Vitamin B12 Status before and after Outpatient Treatment of Severe Acute Malnutrition in Children Aged 6–59 Months: A Sub-Study of a Randomized Controlled Trial in Burkina Faso
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Settings
2.2. Study Participants
2.3. Intervention
2.4. Data Collection
2.5. Blood Sampling and Storage
2.6. Biochemical Analyses
2.7. Data Management
2.8. Data Analyses
2.9. Ethical Considerations
3. Results
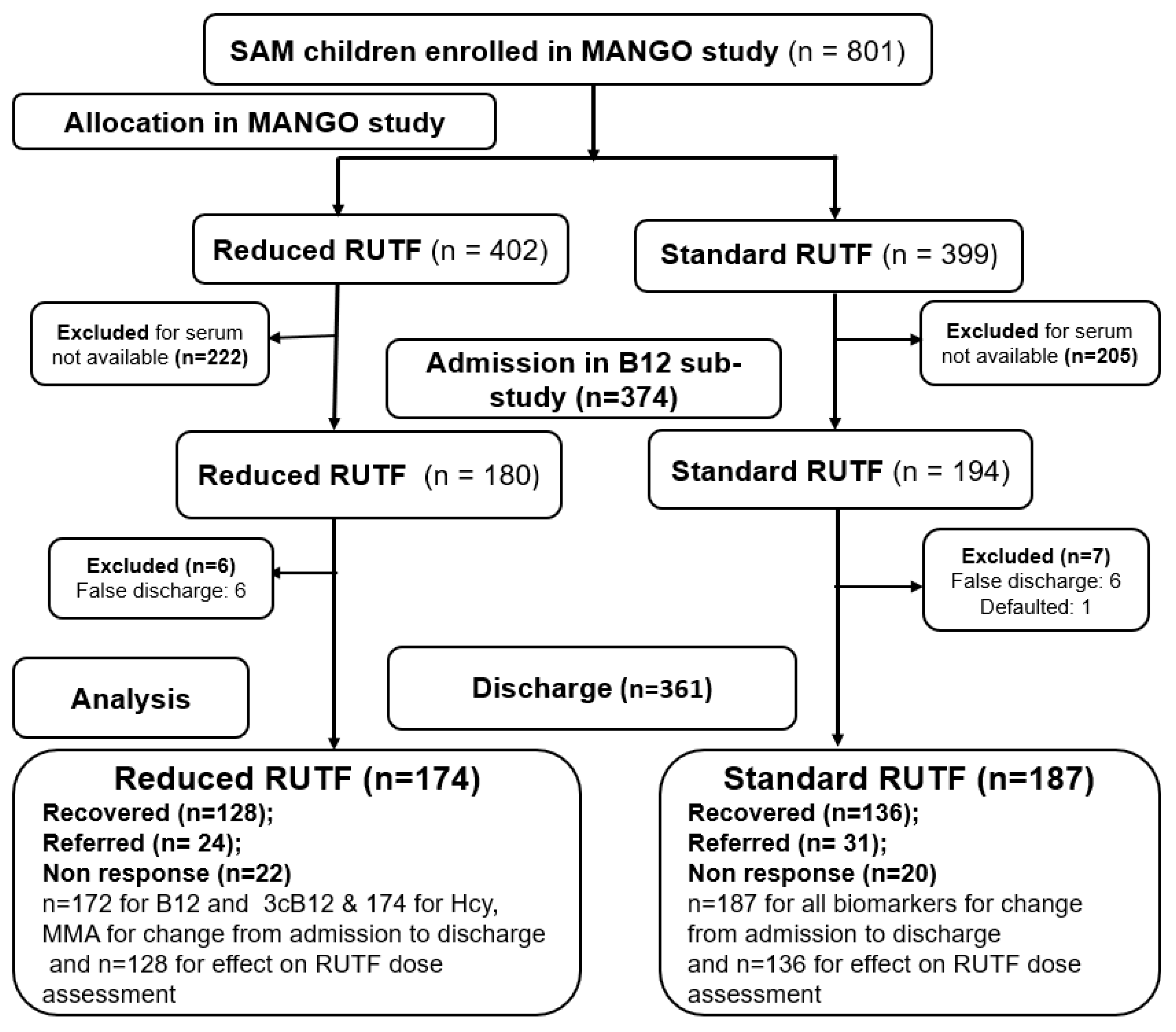
3.1. Characteristics of Children in Vitamin B12 Sub-Study
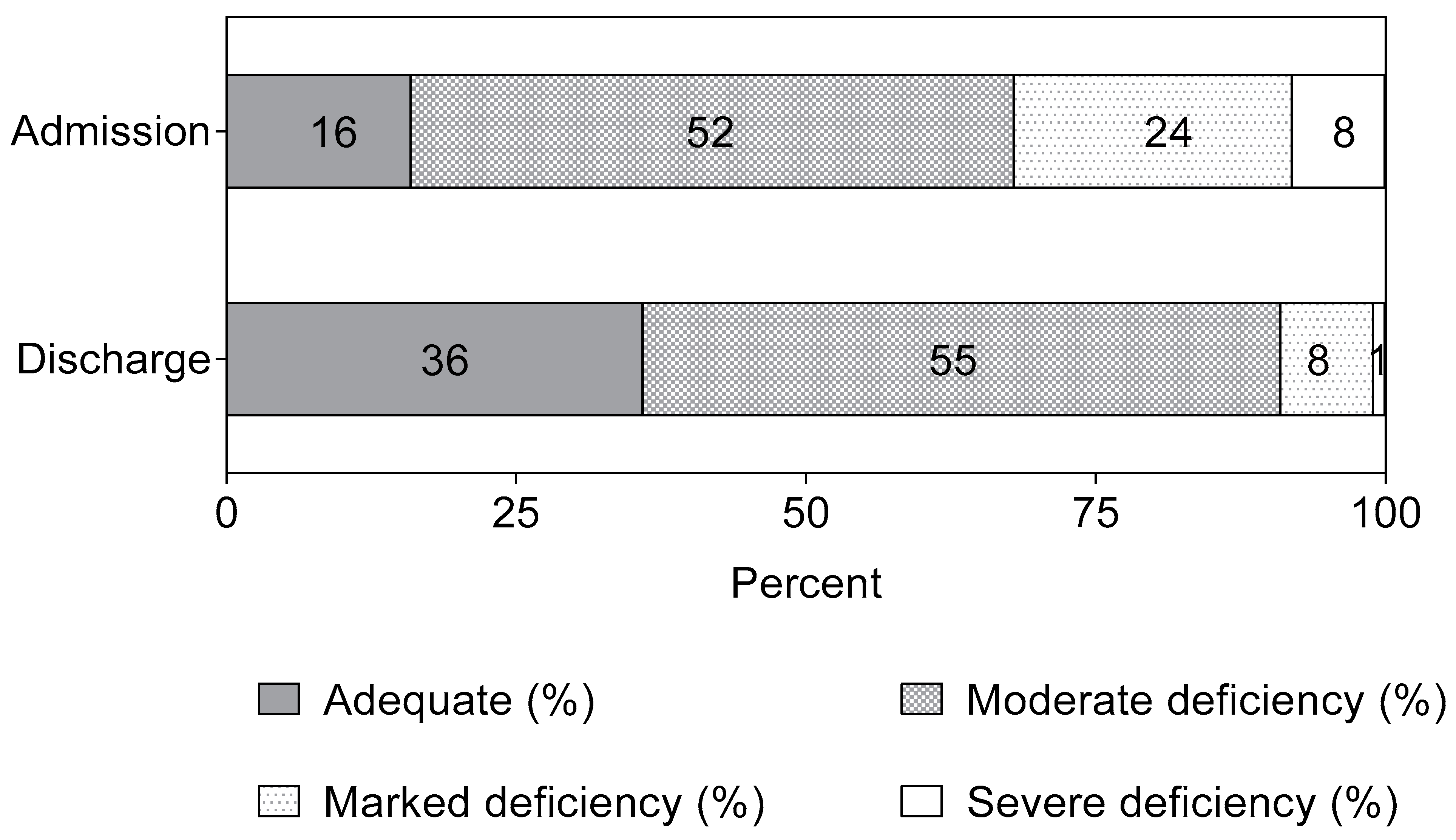
3.2. Biomarkers of Vitamin B12 Status in Children with SAM Treated with RUTF
3.3. Vitamin B12 Biomarkers with a Reduced or a Standard RUTF Dose
3.4. Factors Associated with B12 Status
4. Discussion
4.1. Strengths and Limitations
4.2. Generalizability
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stabler, S.P. Vitamin B12 Deficiency. N. Engl. J. Med. 2013, 368, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Briend, A.; Akomo, P.; Bahwere, P.; de Pee, S.; Dibari, F.; Golden, M.H.; Manary, M.; Ryan, K. Developing Food Supplements for Moderately Malnourished Children: Lessons Learned from Ready-to-Use Therapeutic Foods. Food Nutr. Bull. 2015, 36, S53–S58. [Google Scholar] [CrossRef] [PubMed]
- Carmel, R. Chapter 27: Cobalamin (Vitamin B12). In Modern Nutrition in Health and Disease, 11th ed.; Ross, A.C., Ed.; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2014; pp. 369–389. [Google Scholar]
- Rush, E.C.; Katre, P.; Yajnik, C.S. Vitamin B12: One Carbon Metabolism, Fetal Growth and Programming for Chronic Disease. Eur. J. Clin. Nutr. 2014, 68, 2–7. [Google Scholar] [CrossRef]
- Vogiatzoglou, A.; Oulhaj, A.; Smith, A.D.; Nurk, E.; Drevon, C.A.; Ueland, P.M.; Vollset, S.E.; Tell, G.S.; Refsum, H. Determinants of Plasma Methylmalonic Acid in a Large Population: Implications for Assessment of Vitamin B12 Status. Clin. Chem. 2009, 55, 2198–2206. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.L.; Carmel, R.; Green, R.; Pfeiffer, C.M.; Cogswell, M.E.; Osterloh, J.D.; Sempos, C.T.; Yetley, E.A. Monitoring of Vitamin B-12 Nutritional Status in the United States by Using Plasma Methylmalonic Acid and Serum Vitamin B-12. Am. J. Clin. Nutr. 2011, 94, 552–561. [Google Scholar] [CrossRef]
- Green, R. Indicators for Assessing Folate and Vitamin B12 Status and for Monitoring the Efficacy of Intervention Strategies. Food Nutr. Bull. 2008, 29 (Suppl. 1), S52–S63. [Google Scholar] [CrossRef]
- Carmel, R. Biomarkers of Cobalamin (Vitamin B-12) Status in the Epidemiologic Setting: A Critical Overview of Context, Applications, and Performance Characteristics of Cobalamin, Methylmalonic Acid, and Holotranscobalamin II. Am. J. Clin. Nutr. 2011, 94, 348S–358S. [Google Scholar] [CrossRef]
- Morris, M.S. The Role of B Vitamins in Preventing and Treating Cognitive Impairment and Decline. Adv. Nutr. 2012, 3, 801–812. [Google Scholar] [CrossRef]
- Green, R.; Allen, L.H.; Bjørke-Monsen, A.-L.; Brito, A.; Guéant, J.-L.; Miller, J.W.; Molloy, A.M.; Nexo, E.; Stabler, S.; Toh, B.-H.; et al. Vitamin B12 Deficiency. Nat. Rev. Dis. Prim. 2017, 3, 17040. [Google Scholar] [CrossRef]
- Harrington, D.J. Methods for Assessment of Vitamin B12. In Laboratory Assessment of Vitamin Status; Elsevier: Amsterdam, The Netherlands, 2019; pp. 265–299. ISBN 978-0-12-813050-6. [Google Scholar]
- Jarquin Campos, A.; Risch, L.; Nydegger, U.; Wiesner, J.; Vazquez Van Dyck, M.; Renz, H.; Stanga, Z.; Risch, M. Diagnostic Accuracy of Holotranscobalamin, Vitamin B12, Methylmalonic Acid, and Homocysteine in Detecting B12 Deficiency in a Large, Mixed Patient Population. Dis. Markers 2020, 2020, 7468506. [Google Scholar] [CrossRef]
- Fedosov, S.N. Metabolic Signs of Vitamin B12 Deficiency in Humans: Computational Model and Its Implications for Diagnostics. Metabolism 2010, 59, 1124–1138. [Google Scholar] [CrossRef] [PubMed]
- Fedosov, S.N.; Brito, A.; Miller, J.W.; Green, R.; Allen, L.H. Combined Indicator of Vitamin B12 Status: Modification for Missing Biomarkers and Folate Status and Recommendations for Revised Cut-Points. Clin. Chem. Lab. Med. 2015, 53, 1215–1225. [Google Scholar] [CrossRef] [PubMed]
- Kangas, S.T.; Salpéteur, C.; Nikièma, V.; Talley, L.; Ritz, C.; Friis, H.; Briend, A.; Kaestel, P. Impact of Reduced Dose of Ready-to-Use Therapeutic Foods in Children with Uncomplicated Severe Acute Malnutrition: A Randomised Non-Inferiority Trial in Burkina Faso. PLoS Med. 2019, 16, e1002887. [Google Scholar] [CrossRef] [PubMed]
- Ministère de La Santé. Enquête Nutritionnelle Nationale SMART 2016 Burkina Faso. 2016. Available online: https://www.humanitarianresponse.info/sites/www.humanitarianresponse.info/files/documents/files/smart_2016.pdf (accessed on 28 May 2023).
- Ministère de la Santé. Enquête Nutritionnelle Nationale SMART 2018 Burkina Faso. 2018. Available online: https://www.humanitarianresponse.info/sites/www.humanitarianresponse.info/files/documents/files/bfa_rapport_smart_2020_vf.pdf (accessed on 28 May 2023).
- World Health Organization. Guideline: Updates on the Management of Severe Acute Malnutrition in Infants and Children; World Health Organization: Geneva, Switzerland, 2022; ISBN 978-92-4-004812-6. [Google Scholar]
- Zangenberg, M.; Abdissa, A.; Johansen, Ø.H.; Tesfaw, G.; Friis, H.; Briend, A.; Eshetu, B.; Kurtzhals, J.A.L.; Girma, T. Critical Evaluation of the Appetite Test for Children with Severe Acute Malnutrition. Trop. Med. Int. Health 2020, 25, 424–432. [Google Scholar] [CrossRef]
- Kangas, S.T.; Salpéteur, C.; Nikièma, V.; Talley, L.; Briend, A.; Ritz, C.; Friis, H.; Kaestel, P. Vitamin A and Iron Status of Children before and after Treatment of Uncomplicated Severe Acute Malnutrition. Clin. Nutr. 2020, 39, 3512–3519. [Google Scholar] [CrossRef]
- Ministère de la Santé. Protocole National Prise en Charge Intégrée de la Malnutrition Aiguë (PCIMA). Burkina Faso 2014. Available online: https://www.humanitarianresponse.info/sites/www.humanitarianresponse.info/files/documents/files/protocole_pcima_bf_janv_2015.pdf (accessed on 28 May 2023).
- UNICEF; WHO; WFP. Community-Based Management of Severe Acute Malnutrition; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Nikièma, V.; Kangas, S.T.; Salpéteur, C.; Ouédraogo, A.; Lachat, C.; Bassolé, N.H.I.; Fogny, N.F. Adequacy of Nutrient Intakes of Severely and Acutely Malnourished Children Treated with Different Doses of Ready-To-Use Therapeutic Food in Burkina Faso. J. Nutr. 2021, 151, 1008–1017. [Google Scholar] [CrossRef]
- Nikièma, V.; Fogny, N.F.; Salpéteur, C.; Lachat, C.; Kangas, S.T. Complementary Feeding Practices and Associated Factors of Dietary Diversity among Uncomplicated Severe Acute Malnourished Children Aged 6–23 Months in Burkina Faso. Matern. Child Nutr. 2021, 17, e13220. [Google Scholar] [CrossRef]
- Kelleher, B.P.; Broin, S.D. Microbiological Assay for Vitamin B12 Performed in 96-Well Microtitre Plates. J. Clin. Pathol. 1991, 44, 592–595. [Google Scholar] [CrossRef]
- Midttun, Ø.; McCann, A.; Aarseth, O.; Krokeide, M.; Kvalheim, G.; Meyer, K.; Ueland, P.M. Combined Measurement of 6 Fat-Soluble Vitamins and 26 Water-Soluble Functional Vitamin Markers and Amino Acids in 50 ΜL of Serum or Plasma by High-Throughput Mass Spectrometry. Anal. Chem. 2016, 88, 10427–10436. [Google Scholar] [CrossRef]
- Erhardt, J.G.; Estes, J.E.; Pfeiffer, C.M.; Biesalski, H.K.; Craft, N.E. Combined Measurement of Ferritin, Soluble Transferrin Receptor, Retinol Binding Protein, and C-Reactive Protein by an Inexpensive, Sensitive, and Simple Sandwich Enzyme-Linked Immunosorbent Assay Technique. J. Nutr. 2004, 134, 3127–3132. [Google Scholar] [CrossRef]
- WHO. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age; Methods and Development; WHO Press: Geneva, Switzerland, 2006; ISBN 978-92-4-154693-5. [Google Scholar]
- Ugwu, C.L.J.; Zewotir, T.T. Using Mixed Effects Logistic Regression Models for Complex Survey Data on Malaria Rapid Diagnostic Test Results. Malar. J. 2018, 17, 453. [Google Scholar] [CrossRef]
- Taneja, S.; Bhandari, N.; Strand, T.A.; Sommerfelt, H.; Refsum, H.; Ueland, P.M.; Schneede, J.; Bahl, R.; Bhan, M.K. Cobalamin and Folate Status in Infants and Young Children in a Low-to-Middle Income Community in India. Am. J. Clin. Nutr. 2007, 86, 1302–1309. [Google Scholar] [CrossRef]
- Gupta, S.; Haldar, P.; Singh, A.; Malhotra, S.; Kant, S. Prevalence of Serum Cobalamin and Folate Deficiency among Children Aged 6–59 Months: A Hospital-Based Cross-Sectional Study from Northern India. J. Fam. Med. Prim. Care 2022, 11, 1063. [Google Scholar] [CrossRef]
- Friis, H.; Cichon, B.; Fabiansen, C.; Iuel-Brockdorff, A.-S.; Yaméogo, C.W.; Ritz, C.; Frikke-Schmidt, R.; Briend, A.; Michaelsen, K.F.; Christensen, V.B.; et al. Serum Cobalamin in Children with Moderate Acute Malnutrition in Burkina Faso: Secondary Analysis of a Randomized Trial. PLoS Med. 2022, 19, e1003943. [Google Scholar] [CrossRef] [PubMed]
- Sudfeld, C.R.; McCoy, D.C.; Fink, G.; Muhihi, A.; Bellinger, D.C.; Masanja, H.; Smith, E.R.; Danaei, G.; Ezzati, M.; Fawzi, W.W. Malnutrition and Its Determinants Are Associated with Suboptimal Cognitive, Communication, and Motor Development in Tanzanian Children. J. Nutr. 2015, 145, 2705–2714. [Google Scholar] [CrossRef] [PubMed]
- De Benoist, B. Conclusions of a WHO Technical Consultation on Folate and Vitamin B12 Deficiencies. Food Nutr. Bull. 2008, 29, S238–S244. [Google Scholar] [CrossRef] [PubMed]
- EVM (Expert Group on Vitamins and Minerals). Safe Upper Levels for Vitamins and Minerals. 2003. Available online: http://cot.food.gov.uk/sites/default/files/vitmin2003.pdf (accessed on 28 May 2023).
- Aguayo, G.A.; Zhang, L.; Vaillant, M.; Ngari, M.; Perquin, M.; Moran, V.; Huiart, L.; Krüger, R.; Azuaje, F.; Ferdynus, C.; et al. Correction: Machine Learning for Predicting Neurodegenerative Diseases in the General Older Population: A Cohort Study. BMC Med. Res. Methodol. 2023, 23, 32. [Google Scholar] [CrossRef]
- WHO; FAO. Vitamin and Mineral Requirements in Human Nutrition, 2nd ed.; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Berlin, H.; Berlin, R.; Brante, G. Oral Treatment of Pernicious Anemia with High Doses of Vitamin B12 without Intrinsic Factor. Acta Med. Scand. 1968, 184, 247–258. [Google Scholar] [CrossRef]
- Chanarin, I. The Megaloblastic Anaemias, 2nd ed.; Blackwell Scientific Publications: Oxford, UK, 1979. [Google Scholar]
- Williams, A.M.; Chantry, C.J.; Young, S.L.; Achando, B.S.; Allen, L.H.; Arnold, B.F.; Colford, J.M.; Dentz, H.N.; Hampel, D.; Kiprotich, M.C.; et al. Vitamin B-12 Concentrations in Breast Milk Are Low and Are Not Associated with Reported Household Hunger, Recent Animal-Source Food, or Vitamin B-12 Intake in Women in Rural Kenya. J. Nutr. 2016, 146, 1125–1131. [Google Scholar] [CrossRef]
- Neumann, C.G.; Oace, S.M.; Chaparro, M.P.; Herman, D.; Drorbaugh, N.; Bwibo, N.O. Low Vitamin B12 Intake during Pregnancy and Lactation and Low Breastmilk Vitamin B12 Content in Rural Kenyan Women Consuming Predominantly Maize Diets. Food Nutr. Bull. 2013, 34, 151–159. [Google Scholar] [CrossRef]
- Siddiqua, T.J. Vitamin B12 Deficiency in Pregnancy and Lactation: Is There a Need for Pre and Post-Natal Supplementation? J. Nutr. Disord. Ther. 2014, 4, 142. [Google Scholar] [CrossRef]
- Dror, D.K.; Allen, L.H. Vitamin B-12 in Human Milk: A Systematic Review. Adv. Nutr. 2018, 9, 358S–366S. [Google Scholar] [CrossRef]
- Food and Nutrition Board. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; National Academy Press: Washington, DC, USA, 1998. [Google Scholar]
- Greibe, E.; Lildballe, D.L.; Streym, S.; Vestergaard, P.; Rejnmark, L.; Mosekilde, L.; Nexo, E. Cobalamin and Haptocorrin in Human Milk and Cobalamin-Related Variables in Mother and Child: A 9-Mo Longitudinal Study. Am. J. Clin. Nutr. 2013, 98, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.H. Vitamin B12 Metabolism and Status during Pregnancy, Lactation and Infancy. In Nutrient Regulation during Pregnancy, Lactation, and Infant Growth; Allen, L., King, J., Lönnerdal, B., Eds.; Springer: Boston, MA, USA, 1994; pp. 173–186. [Google Scholar] [CrossRef]
- Anaya-Loyola, M.A.; Brito, A.; Brown, K.H.; Allen, L.H. Breast Milk Provides Inadequate Amounts of Vitamin B12 for Predominantly Breastfed Guatemalan Infants. Int. J. Vitam. Nutr. Res. 2020, 90, 395–402. [Google Scholar] [CrossRef]
- Shrestha, P.N.; Khanal, B.; Joshi, P. Chronic Diarrhoea: A Rare Presentation of Vitamin B12 Deficiency Anemia In Children. J. Nepal Paediatr. Soc. 2021, 41, 278–281. [Google Scholar] [CrossRef]
- Kumar, K.J.; Prudhvi, S.; Balaji, K.; Rahul, R. Persistent Diarrhea, Hemolytic Anemia, and Splenohepatomegaly Due to Vitamin B12 Deficiency in an Infant. J. Appl. Hematol. 2018, 9, 148. [Google Scholar] [CrossRef]
- Jansen, E.H.J.M.; Beekhof, P.K. Stability of Folate and Vitamin B12 in Human Serum after Long-Term Storage: A Follow-Up after 13 Years. J. Nutr. Metab. 2018, 2018, 9834181. [Google Scholar] [CrossRef] [PubMed]
- Midttun, Ø.; Townsend, M.K.; Nygård, O.; Tworoger, S.S.; Brennan, P.; Johansson, M.; Ueland, P.M. Most Blood Biomarkers Related to Vitamin Status, One-Carbon Metabolism, and the Kynurenine Pathway Show Adequate Preanalytical Stability and Within-Person Reproducibility to Allow Assessment of Exposure or Nutritional Status in Healthy Women and Cardiovascular Patients. J. Nutr. 2014, 144, 784–790. [Google Scholar] [CrossRef]
- Allen, L.H.; Miller, J.W.; de Groot, L.; Rosenberg, I.H.; Smith, A.D.; Refsum, H.; Raiten, D.J. Biomarkers of Nutrition for Development (BOND): Vitamin B-12 Review. J. Nutr. 2018, 148, 1995S–2027S. [Google Scholar] [CrossRef]
| Childs Weight (kg) | Standard RUTF Dose | Reduced 1 RUTF Dose | ||
|---|---|---|---|---|
| RUTF Quantity/Day (Sachets) | B12 Content of Daily RUTF Dose (µg) | RUTF Quantity/Day (Sachets) | B12 Content of Daily RUTF Dose (µg) | |
| 3.0–3.4 | 1.1 | 1.62 | 1.0 | 1.47 |
| 3.5–4.9 | 1.4 | 2.06 | 1.0 | 1.47 |
| 5.0–6.9 | 2.1 | 3.09 | 1.0 | 1.47 |
| 7.0–9.9 | 2.9 | 4.26 | 2.0 | 2.94 |
| 10.0–14.9 | 4.3 | 6.32 | 2.0 | 2.94 |
| Variables | n | Excluded (n = 427) | Included (n = 374) | p-Value 2 |
|---|---|---|---|---|
| Age (months) | 801 | 9.5 [7.2; 15.9] | 11.0 [7.7; 16.9] | 0.34 |
| Female | 801 | 52 (221) | 49 (184) | 0.47 |
| Breastfeeding | 801 | 86 (368) | 86 (321) | 0.89 |
| Weight (kg) | 801 | 6.2 (1.3) | 6.3 (1.3) | 0.09 |
| Height (cm) | 801 | 68.8 (7.7) | 69.5 (7.7) | 0.25 |
| MUAC (mm) | 801 | 113 (6.8) | 113 (6.1) | 0.14 |
| Acute illnesses % (n) | ||||
| Malaria | 801 | 29 (125) | 37 (138) | 0.02 |
| Acute respiratory illness | 801 | 36 (153) | 26 (98) | <0.01 |
| Diarrhea | 801 | 25 (105) | 25 (95) | 0.79 |
| Fever | 801 | 27 (116) | 25 (92) | 0.41 |
| Biochemical profile | ||||
| Hemoglobin (g/L) | 801 | 97.0 [84.0; 108] | 99.0 [86.0; 108] | 0.14 |
| <110 g/L | 801 | 79 (335) | 78 (293) | 0.97 |
| Serum ferritin (µg/L) | 754 1 | 14.2 [6.6; 32.3] | 15.9 [7.8; 30.6] | 0.01 |
| <12 µg/L | 754 1 | 43 (164) | 40 (150) | 0.58 |
| Serum soluble transferrin receptor (mg/L) | 754 1 | 8.3 [6.1; 12.8] | 8.5 [6.6; 12.8] | 0.24 |
| >8.3 mg/L | 754 1 | 50 (190) | 52 (194) | 0.31 |
| Iron deficiency anemia | 754 1 | 39 (147) | 39 (144) | 0.83 |
| Serum Biomarkers | Admission (n = 374) | Discharge (n = 361) | Difference 2 | |||
|---|---|---|---|---|---|---|
| n | Median [IQR] | n | Median [IQR] | Mean [95% CI] | p Value | |
| B12, pmol/L | 374 | 188 [125; 266] | 359 1 | 279 [207; 365] | 66 [55; 77] | <0.0001 |
| tHcy, µmol/L | 374 | 11 [7; 15] | 361 | 8 [6; 10] | −4 [−4.4; −3.2] | <0.0001 |
| MMA, µmol/L | 374 | 0.59 [0.31; 1.12] | 361 | 0.37 [0.25; 0.73] | −0.26 [−0.36; −0.17] | <0.0001 |
| 3cB12 | 374 | −1 [−1.6; −0.4] | 359 1 | −0.3 [−0.9; 0.2] | 0.5 [0.4; 0.6] | <0.0001 |
| Outcomes | Admission | Recovery | ||||||
|---|---|---|---|---|---|---|---|---|
| Reduced RUTF (n = 180) | Standard RUTF (n = 194) | Difference [95% CI] 1 | p-Value | Reduced RUTF (n = 128) | Standard RUTF (n = 136) | Difference [95% CI] 2 | p-Value | |
| B12 (pmol/L) | 198 [124; 286] | 174 [125; 251] | −17 [−40; 5] | 0.14 | 290 [209; 363] | 267 [193; 350] | −6 [−29; 17] | 0.61 |
| tHcy (µg/L) | 11 [8; 15] | 11 [7; 16] | 0.6 [−0.8; 2.0] | 0.39 | 8 [6; 10] | 8 [6; 10] | 0.1 [−0.5; 0.8] | 0.68 |
| MMA (µmol/L) | 0.71[0.33; 1.22] | 0.56 [0.31; 1.00] | −0.10 [−0.30; 0.11] | 0.35 | 0.41 [0.24; 0.71] 3 | 0.37 [0.25; 0.76] | 0.05 [−0.07; 0.16] | 0.42 |
| 3cB12 | −1.1 [−1.7; −0.4] | −0.9[−1.5; −0.4] | 0.01 [−0.16; 0.18] | 0.93 | −0.27 [−0.82; 0.24] 3 | −0.40 [−0.90; 0.14] | −0.07 [−0.20; 0.05] | 0.26 |
| Characteristics | Admission (n = 374) | Discharge (n = 361) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3cB12, Median [IQR] | Unadjusted | Adjusted 1 | 3cB12, Median [IQR] | Unadjusted | Adjusted 1 | |||||
| Child’s Sex | β | p-Value | β | p-Value | β | p-Value | β | p-Value | ||
| Male | −0.91 [−1.63; −0.43] | Ref | Ref | −0.35 [−0.85; 0.08] | Ref | Ref | ||||
| Female | −1.05 [−1.63; −0.23] | 0.01 | 0.94 | 0.05 | 0.53 | −0.34 [−0.87; 0.28] | 0.02 | 0.79 | 0.06 | 0.44 |
| Breastfeeding status | ||||||||||
| No | −0.18 [−0.75; 0.47] | Ref | Ref | 0.19 [−0.31; 0.58] | Ref | Ref | ||||
| Yes | −1.10 [−1.70; −0.48] | −0.91 | <0.001 | −0.51 | <0.01 | −0.44 [−0.95; 0.07] | −0.57 | <0.001 | −0.33 | 0.01 |
| Malaria | ||||||||||
| No | −0.92 [−1.64; −0.44] | Ref | Ref | −0.36 [−0.86; 0.18] | Ref | Ref | ||||
| Yes | −1.06 [−1.56; −0.18] | 0.14 | 0.16 | 0.00 | 0.99 | −0.18 [−0.84; 0.29] | 0.05 | 0.78 | 0.08 | 0.62 |
| Acute Respiratory illness | ||||||||||
| No | −0.99 [−1.66; −0.40] | Ref | Ref | −0.32 [−0.85; 0.19] | Ref | Ref | ||||
| Yes | −0.92 [−1.45; −0.34] | 0.18 | 0.10 | 0.15 | 0.14 | −0.49 [−1.06; 0.10] | −0.07 | 0.70 | −0.05 | 0.77 |
| Diarrhea | ||||||||||
| No | −1.10 [−1.68; −0.45] | Ref | Ref | −0.36 [−0.86; 0.18] | Ref | Ref | ||||
| Yes | −0.69 [−1.33; −0.11] | 0.29 | 0.01 | 0.24 | 0.01 | −0.11 [−0.34; 0.26] | 0.23 | 0.25 | 0.27 | 0.16 |
| Fever | ||||||||||
| No | −0.98 [−1.68; −0.37] | Ref | Ref | −0.36 [−0.87; 0.19] | Ref | Ref | ||||
| Yes | −0.94 [−1.37; −0.42] | 0.12 | 0.27 | 0.09 | 0.37 | −0.27 [−0.47; 0.20] | 0.09 | 0.61 | 0.05 | 0.78 |
| Hemoglobin | ||||||||||
| <110 g/L | −0.80 [−1.46; −0.28] | Ref | Ref | −0.24 [−0.72; 0.20] | Ref | Ref | ||||
| ≥110 g/L | −1.01 [−1.63; −0.41] | −0.09 | 0.45 | −0.08 | 0.47 | −0.49 [−0.95; 0.16] | −0.10 | 0.21 | −0.11 | 0.16 |
| Ferritin | ||||||||||
| <12 µg/L | −0.96 [−1.58; −0.40] | Ref | Ref | −0.31 [−0.74; 0.21] | Ref | Ref | ||||
| ≥12 µg/L | −0.97 [−1.68; −0.38] | −0.03 | 0.79 | −0.02 | 0.86 | −0.38 [−1.00; 0.12] | −0.10 | 0.20 | −0.08 | 0.25 |
| Soluble transferrin receptor (STfR) | ||||||||||
| <8.3 mL/L | −1.07 [−1.61; −0.44] | Ref | Ref | −0.32 [−0.82; 0.19] | Ref | Ref | ||||
| ≥8.3 mL/L | −0.89 [−1.63; −0.29] | 0.05 | 0.57 | 0.06 | 0.50 | −0.36 [−0.87; 0.16] | −0.04 | 0.63 | −0.02 | 0.77 |
| Iron deficiency anemia (IDA) | ||||||||||
| No | −0.93 [−1.55; −0.40] | Ref | Ref | −0.30 [−0.74; 0.19] | Ref | Ref | ||||
| Yes | −0.97 [−1.72; −0.38] | −0.06 | 0.55 | −0.05 | 0.57 | −0.69 [−1.18; 0.15] | −0.21 | 0.02 | −0.20 | 0.03 |
| Length of treatment | ||||||||||
| <8 weeks | −0.93 [−1.63; −0.34] | Ref | Ref | −0.30 [−0.85; 0.16] | Ref | Ref | ||||
| ≥8 weeks | −1.01 [−1.61; −0.41] | −0.03 | 0.74 | 0.10 | 0.25 | −0.37 [−0.86; 0.19] | 0.02 | 0.84 | 0.06 | 0.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikièma, V.; Kangas, S.T.; Salpeteur, C.; Briend, A.; Talley, L.; Friis, H.; Ritz, C.; Nexo, E.; McCann, A. Vitamin B12 Status before and after Outpatient Treatment of Severe Acute Malnutrition in Children Aged 6–59 Months: A Sub-Study of a Randomized Controlled Trial in Burkina Faso. Nutrients 2023, 15, 3496. https://doi.org/10.3390/nu15163496
Nikièma V, Kangas ST, Salpeteur C, Briend A, Talley L, Friis H, Ritz C, Nexo E, McCann A. Vitamin B12 Status before and after Outpatient Treatment of Severe Acute Malnutrition in Children Aged 6–59 Months: A Sub-Study of a Randomized Controlled Trial in Burkina Faso. Nutrients. 2023; 15(16):3496. https://doi.org/10.3390/nu15163496
Chicago/Turabian StyleNikièma, Victor, Suvi T. Kangas, Cécile Salpeteur, André Briend, Leisel Talley, Henrik Friis, Christian Ritz, Ebba Nexo, and Adrian McCann. 2023. "Vitamin B12 Status before and after Outpatient Treatment of Severe Acute Malnutrition in Children Aged 6–59 Months: A Sub-Study of a Randomized Controlled Trial in Burkina Faso" Nutrients 15, no. 16: 3496. https://doi.org/10.3390/nu15163496
APA StyleNikièma, V., Kangas, S. T., Salpeteur, C., Briend, A., Talley, L., Friis, H., Ritz, C., Nexo, E., & McCann, A. (2023). Vitamin B12 Status before and after Outpatient Treatment of Severe Acute Malnutrition in Children Aged 6–59 Months: A Sub-Study of a Randomized Controlled Trial in Burkina Faso. Nutrients, 15(16), 3496. https://doi.org/10.3390/nu15163496








