforming protein 2 |
| Name of the Pathway | ES | NES | p-Value | FDR q-Value |
|---|---|---|---|---|
| Biocarta—FCεRI | 0.90 | 1.88 | <0.001 | <0.001 |
| GO—Activation of innate immune response | 0.71 | 1.87 | <0.001 | <0.001 |
| Hallmark—Complement | 0.70 | 1.84 | <0.001 | <0.001 |
| GO—Interferon Gamma response | 0.68 | 1.78 | <0.001 | <0.001 |
| GO—Regulation of interferon beta production | 0.80 | 1.71 | <0.001 | 0.002 |
| GO—Positive regulation of interferon beta production | 0.86 | 1.70 | <0.001 | 0.002 |
| GO—Leukocyte chemotaxis | 0.67 | 1.64 | <0.001 | 0.013 |
| GO—Positive regulation of interferon alpha production | 0.89 | 1.59 | 0.002 | 0.032 |
| Hallmark—IL6/JAK/STAT3 signaling | 0.66 | 1.58 | <0.001 | 0.032 |
| GO—Activation of immune response | 0.57 | 1.57 | <0.001 | 0.032 |
| GO—Response to vitamin D | 0.77 | 1.57 | 0.012 | 0.031 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christofyllakis, K.; Neumann, F.; Bewarder, M.; Thurner, L.; Kaddu-Mulindwa, D.; Kos, I.A.; Lesan, V.; Bittenbring, J.T. Vitamin D Enhances Immune Effector Pathways of NK Cells Thus Providing a Mechanistic Explanation for the Increased Effectiveness of Therapeutic Monoclonal Antibodies. Nutrients 2023, 15, 3498. https://doi.org/10.3390/nu15163498
Christofyllakis K, Neumann F, Bewarder M, Thurner L, Kaddu-Mulindwa D, Kos IA, Lesan V, Bittenbring JT. Vitamin D Enhances Immune Effector Pathways of NK Cells Thus Providing a Mechanistic Explanation for the Increased Effectiveness of Therapeutic Monoclonal Antibodies. Nutrients. 2023; 15(16):3498. https://doi.org/10.3390/nu15163498
Chicago/Turabian StyleChristofyllakis, Konstantinos, Frank Neumann, Moritz Bewarder, Lorenz Thurner, Dominic Kaddu-Mulindwa, Igor Age Kos, Vadim Lesan, and Joerg Thomas Bittenbring. 2023. "Vitamin D Enhances Immune Effector Pathways of NK Cells Thus Providing a Mechanistic Explanation for the Increased Effectiveness of Therapeutic Monoclonal Antibodies" Nutrients 15, no. 16: 3498. https://doi.org/10.3390/nu15163498
APA StyleChristofyllakis, K., Neumann, F., Bewarder, M., Thurner, L., Kaddu-Mulindwa, D., Kos, I. A., Lesan, V., & Bittenbring, J. T. (2023). Vitamin D Enhances Immune Effector Pathways of NK Cells Thus Providing a Mechanistic Explanation for the Increased Effectiveness of Therapeutic Monoclonal Antibodies. Nutrients, 15(16), 3498. https://doi.org/10.3390/nu15163498








