Association of Serum Calcium with the Risk of Chronic Obstructive Pulmonary Disease: A Prospective Study from UK Biobank
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Assessment of Biomarkers
2.3. Ascertainment of Outcomes
2.4. Ascertainment of Covariates
2.5. Statistical Analysis
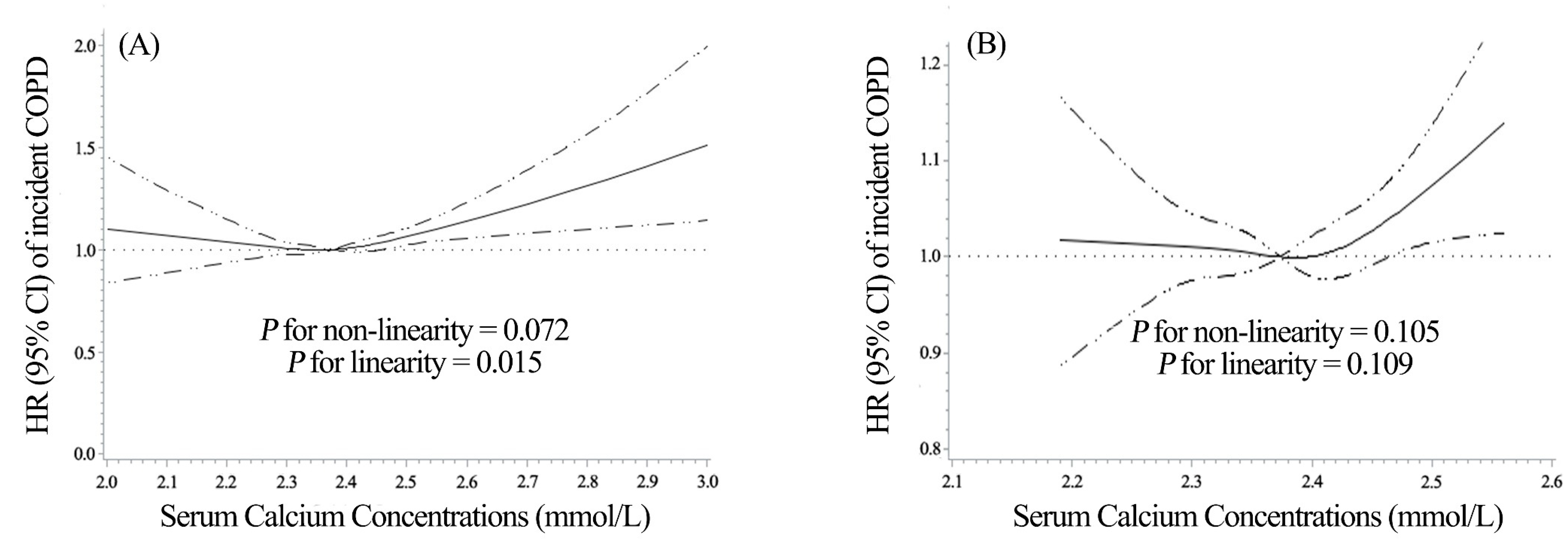
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baird, G.S. Ionized calcium. Clin. Chim. Acta 2011, 412, 696–701. [Google Scholar] [CrossRef]
- Vannucci, L.; Fossi, C.; Quattrini, S.; Guasti, L.; Pampaloni, B.; Gronchi, G.; Giusti, F.; Romagnoli, C.; Cianferotti, L.; Marcucci, G.; et al. Calcium Intake in Bone Health: A Focus on Calcium-Rich Mineral Waters. Nutrients 2018, 10, 1930. [Google Scholar] [CrossRef]
- Cheng, H.-P.; Wei, S.; Wei, L.-P.; Verkhratsky, A. Calcium signaling in physiology and pathophysiology. Acta Pharmacol. Sin. 2006, 27, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Berchtold, M.W.; Brinkmeier, H.; Müntener, M.; VanderVeen, B.N.; Hardee, J.P.; Fix, D.K.; Carson, J.A.; Fajardo, V.A.; Rietze, B.A.; Chambers, P.J.; et al. Calcium Ion in Skeletal Muscle: Its Crucial Role for Muscle Function, Plasticity, and Disease. Physiol. Rev. 2000, 80, 1215–1265. [Google Scholar] [CrossRef]
- Nanou, E.; Catterall, W.A. Calcium Channels, Synaptic Plasticity, and Neuropsychiatric Disease. Neuron 2018, 98, 466–481. [Google Scholar] [CrossRef]
- Clapham, D.E. Calcium Signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Peacock, M. Calcium Metabolism in Health and Disease. Clin. J. Am. Soc. Nephrol. 2010, 5 (Suppl. S1), S23–S30. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Gao, T.; Fang, W.; Xian-Yu, C.; Deng, N.; Zhang, C.; Niu, Y. Global, regional and national burden of chronic obstructive pulmonary disease over a 30-year period: Estimates from the 1990 to 2019 Global Burden of Disease Study. Respirology 2023, 28, 29–36. [Google Scholar] [CrossRef]
- Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2020 Report. Available online: https://goldcopd.org/gold-reports/ (accessed on 17 June 2023).
- Stockley, R.A.; Halpin, D.M.G.; Celli, B.R.; Singh, D. Chronic Obstructive Pulmonary Disease Biomarkers and Their Interpretation. Am. J. Respir. Crit. Care Med. 2019, 199, 1195–1204. [Google Scholar] [CrossRef]
- Sagar, S.; Kapoor, H.; Chaudhary, N.; Roy, S.S. Cellular and mitochondrial calcium communication in obstructive lung disorders. Mitochondrion 2021, 58, 184–199. [Google Scholar] [CrossRef]
- Yang, M.; Miao, J.; Du, L.; Wang, J.; Yang, J.; Lu, J.; Fan, X.; Huang, C.; Fu, Z.; Xu, Z.; et al. Serum Calcium Concentrations and Risk of All-Cause and Cause-Specific Mortality: Results From 2 Prospective Cohorts. J. Clin. Endocrinol. Metab. 2023, 108, e527–e535. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Hu, J.; Liu, Z.; Wang, E.; Guo, Q.; Zhang, Z.; Song, Z. L-shaped association of serum calcium with all-cause and CVD mortality in the US adults: A population-based prospective cohort study. Front. Nutr. 2022, 9, 1097488. [Google Scholar] [CrossRef]
- Truong, L.; Zheng, Y.-M.; Kandhi, S.; Wang, Y.-X. Overview on Interactive Role of Inflammation, Reactive Oxygen Species, and Calcium Signaling in Asthma, Copd, and Pulmonary Hypertension. Adv. Exp. Med. Biol. 2021, 1304, 147–164. [Google Scholar] [CrossRef]
- Quanjer, P.H.; Stanojevic, S.; Cole, T.J.; Baur, X.; Hall, G.L.; Culver, B.H.; Enright, P.L.; Hankinson, J.L.; Ip, M.S.M.; Zheng, J.; et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: The global lung function 2012 equations. Eur. Respir. J. 2012, 40, 1324–1343. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- UK Biobank: Protocol for a Large-Scale Prospective Epidemiological Resource. Available online: https://www.ukbiobank.ac.uk/media/gnkeyh2q/study-rationale.pdf (accessed on 17 June 2023).
- Schini, M.; Hannan, F.M.; Walsh, J.S.; Eastell, R. Reference interval for albumin-adjusted calcium based on a large UK population. Clin. Endocrinol. 2021, 94, 34–39. [Google Scholar] [CrossRef]
- Vestbo, J.; Hurd, S.S.; Agustí, A.G.; Jones, P.W.; Vogelmeier, C.; Anzueto, A.; Barnes, P.J.; Fabbri, L.M.; Martinez, F.J.; Nishimura, M.; et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease GOLD Executive Summary. Am. J. Respir. Crit. Care Med. 2013, 187, 347–365. [Google Scholar] [CrossRef] [PubMed]
- Hirota, N.; Martin, J.G. Mechanisms of Airway Remodeling. Chest 2013, 144, 1026–1032. [Google Scholar] [CrossRef]
- Yan, F.; Gao, H.; Zhao, H.; Bhatia, M.; Zeng, Y. Roles of airway smooth muscle dysfunction in chronic obstructive pulmonary disease. J. Transl. Med. 2018, 16, 262. [Google Scholar] [CrossRef]
- Malińska, D.; Więckowski, M.R.; Michalska, B.; Drabik, K.; Prill, M.; Patalas-Krawczyk, P.; Walczak, J.; Szymański, J.; Mathis, C.; Van der Toorn, M.; et al. Mitochondria as a possible target for nicotine action. J. Bioenerg. Biomembr. 2019, 51, 259–276. [Google Scholar] [CrossRef]
- Sassano, M.F.; Ghosh, A.; Tarran, R. Tobacco Smoke Constituents Trigger Cytoplasmic Calcium Release. Appl. In Vitro Toxicol. 2017, 3, 193–198. [Google Scholar] [CrossRef]
- Gerzanich, V.; Wang, F.; Kuryatov, A.; Lindstrom, J. alpha 5 Subunit alters desensitization, pharmacology, Ca++ permeability and Ca++ modulation of human neuronal alpha 3 nicotinic receptors. J. Pharmacol. Exp. Ther. 1998, 286, 311–320. [Google Scholar] [PubMed]
- Billington, C.K.; Penn, R.B. Signaling and regulation of G protein-coupled receptors in airway smooth muscle. Respir. Res. 2003, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Gosling, M.; Poll, C.; Li, S. TRP channels in airway smooth muscle as therapeutic targets. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2005, 371, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Ay, B.; Prakash, Y.S.; Pabelick, C.M.; Sieck, G.C. Store-operated Ca2+ entry in porcine airway smooth muscle. Am. J. Physiol. Cell. Mol. Physiol. 2004, 286, L909–L917. [Google Scholar] [CrossRef]
- Zhu, G.; Gulsvik, A.; Bakke, P.; Ghatta, S.; Anderson, W.; Lomas, D.A.; Silverman, E.K.; Pillai, S.G. ICGN Investigators Association of TRPV4 gene polymorphisms with chronic obstructive pulmonary disease. Hum. Mol. Genet. 2009, 18, 2053–2062. [Google Scholar] [CrossRef]
- Jha, A.; Sharma, P.; Anaparti, V.; Ryu, M.H.; Halayko, A.J. A role for transient receptor potential ankyrin 1 cation channel (TRPA1) in airway hyper-responsiveness? Can. J. Physiol. Pharmacol. 2015, 93, 171–176. [Google Scholar] [CrossRef]
- Pan, S.; Conaway, S., Jr.; Deshpande, D.A. Mitochondrial regulation of airway smooth muscle functions in health and pulmonary diseases. Arch. Biochem. Biophys. 2019, 663, 109–119. [Google Scholar] [CrossRef]
- Hough, K.; Curtiss, M.L.; Blain, T.J.; Liu, R.-M.; Trevor, J.; Deshane, J.S.; Thannickal, V.J. Airway Remodeling in Asthma. Front. Med. 2020, 7, 191. [Google Scholar] [CrossRef]
- Durup, D.; Jørgensen, H.L.; Christensen, J.; Schwarz, P.; Heegaard, A.M.; Lind, B. A Reverse J-Shaped Association of All-Cause Mortality with Serum 25-Hydroxyvitamin D in General Practice: The CopD Study. J. Clin. Endocrinol. Metab. 2012, 97, 2644–2652. [Google Scholar] [CrossRef]
- Lu, J.L.; Molnar, M.Z.; Ma, J.Z.; George, L.K.; Sumida, K.; Kalantar-Zadeh, K.; Kovesdy, C.P. Racial Differences in Association of Serum Calcium with Mortality and Incident Cardio- and Cerebrovascular Events. J. Clin. Endocrinol. Metab. 2016, 101, 4851–4859. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Papagerakis, P.; Mitsiadis, T.A.; Rosen, C.J.; Compston, J.E.; Lian, J.B. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 171–179. ISBN 9780977888214. [Google Scholar]
- NICE. Hyperparathyroidism (Primary): Diagnosis, Assessment and Initial Management; National Institute for Health and Care Excellence: London, UK, 2019. Available online: https://www.ncbi.nlm.nih.gov/books/NBK542087/ (accessed on 17 June 2023).
- Crosswhite, P.; Sun, Z. Molecular Mechanisms of Pulmonary Arterial Remodeling. Mol. Med. 2014, 20, 191–201. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Albumin-Corrected Calcium Concentrations (mmol/L) | |||
|---|---|---|---|---|
| Total | Hypocalcemia (<2.19) | Normal (2.19–2.56) | Hypercalcemia (≥2.56) | |
| N | 386,844 | 2272 | 375,897 | 8675 |
| Age, mean (SD), y | 56.48 (8.1) | 54.82 (8.5) | 56.43 (8.1) | 59.30 (7.0) |
| Follow-up time, mean (SD), y | 11.75 (2.2) | 11.76 (2.4) | 11.76 (2.2) | 11.53 (2.3) |
| Female, No. (%) | 209,724(54.2) | 994 (43.8) | 202,191 (53.8) | 6539 (75.4) |
| White race, No. (%) | 365,358 (94.5) | 2097 (92.5) | 355,128 (94.6) | 8133 (93.8) |
| College or university degree, No. (%) | 127,174 (32.9) | 893 (39.4) | 123,971 (33.0) | 2310 (26.7) |
| Fasting before blood draw, No. (%) | 15,471 (4.0) | 149 (6.6) | 15,051 (4.0) | 271 (3.1) |
| Townsend index, mean (SD) | −1.39 (3.0) | −1.00 (3.2) | −1.39 (3.0) | −1.34 (3.0) |
| Smoking status, No. (%) a | ||||
| Never | 216,063 (55.9) | 1323 (58.3) | 209,932 (55.9) | 4808 (55.5) |
| Previous | 133,061 (34.4) | 734 (32.4) | 129,290 (34.4) | 3037 (35.0) |
| Current, pack-years <10 | 4811 (1.2) | 32 (1.4) | 4704 (1.3) | 75 (0.9) |
| Current, pack-years ≥10 and <20 | 6799 (1.8) | 30 (1.3) | 6623 (1.8) | 146 (1.7) |
| Current, pack-years ≥20 and <30 | 6264 (1.6) | 39 (1.7) | 6070 (1.6) | 155 (1.8) |
| Current, pack-years ≥30 | 9853 (2.6) | 44 (1.9) | 9560 (2.6) | 249 (2.9) |
| Passive smoking, No. (%) a | ||||
| Never | 257,510 (71.4) | 1510 (71.0) | 250,372 (71.4) | 5628 (69.7) |
| <20 h a week | 69,187 (19.2) | 430 (20.2) | 67,207 (19.17) | 1550 (19.2) |
| ≥20 h a week | 4229 (1.2) | 28 (1.3) | 4115 (1.2) | 86 (1.1) |
| PM2.5, mean (SD), μg/m3 | 9.97 (1.1) | 10.02 (1.1) | 9.97 (1.1) | 9.97 (1.0) |
| Alcohol intake frequency, No. (%) a | ||||
| Never or special occasions only | 73,738 (19.1) | 448 (19.8) | 70,983 (18.9) | 2307 (26.6) |
| Once a month to twice a week | 143,781 (37.2) | 828 (36.5) | 139,715 (37.2) | 3238 (37.4) |
| Three times a week to daily | 168,518 (43.6) | 989 (43.6) | 164,422 (43.8) | 3107 (35.9) |
| Body mass index, mean (SD), kg/m2 | 27.50 (4.8) | 27.31 (5.0) | 27.48 (4.8) | 27.99 (5.1) |
| eGFR, mL/min/1.73 m2 | 90.89 (13.4) | 94.12 (16.1) | 90.97 (13.30) | 86.55 (14.9) |
| 25(OH)D, nmol/L | 48.72 (21.0) | 43.41 (20.7) | 48.74 (21.0) | 49.54 (21.0) |
| Physical activity, mean (SD), MET-hour/week | 15.54 (20.4) | 13.97 (18.8) | 15.55 (20.4) | 15.77 (20.5) |
| Family history of respiratory disease, No. (%) | 57,328 (14.8) | 309 (13.6) | 55,532 (14.8) | 1487 (17.1) |
| Prevalent Asthma, No. (%) | 39,142 (10.1) | 221 (9.7) | 37,972 (10.1) | 949 (10.9) |
| Occupations associated with COPD, No. (%) | 7259 (1.9) | 48 (2.1) | 7029 (1.9) | 182 (2.1) |
| Groups | No. of COPD /Person-Years | Basic Model a | Fully Adjusted Model b |
|---|---|---|---|
| Deciles of albumin-corrected calcium concentrations (mmol/L) | |||
| Decile 1 (1.12–2.28) | 916/458,796 | 0.96 (0.88–1.05) | 1.02 (0.93–1.12) |
| Decile 2 (2.28–2.31) | 943/459,001 | 0.96 (0.88–1.05) | 1.00 (0.92–1.10) |
| Decile 3 (2.31–2.34) | 977/456,543 | 1.00 (0.91–1.09) | 1.04 (0.95–1.14) |
| Decile 4 (2.34–2.36) | 955/456,728 | 0.96 (0.88–1.05) | 0.98 (0.90–1.07) |
| Decile 5 (2.36–2.38) | 1000/456,149 | ref | ref |
| Decile 6 (2.38–2.39) | 1071/454,612 | 1.06 (0.97–1.15) | 1.05 (0.96–1.14) |
| Decile 7 (2.39–2.41) | 1063/453,479 | 1.04 (0.96–1.14) | 0.99 (0.91–1.08) |
| Decile 8 (2.41–2.44) | 1085/452,444 | 1.06 (0.98–1.16) | 1.00 (0.91–1.09) |
| Decile 9 (2.44–2.48) | 1237/450,688 | 1.20 (1.10–1.30) | 1.07 (0.98–1.16) |
| Decile 10 (2.48–3.56) | 1335/448,147 | 1.25 (1.15–1.36) | 1.10 (1.01–1.20) |
| Reference interval for UK Biobank population (mmol/L) | |||
| Hypocalcemia (<2.19) | 59/26,723 | 1.02 (0.79–1.32) | 1.07 (0.83–1.39) |
| Normal (2.19–2.56) | 10,188/4,419,880 | ref | ref |
| Hypercalcemia (≥2.56) | 335/99,983 | 1.30 (1.16–1.45) | 1.14 (1.02–1.27) |
| p for trend | <0.0001 | 0.020 | |
| HR per 1-SD increment c | 1.09 (1.07–1.11) | 1.02 (1.00–1.04) | |
| Model | Reference Interval for UK Biobank Population (mmol/L) | ||||
|---|---|---|---|---|---|
| Hypocalcemia (<2.19) | Normal (2.19–2.56) | Hypercalcemia (>2.56) | p for Trend | HR per 1-SD Increment c | |
| All-cause | |||||
| death cases/total cases | 21/59 | 2544/10,188 | 97/335 | ||
| Basic model a | 1.19 (0.77–1.83) | Ref | 1.29 (1.05–1.58) | <0.0001 | 1.09 (1.05–1.13) |
| Fully-adjusted model b | 1.23 (0.80–1.89) | Ref | 1.24 (1.01–1.53) | <0.0001 | 1.09 (1.05–1.13) |
| COPD-specific | |||||
| death cases/total cases | 3/59 | 200/10,188 | 12/335 | ||
| Basic model a | 1.89 (0.60–5.97) | Ref | 2.17 (1.20–3.90) | 0.343 | 1.07 (0.93–1.22) |
| Fully-adjusted model b | 1.80 (0.56–5.78) | Ref | 2.09 (1.15–3.81) | 0.254 | 1.08 (0.94–1.24) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, X.; Chen, L.; Zhu, Z.; Luo, P.; Hang, D.; Su, J.; Tao, R.; Zhou, J.; Fan, X. Association of Serum Calcium with the Risk of Chronic Obstructive Pulmonary Disease: A Prospective Study from UK Biobank. Nutrients 2023, 15, 3439. https://doi.org/10.3390/nu15153439
Wan X, Chen L, Zhu Z, Luo P, Hang D, Su J, Tao R, Zhou J, Fan X. Association of Serum Calcium with the Risk of Chronic Obstructive Pulmonary Disease: A Prospective Study from UK Biobank. Nutrients. 2023; 15(15):3439. https://doi.org/10.3390/nu15153439
Chicago/Turabian StyleWan, Xinglin, Lulu Chen, Zheng Zhu, Pengfei Luo, Dong Hang, Jian Su, Ran Tao, Jinyi Zhou, and Xikang Fan. 2023. "Association of Serum Calcium with the Risk of Chronic Obstructive Pulmonary Disease: A Prospective Study from UK Biobank" Nutrients 15, no. 15: 3439. https://doi.org/10.3390/nu15153439
APA StyleWan, X., Chen, L., Zhu, Z., Luo, P., Hang, D., Su, J., Tao, R., Zhou, J., & Fan, X. (2023). Association of Serum Calcium with the Risk of Chronic Obstructive Pulmonary Disease: A Prospective Study from UK Biobank. Nutrients, 15(15), 3439. https://doi.org/10.3390/nu15153439





