Trabecular Bone Score Preceding and during a 2-Year Follow-Up after Sleeve Gastrectomy: Pitfalls and New Insights
Abstract
1. Introduction
2. Methods
2.1. Study Overview
2.2. Measures and Outcome Variables
2.3. Statistical Analysis
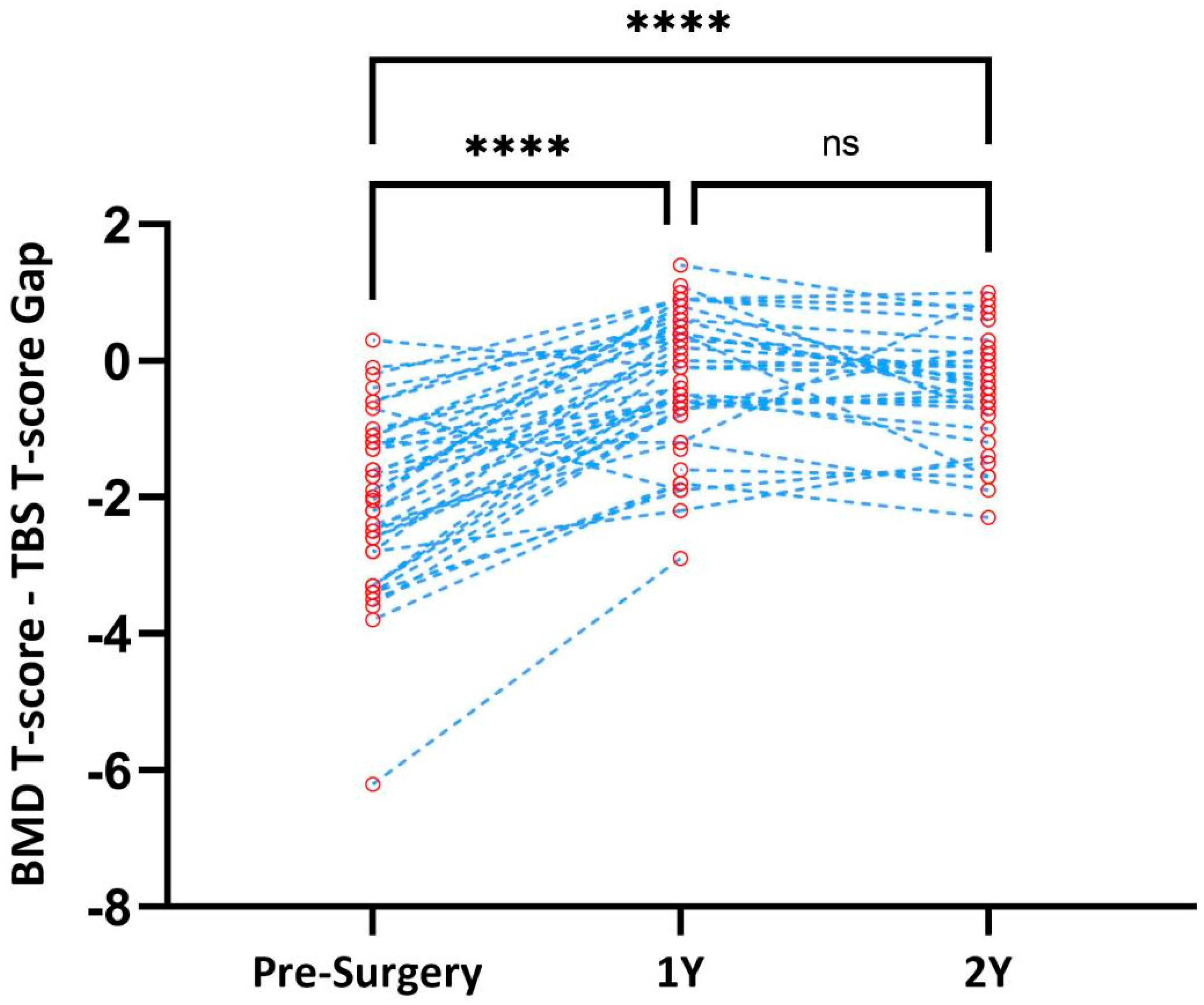
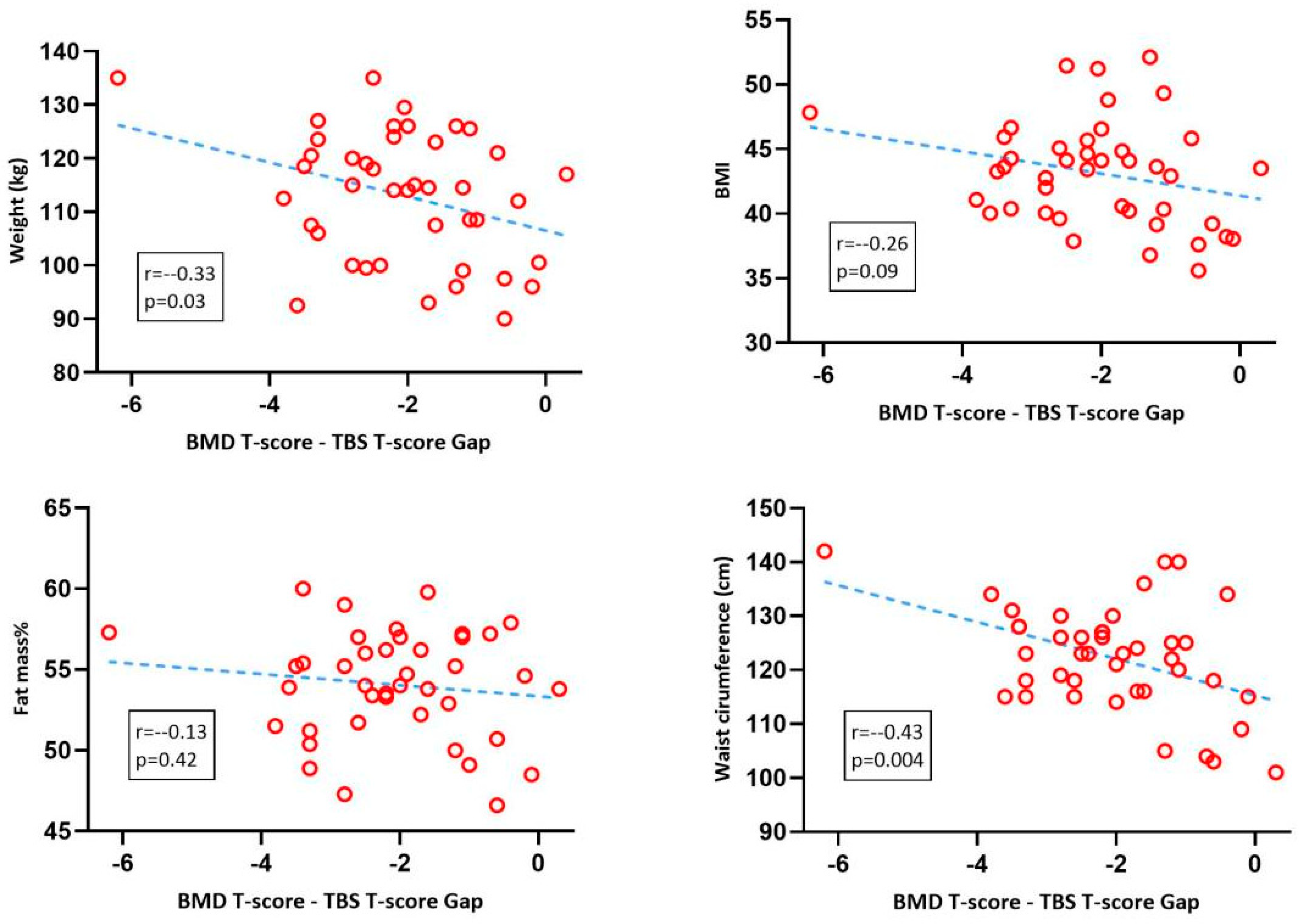
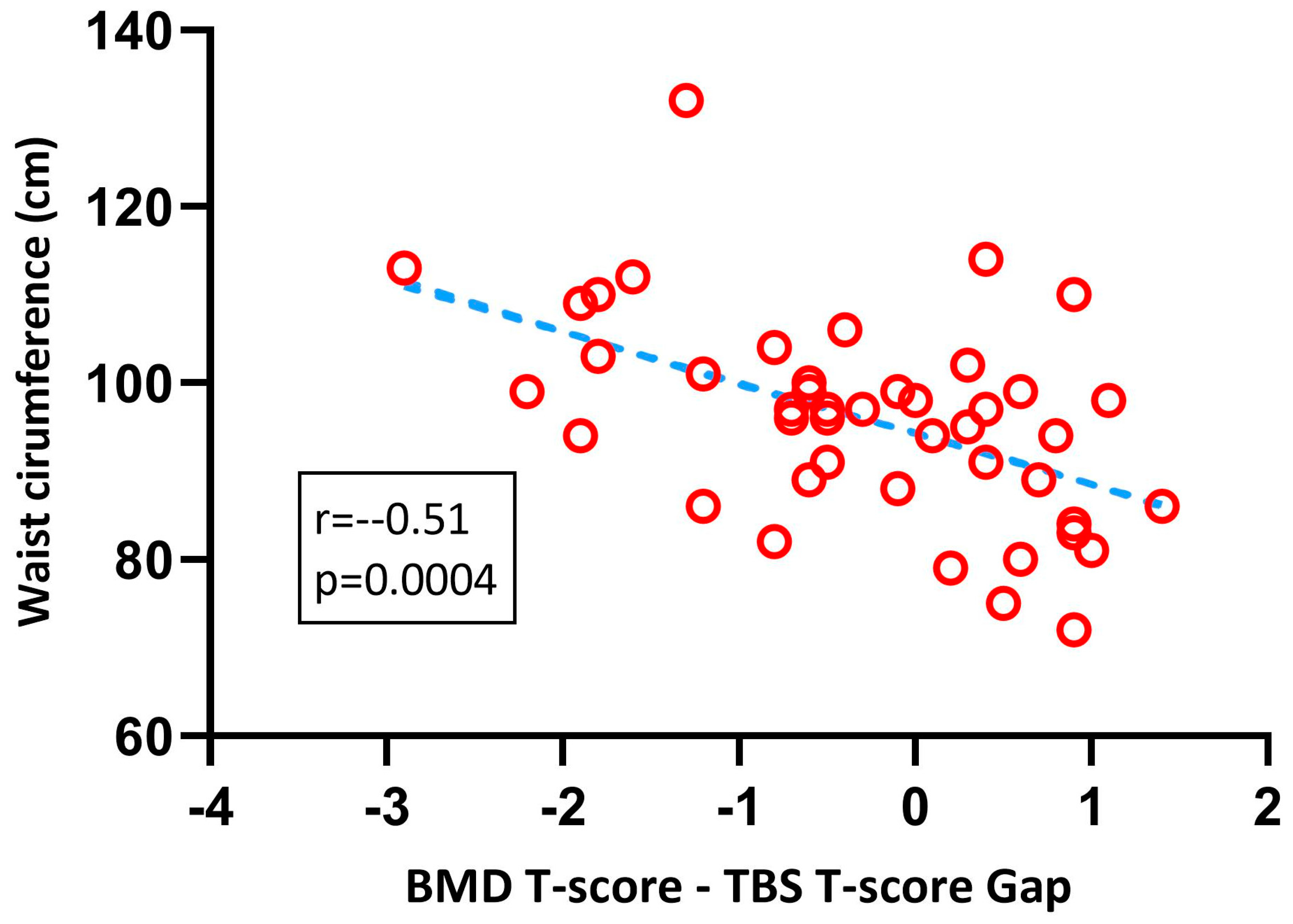
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arterburn, D.E.; Telem, D.A.; Kushner, R.F.; Courcoulas, A.P. Benefits and Risks of Bariatric Surgery in Adults: A Review. J. Am. Med. Assoc. 2020, 324, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Nimeri, A.; Khorgami, Z.; El Chaar, M.; Lima, A.G.; Vosburg, R.W.; on behalf of the American Society for Metabolic and Bariatric Surgery (ASMBS) Clinical Issues Committee. Metabolic bone changes after bariatric surgery: 2020 update, American Society for Metabolic and Bariatric Surgery Clinical Issues Committee position statement. Surg. Obes. Relat. Dis. 2021, 17, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ben-Porat, T.; Elazary, R.; Sherf-Dagan, S.; Goldenshluger, A.; Brodie, R.; Mintz, Y.; Weiss, R. Bone Health following Bariatric Surgery: Implications for Management Strategies to Attenuate Bone Loss. Adv. Nutr. 2018, 9, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Aaseth, J.O.; Alexander, J. Postoperative Osteoporosis in Subjects with Morbid Obesity Undergoing Bariatric Surgery with Gastric Bypass or Sleeve Gastrectomy. Nutrients 2023, 15, 1302. [Google Scholar] [CrossRef]
- Siris, E.S.; Adler, R.; Bilezikian, J.; Bolognese, M.; Dawson-Hughes, B.; Favus, M.J.; Harris, S.T.; Jan de Beur, S.M.; Khosla, S.; Lane, N.E.; et al. The clinical diagnosis of osteoporosis: A position statement from the National Bone Health Alliance Working Group. Osteoporos. Int. 2014, 25, 1439–1443. [Google Scholar] [CrossRef]
- LeBoff, M.S.; Greenspan, S.L.; Insogna, K.L.; Lewiecki, E.M.; Saag, K.G.; Singer, A.J.; Siris, E.S. The clinician’s guide to prevention and treatment of osteoporosis. Osteoporos. Int. 2022, 33, 2049–2102. [Google Scholar] [CrossRef]
- Mele, C.; Caputo, M.; Ferrero, A.; Daffara, T.; Cavigiolo, B.; Spadaccini, D.; Nardone, A.; Prodam, F.; Aimaretti, G.; Marzullo, P. Bone Response to Weight Loss Following Bariatric Surgery. Front. Endocrinol. Lausanne 2022, 13, 921353. [Google Scholar] [CrossRef]
- Silva, B.C.; Leslie, W.D.; Resch, H.; Lamy, O.; Lesnyak, O.; Binkley, N.; McCloskey, E.V.; Kanis, J.A.; Bilezikian, J.P. Trabecular bone score: A noninvasive analytical method based upon the DXA image. J. Bone Miner. Res. 2014, 29, 518–530. [Google Scholar] [CrossRef]
- Weber, D.R.; Boyce, A.; Gordon, C.; Högler, W.; Kecskemethy, H.H.; Misra, M.; Swolin-Eide, D.; Tebben, P.; Ward, L.M.; Wasserman, H.; et al. The Utility of DXA Assessment at the Forearm, Proximal Femur, and Lateral Distal Femur, and Vertebral Fracture Assessment in the Pediatric Population: 2019 ISCD Official Position. J. Clin. Densitom. 2019, 22, 567–589. [Google Scholar] [CrossRef]
- Marengo, A.P.; Guerrero Perez, F.; San Martin, L.; Monseny, R.; Casajoana, A.; Valera, R.; Virgili, N.; Simo Servat, A.; Prats, A.; Gomez-Vaquero, C.; et al. Is Trabecular Bone Score Valuable in Bone Microstructure Assessment after Gastric Bypass in Women with Morbid Obesity? Nutrients 2017, 9, 1314. [Google Scholar] [CrossRef]
- Ben-Porat, T.; Weiss, R.; Khalaileh, A.; Abu Gazala, M.; Kaluti, D.; Mintz, Y.; Sherf-Dagan, S.; Yackobovitch-Gavan, M.; Rottenstreich, A.; Brodie, R.; et al. The impact of preoperative vitamin administration on skeletal status following sleeve gastrectomy in young and middle-aged women: A randomized controlled trial. Int. J. Obes. Lond. 2021, 45, 1925–1936. [Google Scholar] [CrossRef]
- Ben-Porat, T.; Peretz, S.; Rottenstreich, A.; Weiss, R.; Szalat, A.; Elazary, R.; Abu Gazala, M. Changes in bone mineral density following laparoscopic sleeve gastrectomy: 2-year outcomes. Surg. Obes. Relat. Dis. 2022, 18, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Sherf Dagan, S.; Goldenshluger, A.; Globus, I.; Schweiger, C.; Kessler, Y.; Kowen Sandbank, G.; Ben-Porat, T.; Sinai, T. Nutritional Recommendations for Adult Bariatric Surgery Patients: Clinical Practice. Adv. Nutr. 2017, 8, 382–394. [Google Scholar] [CrossRef] [PubMed]
- Parrott, J.; Frank, L.; Rabena, R.; Craggs-Dino, L.; Isom, K.A.; Greiman, L. American Society for Metabolic and Bariatric Surgery Integrated Health Nutritional Guidelines for the Surgical Weight Loss Patient 2016 Update: Micronutrients. Surg. Obes. Relat. Dis. Off. J. Am. Soc. Bariatr. Surg. 2017, 13, 727–741. [Google Scholar] [CrossRef]
- Shuhart, C.R.; Yeap, S.S.; Anderson, P.A.; Jankowski, L.G.; Lewiecki, E.M.; Morse, L.R.; Rosen, H.N.; Weber, D.R.; Zemel, B.S.; Shepherd, J.A. Executive Summary of the 2019 ISCD Position Development Conference on Monitoring Treatment, DXA Cross-calibration and Least Significant Change, Spinal Cord Injury, Peri-prosthetic and Orthopedic Bone Health, Transgender Medicine, and Pediatrics. J. Clin. Densitom. 2019, 22, 453–471. [Google Scholar] [CrossRef] [PubMed]
- Amnuaywattakorn, S.; Sritara, C.; Utamakul, C.; Chamroonrat, W.; Kositwattanarerk, A.; Thamnirat, K.; Ongphiphadhanakul, B. Simulated increased soft tissue thickness artefactually decreases trabecular bone score: A phantom study. BMC Musculoskelet. Disord. 2016, 17, 17. [Google Scholar] [CrossRef]
- Messina, C.; Poloni, A.; Chianca, V.; Albano, D.; Piodi, L.P.; Ulivieri, F.M.; Sconfienza, L.M. Increasing soft tissue thickness does not affect trabecular bone score reproducibility: A phantom study. Endocrine 2018, 61, 336–342. [Google Scholar] [CrossRef]
- Binkley, N.; Krueger, D.; Leslie, W.D. Accurate Weight and Height Measurement is Essential for Correct Trabecular Bone Score Determination. J. Clin. Densitom. 2023, 26, 52–54. [Google Scholar] [CrossRef]
- Mazzetti, G.; Berger, C.; Leslie, W.D.; Hans, D.; Langsetmo, L.; Hanley, D.A.; Kovacs, C.S.; Prior, J.C.; Kaiser, S.M.; Davison, K.S.; et al. Densitometer-Specific Differences in the Correlation Between Body Mass Index and Lumbar Spine Trabecular Bone Score. J. Clin. Densitom. 2017, 20, 233–238. [Google Scholar] [CrossRef]
- Shevroja, E.; Aubry-Rozier, B.; Hans, G.; Gonzalez-Rodriguez, E.; Stoll, D.; Lamy, O.; Hans, D. Clinical Performance of the Updated Trabecular Bone Score (TBS) Algorithm, Which Accounts for the Soft Tissue Thickness: The OsteoLaus Study. J. Bone Miner. Res. 2019, 34, 2229–2237. [Google Scholar] [CrossRef]
- Schacter, G.I.; Leslie, W.D.; Majumdar, S.R.; Morin, S.N.; Lix, L.M.; Hans, D. Clinical performance of an updated trabecular bone score (TBS) algorithm in men and women: The Manitoba BMD cohort. Osteoporos. Int. 2017, 28, 3199–3203. [Google Scholar] [CrossRef] [PubMed]
| Mean | SD | |
|---|---|---|
| Age at surgery (years) | 33.05 | 11.05 |
| Height (cm) | 161.4 | 4.86 |
| Weight (kg) | 112.80 | 12.11 |
| BMI | 43.27 | 4.06 |
| Body fat mass% | 54.54 | 3.33 |
| Waist circumference (cm) | 122.77 | 10.72 |
| BMD T < −2.5 | −2.5 < BMD T< −1.0 | BMD T > −1.0 | TBS < 1.2 | 1.2 < TBS < 1.3 | TBS > 1.3 | |
|---|---|---|---|---|---|---|
| Baseline | 1/52 (1.9%) | 8/52 (15.4%) | 43/52 (82.7%) | 9/42 (21.5%) | 17/42 (40.5%) | 16/42 (38.0%) |
| 1 year | 2/52 (3.8%) | 8/52 (15.4%) | 42/52 (80.8%) | 0/48 (0.0%) | 6/48 (12.5%) | 42/48 (87.5%) |
| 2 years | 1/31 (3.2%) | 3/31 (9.7%) | 27/31 (64.5%) | 0/31 (0.0%) | 8/31 (25.8%) | 23/31 (74.2%) |
| Number of Patients with BMI > 37 kg/m2 | Number of Patients with 35 < BMI < 37 kg/m2 | Number of Patients with BMI < 35 kg/m2 | |
|---|---|---|---|
| Baseline | 49/52 (94.2%) | 3/52 (5.7%) | 0/52 (0.0%) |
| 1 year | 6/52 (11.5%) | 6/52 (11.5%) | 40/52 (76.9%) |
| 2 years | 630 (20%) | 3/30 (10%) | 21/30 (70%) |
| LS BMD | LS T-Score | TBS | TBS- T-Score | T-Gap | |
|---|---|---|---|---|---|
| Baseline | 1.018 ± 0.09 | −0.4 ± 0.93 | 1.25 ± 0.12 (N = 42) | −2.3 ± 1.33 | −2.05 ± 1.26 |
| 1 year | 1.01 ± 0.1 (vs. baseline: −0.93% ± 3.37, p = 0.06) | 0.33 ± 1.00 | 1.41 ± 0.08 (vs. baseline: +12.12% ± 9.34, p < 1.5 × 10−10) | −0.62 ± 0.98 | −0.296 ± 1.03 |
| 2 years | 0.95 ± 0.12 (vs. 1-year: −0.31% ± 4.23) | −0.67 ± 1.00 | 1.36 ± 0.10 (vs. 1-year: −1.78% ± 5.16, p = 0.06) | −1.15 ± 1.12 | −0.44 ± 0.85 |
| BMI kg/m2 (Mean ± SD) | WC in cm (Mean ± SD) | |
|---|---|---|
| Baseline | 43.26 ± 4.1 | 122.7 ± 10 |
| 1 year | 31.25 ± 4.8 | 95.9 ± 11.8 |
| 2 years | 32.74 ± 4.7 | 96.7 ± 11.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stokar, J.; Ben-Porat, T.; Kaluti, D.; Abu-Gazala, M.; Weiss, R.; Mintz, Y.; Elazari, R.; Szalat, A. Trabecular Bone Score Preceding and during a 2-Year Follow-Up after Sleeve Gastrectomy: Pitfalls and New Insights. Nutrients 2023, 15, 3481. https://doi.org/10.3390/nu15153481
Stokar J, Ben-Porat T, Kaluti D, Abu-Gazala M, Weiss R, Mintz Y, Elazari R, Szalat A. Trabecular Bone Score Preceding and during a 2-Year Follow-Up after Sleeve Gastrectomy: Pitfalls and New Insights. Nutrients. 2023; 15(15):3481. https://doi.org/10.3390/nu15153481
Chicago/Turabian StyleStokar, Joshua, Tair Ben-Porat, Donia Kaluti, Mahmud Abu-Gazala, Ram Weiss, Yoav Mintz, Ram Elazari, and Auryan Szalat. 2023. "Trabecular Bone Score Preceding and during a 2-Year Follow-Up after Sleeve Gastrectomy: Pitfalls and New Insights" Nutrients 15, no. 15: 3481. https://doi.org/10.3390/nu15153481
APA StyleStokar, J., Ben-Porat, T., Kaluti, D., Abu-Gazala, M., Weiss, R., Mintz, Y., Elazari, R., & Szalat, A. (2023). Trabecular Bone Score Preceding and during a 2-Year Follow-Up after Sleeve Gastrectomy: Pitfalls and New Insights. Nutrients, 15(15), 3481. https://doi.org/10.3390/nu15153481







