A Three-Arm, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Safety of Lactobacillus salivarius AP-32 and Bifidobacterium animalis CP-9 Used Individually in Healthy Infants
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Products
2.2. Study Design
2.3. Study Outcome and Sample Collection
2.4. Statistical Analysis
2.5. Fecal DNA Extraction
2.6. Next-Generation Sequencing (NGS) Analysis
2.7. Bioinformatics Analysis and Statistics
3. Results
3.1. Analysis Population
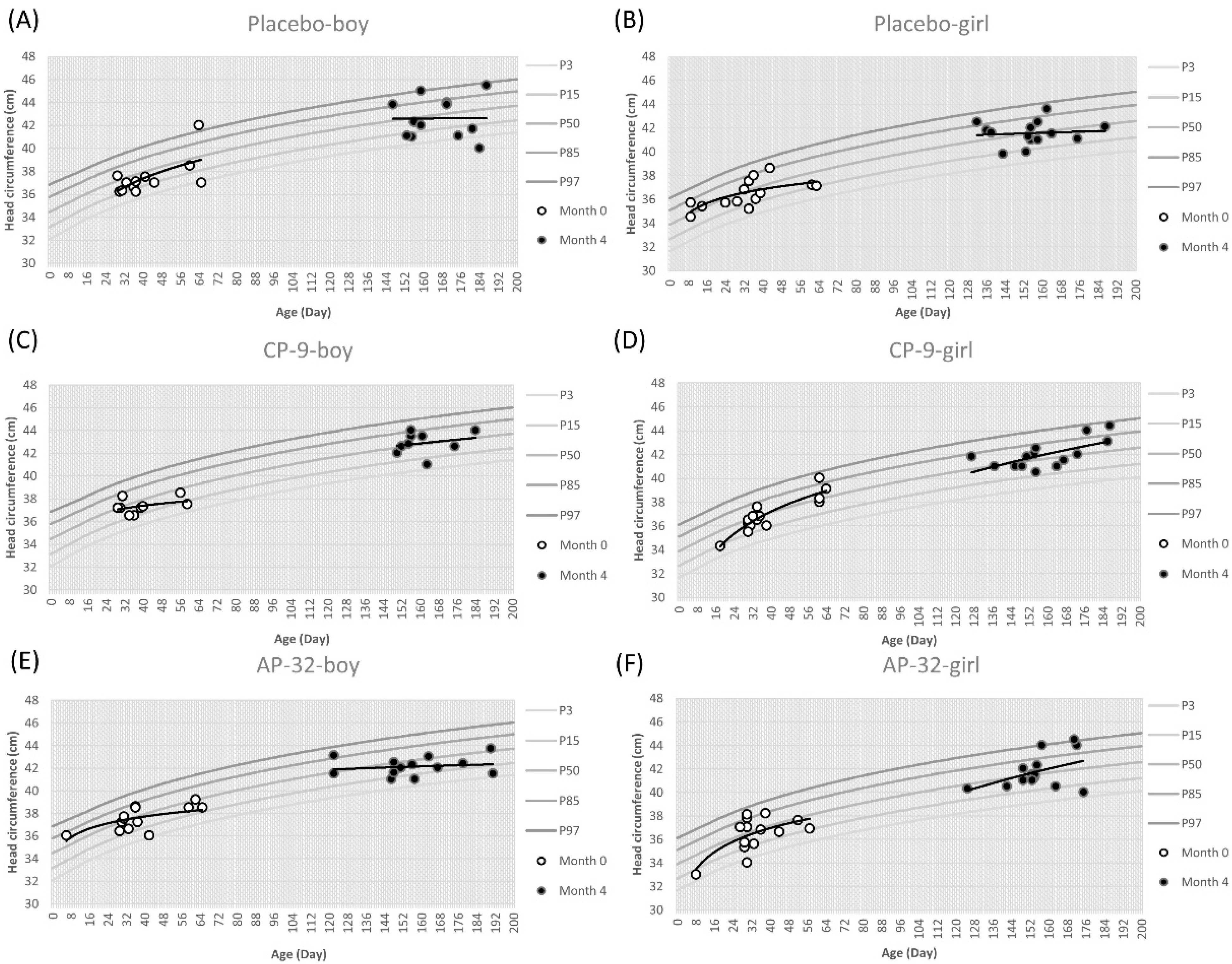
3.2. Growth of Infants
3.3. Infant Health
3.4. Gut Microbiota Modulation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Snydman, D.R. The safety of probiotics. Clin. Infect. Dis. 2008, 46 (Suppl. 2), S104–S111; discussion S144–S151. [Google Scholar] [CrossRef] [PubMed]
- Liong, M.T. Safety of probiotics: Translocation and infection. Nutr. Rev. 2008, 66, 192–202. [Google Scholar] [CrossRef]
- Roberts, A.; Haighton, L.A. A hard look at FDA’s review of GRAS notices. Regul. Toxicol. Pharmacol. 2016, 79 (Suppl. 2), S124–S128. [Google Scholar] [CrossRef]
- Malmuthuge, N.; Griebel, P.J. Fetal environment and fetal intestine are sterile during the third trimester of pregnancy. Vet. Immunol. Immunopathol. 2018, 204, 59–64. [Google Scholar] [CrossRef]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The impact of the gut microbiota on human health: An integrative view. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef]
- Le Doare, K.; Holder, B.; Bassett, A.; Pannaraj, P.S. Mother’s Milk: A Purposeful Contribution to the Development of the Infant Microbiota and Immunity. Front. Immunol. 2018, 9, 361. [Google Scholar] [CrossRef]
- Pannaraj, P.S.; Li, F.; Cerini, C.; Bender, J.M.; Yang, S.; Rollie, A.; Adisetiyo, H.; Zabih, S.; Lincez, P.J.; Bittinger, K.; et al. Association Between Breast Milk Bacterial Communities and Establishment and Development of the Infant Gut Microbiome. JAMA Pediatr. 2017, 171, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.; Heilig, G.H.; Zoetendal, E.G.; Smidt, H.; Rodriguez, J.M. Diversity of the Lactobacillus group in breast milk and vagina of healthy women and potential role in the colonization of the infant gut. J. Appl. Microbiol. 2007, 103, 2638–2644. [Google Scholar] [CrossRef] [PubMed]
- Solis, G.; de Los Reyes-Gavilan, C.G.; Fernandez, N.; Margolles, A.; Gueimonde, M. Establishment and development of lactic acid bacteria and bifidobacteria microbiota in breast-milk and the infant gut. Anaerobe 2010, 16, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Mugambi, M.N.; Musekiwa, A.; Lombard, M.; Young, T.; Blaauw, R. Synbiotics, probiotics or prebiotics in infant formula for full term infants: A systematic review. Nutr. J. 2012, 11, 81. [Google Scholar] [CrossRef]
- van den Nieuwboer, M.; Claassen, E.; Morelli, L.; Guarner, F.; Brummer, R.J. Probiotic and synbiotic safety in infants under two years of age. Benef. Microbes 2014, 5, 45–60. [Google Scholar] [CrossRef]
- Chi, C.; Li, C.; Buys, N.; Wang, W.; Yin, C.; Sun, J. Effects of Probiotics in Preterm Infants: A Network Meta-analysis. Pediatrics 2021, 147, e20200706. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.Y.; Wang, Y.C.; Liao, C.A.; Su, C.Y.; Huang, C.H.; Chiu, M.H.; Yeh, Y.T. Safety and the probiotic potential of Bifidobacterium animalis CP-9. J. Food Sci. 2022, 87, 2211–2228. [Google Scholar] [CrossRef]
- Lin, W.Y.; Lin, J.H.; Kuo, Y.W.; Chiang, P.R.; Ho, H.H. Probiotics and their Metabolites Reduce Oxidative Stress in Middle-Aged Mice. Curr. Microbiol. 2022, 79, 104. [Google Scholar] [CrossRef]
- Hsieh, P.S.; Tsai, Y.C.; Chen, Y.C.; Teh, S.F.; Ou, C.M.; King, V.A. Eradication of Helicobacter pylori infection by the probiotic strains Lactobacillus johnsonii MH-68 and L. salivarius ssp. salicinius AP-32. Helicobacter 2012, 17, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Nurrahma, B.A.; Tsao, S.P.; Wu, C.H.; Yeh, T.H.; Hsieh, P.S.; Panunggal, B.; Huang, H.Y. Probiotic Supplementation Facilitates Recovery of 6-OHDA-Induced Motor Deficit via Improving Mitochondrial Function and Energy Metabolism. Front. Aging Neurosci. 2021, 13, 668775. [Google Scholar] [CrossRef] [PubMed]
- Tsao, S.P.; Nurrahma, B.A.; Kumar, R.; Wu, C.H.; Yeh, T.H.; Chiu, C.C.; Lee, Y.P.; Liao, Y.C.; Huang, C.H.; Yeh, Y.T.; et al. Probiotic Enhancement of Antioxidant Capacity and Alterations of Gut Microbiota Composition in 6-Hydroxydopamin-Induced Parkinson’s Disease Rats. Antioxidants 2021, 10, 1823. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Hsieh, P.S.; Ho, H.H.; Hsieh, S.H.; Kuo, Y.W.; Yang, S.F.; Lin, C.W. Antibacterial activity of viable and heat-killed probiotic strains against oral pathogens. Lett. Appl. Microbiol. 2020, 70, 310–317. [Google Scholar] [CrossRef]
- Lin, W.Y.; Kuo, Y.W.; Chen, C.W.; Huang, Y.F.; Hsu, C.H.; Lin, J.H.; Liu, C.R.; Chen, J.F.; Hsia, K.C.; Ho, H.H. Viable and Heat-Killed Probiotic Strains Improve Oral Immunity by Elevating the IgA Concentration in the Oral Mucosa. Curr. Microbiol. 2021, 78, 3541–3549. [Google Scholar] [CrossRef]
- Hsieh, P.S.; Ho, H.H.; Tsao, S.P.; Hsieh, S.H.; Lin, W.Y.; Chen, J.F.; Kuo, Y.W.; Tsai, S.Y.; Huang, H.Y. Multi-strain probiotic supplement attenuates streptozotocin-induced type-2 diabetes by reducing inflammation and beta-cell death in rats. PLoS ONE 2021, 16, e0251646. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Yen, H.R.; Lu, W.L.; Ho, H.H.; Lin, W.Y.; Kuo, Y.W.; Huang, Y.Y.; Tsai, S.Y.; Lin, H.C. Adjuvant Probiotics of Lactobacillus salivarius subsp. salicinius AP-32, L. johnsonii MH-68, and Bifidobacterium animalis subsp. lactis CP-9 Attenuate Glycemic Levels and Inflammatory Cytokines in Patients with Type 1 Diabetes Mellitus. Front Endocrinol 2022, 13, 754401. [Google Scholar] [CrossRef]
- Liao, C.A.; Huang, C.H.; Ho, H.H.; Chen, J.F.; Kuo, Y.W.; Lin, J.H.; Tsai, S.Y.; Tsai, H.Y.; Yeh, Y.T. A Combined Supplement of Probiotic Strains AP-32, bv-77, and CP-9 Increased Akkermansia mucinphila and Reduced Non-Esterified Fatty Acids and Energy Metabolism in HFD-Induced Obese Rats. Nutrients 2022, 14, 527. [Google Scholar] [CrossRef] [PubMed]
- Gil-Campos, M.; López, M.Á.; Rodriguez-Benítez, M.V.; Romero, J.; Roncero, I.; Linares, M.D.; Maldonado, J.; López-Huertas, E.; Berwind, R.; Ritzenthaler, K.L. Lactobacillus fermentum CECT 5716 is safe and well tolerated in infants of 1–6 months of age: A randomized controlled trial. Pharmacol. Res. 2012, 65, 231–238. [Google Scholar] [CrossRef]
- Scientific Committee on Food. Report of the Scientific Committee on Food on the Revision of Essential Requirements of Infant Formulae and Follow-On Formulae (SCF/CS/NUT/IF/65 Final); European Commission: Brussels, Belgium, 2003. [Google Scholar]
- Mukaka, M.M. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Chong, J.; Liu, P.; Zhou, G.; Xia, J. Using MicrobiomeAnalyst for comprehensive statistical, functional, and meta-analysis of microbiome data. Nat. Protoc. 2020, 15, 799–821. [Google Scholar] [CrossRef]
- Dhariwal, A.; Chong, J.; Habib, S.; King, I.L.; Agellon, L.B.; Xia, J. MicrobiomeAnalyst: A web-based tool for comprehensive statistical, visual and meta-analysis of microbiome data. Nucleic Acids Res. 2017, 45, W180–W188. [Google Scholar] [CrossRef]
- McDonald, D.; Price, M.N.; Goodrich, J.; Nawrocki, E.P.; DeSantis, T.Z.; Probst, A.; Andersen, G.L.; Knight, R.; Hugenholtz, P. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 2012, 6, 610–618. [Google Scholar] [CrossRef]
- Damian, M.R.; Cortes-Perez, N.G.; Quintana, E.T.; Ortiz-Moreno, A.; Garfias Noguez, C.; Cruceno-Casarrubias, C.E.; Sanchez Pardo, M.E.; Bermudez-Humaran, L.G. Functional Foods, Nutraceuticals and Probiotics: A Focus on Human Health. Microorganisms 2022, 10, 1065. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Eriksson, B.; Petzold, M.; Bondjers, G.; Tran, T.K.; Nguyen, L.T.; Ascher, H. Factors associated with physical growth of children during the first two years of life in rural and urban areas of Vietnam. BMC Pediatr. 2013, 13, 149. [Google Scholar] [CrossRef]
- Pankaj, S.; Kanchan, K. Correlation of birth weight with other anthropometric parameters of newborns in Himachal Pradesh. Int. J. Cur. Res. Rev. 2017, 9, 49–54. [Google Scholar]
- Mohamed Ahmed Ayed, M.; Mohamed Gad El-Bastwese, R.; Mohamed Mahmoud, T.; Mohamed Thabet, A.; Farouk Mohamed, M. The Relation between Gestational Age and Anthropometric Measurements among Newborns. Egypt. J. Health Care 2021, 12, 404–417. [Google Scholar] [CrossRef]
- Lind, M.V.; Larnkjaer, A.; Molgaard, C.; Michaelsen, K.F. Breastfeeding, Breast Milk Composition, and Growth Outcomes. Nestle Nutr. Inst. Workshop Ser. 2018, 89, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Lakshman, R.; Zhang, J.; Zhang, J.; Koch, F.S.; Marcus, C.; Ludvigsson, J.; Ong, K.K.; Sobko, T. Higher maternal education is associated with favourable growth of young children in different countries. J. Epidemiol. Commun. Health 2013, 67, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Abuya, B.A.; Ciera, J.; Kimani-Murage, E. Effect of mother’s education on child’s nutritional status in the slums of Nairobi. BMC Pediatr. 2012, 12, 80. [Google Scholar] [CrossRef]
- Onubi, O.J.; Poobalan, A.S.; Dineen, B.; Marais, D.; McNeill, G. Effects of probiotics on child growth: A systematic review. J. Health Popul. Nutr. 2015, 34, 8. [Google Scholar] [CrossRef] [PubMed]
- Catania, J.; Pandit, N.G.; Ehrlich, J.M.; Zaman, M.; Stone, E.; Franceschi, C.; Smith, A.; Tanner-Smith, E.; Zackular, J.P.; Bhutta, Z.A.; et al. Probiotic Supplementation for Promotion of Growth in Children: A Systematic Review and Meta-Analysis. Nutrients 2021, 14, 83. [Google Scholar] [CrossRef]
- Thiese, M.S.; Ronna, B.; Ott, U. P value interpretations and considerations. J. Thorac. Dis. 2016, 8, E928–E931. [Google Scholar] [CrossRef] [PubMed]
- Whitley, E.; Ball, J. Statistics review 4: Sample size calculations. Crit. Care 2002, 6, 335–341. [Google Scholar] [CrossRef]
- Miller, L.E.; Ouwehand, A.C. Probiotic supplementation decreases intestinal transit time: Meta-analysis of randomized controlled trials. World J. Gastroenterol. 2013, 19, 4718–4725. [Google Scholar] [CrossRef]
- Chen, K.; Liu, C.; Li, H.; Lei, Y.; Zeng, C.; Xu, S.; Li, J.; Savino, F. Infantile Colic Treated With Bifidobacterium longum CECT7894 and Pediococcus pentosaceus CECT8330: A Randomized, Double-Blind, Placebo-Controlled Trial. Front. Pediatr. 2021, 9, 635176. [Google Scholar] [CrossRef] [PubMed]
- Tazinya, A.A.; Halle-Ekane, G.E.; Mbuagbaw, L.T.; Abanda, M.; Atashili, J.; Obama, M.T. Risk factors for acute respiratory infections in children under five years attending the Bamenda Regional Hospital in Cameroon. BMC Pulm. Med. 2018, 18, 7. [Google Scholar] [CrossRef] [PubMed]
- Du, T.; Lei, A.; Zhang, N.; Zhu, C. The Beneficial Role of Probiotic Lactobacillus in Respiratory Diseases. Front. Immunol. 2022, 13, 908010. [Google Scholar] [CrossRef]
- Turner, R.B.; Woodfolk, J.A.; Borish, L.; Steinke, J.W.; Patrie, J.T.; Muehling, L.M.; Lahtinen, S.; Lehtinen, M.J. Effect of probiotic on innate inflammatory response and viral shedding in experimental rhinovirus infection—A randomised controlled trial. Benef. Microbes 2017, 8, 207–215. [Google Scholar] [CrossRef]
- Groeger, D.; Schiavi, E.; Grant, R.; Kurnik-Lucka, M.; Michalovich, D.; Williamson, R.; Beinke, S.; Kiely, B.; Akdis, C.A.; Hessel, E.M.; et al. Intranasal Bifidobacterium longum protects against viral-induced lung inflammation and injury in a murine model of lethal influenza infection. EBioMedicine 2020, 60, 102981. [Google Scholar] [CrossRef] [PubMed]
- Darbandi, A.; Asadi, A.; Ghanavati, R.; Afifirad, R.; Darb Emamie, A.; Kakanj, M.; Talebi, M. The effect of probiotics on respiratory tract infection with special emphasis on COVID-19: Systemic review 2010-20. Int. J. Infect. Dis. 2021, 105, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Rather, I.A.; Bajpai, V.K.; Kumar, S.; Lim, J.; Paek, W.K.; Park, Y.H. Probiotics and Atopic Dermatitis: An Overview. Front. Microbiol. 2016, 7, 507. [Google Scholar] [CrossRef]
- Umborowati, M.A.; Damayanti, D.; Anggraeni, S.; Endaryanto, A.; Surono, I.S.; Effendy, I.; Prakoeswa, C.R.S. The role of probiotics in the treatment of adult atopic dermatitis: A meta-analysis of randomized controlled trials. J. Health Popul. Nutr. 2022, 41, 37. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef] [PubMed]
- Houtman, T.A.; Eckermann, H.A.; Smidt, H.; de Weerth, C. Gut microbiota and BMI throughout childhood: The role of firmicutes, bacteroidetes, and short-chain fatty acid producers. Sci. Rep. 2022, 12, 3140. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, S.; Nirmalkar, K.; Hoyo-Vadillo, C.; Garcia-Espitia, M.; Ramirez-Sanchez, D.; Garcia-Mena, J. Gut microbiome production of short-chain fatty acids and obesity in children. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 621–625. [Google Scholar] [CrossRef]
- Zeybek, N.; Rastall, R.A.; Buyukkileci, A.O. Utilization of xylan-type polysaccharides in co-culture fermentations of Bifidobacterium and Bacteroides species. Carbohydr. Polym. 2020, 236, 116076. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Hu, J.; Geng, F.; Nie, S. Bacteroides utilization for dietary polysaccharides and their beneficial effects on gut health. Food Sci. Human. Wellness 2022, 11, 1101–1110. [Google Scholar] [CrossRef]
- Schwalm, N.D., 3rd; Groisman, E.A. Navigating the Gut Buffet: Control of Polysaccharide Utilization in Bacteroides spp. Trends Microbiol. 2017, 25, 1005–1015. [Google Scholar] [CrossRef]
- Nishiyama, K.; Yokoi, T.; Sugiyama, M.; Osawa, R.; Mukai, T.; Okada, N. Roles of the Cell Surface Architecture of Bacteroides and Bifidobacterium in the Gut Colonization. Front. Microbiol. 2021, 12, 754819. [Google Scholar] [CrossRef]
- Rogowski, A.; Briggs, J.A.; Mortimer, J.C.; Tryfona, T.; Terrapon, N.; Lowe, E.C.; Basle, A.; Morland, C.; Day, A.M.; Zheng, H.; et al. Glycan complexity dictates microbial resource allocation in the large intestine. Nat. Commun. 2015, 6, 7481. [Google Scholar] [CrossRef]




| Analysis Population | Total | AP-32 | CP-9 | Placebo |
|---|---|---|---|---|
| Intent-to-treat (ITT) | 88 | 29 | 28 | 31 |
| Modified intent-to-treat (mITT) | 86 (97.7%) | 29 (100.0%) | 26 (92.9%) | 31 (100.0%) |
| Per-protocol (PP) | 68 (77.3%) | 24 (82.8%) | 20 (71.4%) | 24 (77.4%) |
| Safety | 86 (97.7%) | 29 (100.0%) | 26 (92.9%) | 31 (100.0%) |
| mITT completers | 75 * (85.2%) | 27 (93.1%) | 21 (75.0%) | 27 (87.1%) |
| PP completers | 61 (69.3%) | 23 (79.3%) | 16 (57.1%) | 22 (71.0%) |
| Total N = 86 | AP-32 N = 29 | CP-9 N = 26 | Placebo N = 31 | |
|---|---|---|---|---|
| Age (month) a | ||||
| Mean (SD) | 1.3 (0.5) | 1.2 (0.5) | 1.3 (0.4) | 1.3 (0.5) |
| Median | 1.2 | 1.1 | 1.2 | 1.2 |
| Min, max | 0, 2 | 0, 2 | 1, 2 | 0, 2 |
| Gender | ||||
| Male | 37 (43.0%) | 14 (48.3%) | 10 (38.5%) | 13 (41.9%) |
| Female | 49 (57.0%) | 15 (51.7%) | 16 (61.5%) | 18 (58.1%) |
| Gestational age (week) b | ||||
| Mean (SD) | 38.5 (1.0) | 38.5 (1.0) | 38.5 (0.9) | 38.6 (1.0) |
| Median | 38.5 | 38.1 | 38.7 | 38.4 |
| Min, max | 36, 41 | 36, 40 | 37, 40 | 37, 41 |
| Delivery method | ||||
| Natural birth | 57 (66.3%) | 17 (58.6%) | 19 (73.1%) | 21 (67.7%) |
| C-section | 29 (33.7%) | 12 (41.4%) | 7 (26.9%) | 10 (32.3%) |
| Weight at birth (g) | ||||
| Mean (SD) | 3157.1 (419.9) | 3182.2 (519.6) | 3154.6 (374.2) | 3135.6 (360.2) |
| Median | 3140.0 | 3100.0 | 3170.0 | 3160.0 |
| Min, max | 2500, 4650 | 2500, 4650 | 2580, 4290 | 2520, 4480 |
| Recumbent length at birth (cm) | ||||
| Mean (SD) | 50.2 (2.1) | 49.7 (2.0) | 50.7 (2.0) | 50.2 (2.1) |
| Median | 50.0 | 49.5 | 50.5 | 50.0 |
| Min, max | 45, 57 | 45, 56 | 45, 54 | 46, 57 |
| Head circumference at birth (cm) | ||||
| Mean (SD) | 33.8 (1.3) | 33.8 (1.5) | 33.8 (1.1) | 33.8 (1.3) |
| Median | 34.0 | 33.5 | 34.0 | 34.0 |
| Min, max | 31, 36 | 31, 36 | 31, 36 | 31, 36 |
| Weight at baseline c (g) | ||||
| Mean (SD) | 4409.8 (733.5) | 4387.6 (811.0) | 4411.5 (676.7) | 4429.0 (726.7) |
| Median | 4300.0 | 4200.0 | 4300.0 | 4300.0 |
| Min, Max | 2600, 6500 | 2600, 6000 | 3100, 5600 | 3100, 6500 |
| Recumbent length at baseline c (cm) | ||||
| Mean (SD) | 53.4 (2.5) | 52.8 (2.6) | 53.3 (2.4) | 54.0 (2.6) |
| Median | 53.4 | 52.7 | 53.4 | 53.8 |
| Min, max | 46, 62 | 46, 58 | 49, 59 | 48, 62 |
| Head circumference at baseline c (cm) | ||||
| Mean (SD) | 36.9 (1.4) | 37.0 (1.4) | 37.0 (1.2) | 36.7 (1.5) |
| Median | 36.8 | 37.0 | 36.8 | 36.6 |
| Min, max | 33, 42 | 33, 39 | 34, 40 | 33, 42 |
| Weight Gain at Month 4 (g) | ||||||
|---|---|---|---|---|---|---|
| Age (Month) | Gender | Statistics | Total | AP-32 | CP-9 | Placebo |
| ≤1 | Male | n | 6 | 3 | 1 | 2 |
| Mean (SD) | 3226.7 (685.5) | 3120.0 (563.2) | 3200.0 (-) | 3400.0 (1272.8) | ||
| Median | 3050.0 | 2900.0 | 3200.0 | 3400.0 | ||
| Min, max | 2500, 4300 | 2700, 3760 | 3200, 3200 | 2500, 4300 | ||
| Female | n | 12 | 4 | 2 | 6 | |
| Mean (SD) | 3450.0 (702.6) | 3375.0 (950.0) | 3300.0 (1414.2) | 3550.0 (345.0) | ||
| Median | 3550.0 | 3400.0 | 3300.0 | 3550.0 | ||
| Min, max | 2300, 4400 | 2300, 4400 | 2300, 4300 | 3100, 4100 | ||
| >1 | Male | n | 28 | 10 | 8 | 10 |
| Mean (SD) | 3160.7 (845.2) | 3030.0 (600.1) | 3125.0 (799.6) | 3320.0 (1112.4) | ||
| Median | 3100.0 | 3000.0 | 3100.0 | 3150.0 | ||
| Min, max | 1700, 5500 | 2000, 4100 | 1700, 4400 | 1700, 5500 | ||
| Female | n | 29 | 10 | 10 | 9 | |
| Mean (SD) | 3075.9 (681.7) | 3010.0 (772.4) | 3250.0 (696.4) | 2955.6 (591.8) | ||
| Median | 3100.0 | 3150.0 | 3200.0 | 2800.0 | ||
| Min, max | 1900, 4600 | 1900, 4600 | 2200, 4300 | 2200, 3800 | ||
| Statistics | AP-32 N = 27 | CP-9 N = 21 | Placebo N = 27 |
|---|---|---|---|
| Recumbent length (cm) | |||
| Mean (SD) | 64.4 (2.7) | 65.2 (1.7) | 66.1 (2.7) |
| Median | 64.6 | 65.2 | 65.7 |
| Min, max | 58, 70 | 63, 68 | 62, 73 |
| p-value | 0.0210 | 0.1640 | 1.0000 |
| Head circumference (cm) | |||
| Mean (SD) | 41.9 (1.2) | 42.3 (1.2) | 42.0 (1.5) |
| Median | 41.6 | 42.0 | 41.8 |
| Min, max | 40, 45 | 41, 44 | 40, 46 |
| p-value | 0.7524 | 0.4920 | 1.0000 |
| Statistics | AP-32 N = 27 | CP-9 N = 21 | Placebo N = 26 * |
|---|---|---|---|
| Crying/fussing episodes (episodes/day) | |||
| Mean (SD) | 2.4 (1.7) | 2.0 (1.7) | 1.9 (1.6) |
| Median | 2.3 | 1.7 | 1.8 |
| Min, max | 0, 6 | 0, 6 | 0, 5 |
| Crying/fussing time (minutes/day) | |||
| Mean (SD) | 25.7 (24.4) | 18.9 (17.9) | 19.4 (23.0) |
| Median | 15.0 | 11.7 | 10.8 |
| Min, max | 0, 90 | 0, 55 | 0, 88 |
| Total N = 86 | AP-32 N = 29 | CP-9 N = 26 | Placebo N = 31 | |
|---|---|---|---|---|
| Infectious diseases | 12 (14.0%) | 5 (17.2%) | 2 (7.7%) | 5 (16.1%) |
| Acarodermatitis | 1 (1.2%) | 0 | 0 | 1 (3.2%) |
| Bronchiolitis | 1 (1.2%) | 0 | 1 (3.8%) | 0 |
| Corona virus infection | 1 (1.2%) | 1 (3.4%) | 0 | 0 |
| Enteritis | 1 (1.2%) | 0 | 0 | 1 (3.2%) |
| Nasopharyngitis | 2 (2.3%) | 0 | 0 | 2 (6.5%) |
| Oral candidiasis | 1 (1.2%) | 1 (3.4%) | 0 | 0 |
| Upper respiratory tract infection | 3 (3.5%) | 1 (3.4%) | 1 (3.8%) | 1 (3.2%) |
| Urinary tract infection | 2 (2.3%) | 2 (6.8%) | 0 | 0 |
| Allergic diseases | 9 (10.5%) | 3 (10.3%) | 3 (11.5%) | 3 (9.6%) |
| Rhinitis allergic | 1 (1.2%) | 1 (3.4%) | 0 | 0 |
| Dermatitis atopic | 4 (4.7%) | 1 (3.4%) | 2 (7.7%) | 1 (3.2%) |
| Dermatitis contact | 3 (3.5%) | 1 (3.4%) | 1 (3.8%) | 1 (3.2%) |
| Infantile eczema | 1 (1.2%) | 0 | 0 | 1 (3.2%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.-F.; Ou-Yang, M.-C.; Hsia, K.-C.; Neonatal Probiotic Use and Safety Research Group; Li, C.-M.; Yeh, Y.-T.; Ho, H.-H. A Three-Arm, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Safety of Lactobacillus salivarius AP-32 and Bifidobacterium animalis CP-9 Used Individually in Healthy Infants. Nutrients 2023, 15, 3426. https://doi.org/10.3390/nu15153426
Chen J-F, Ou-Yang M-C, Hsia K-C, Neonatal Probiotic Use and Safety Research Group, Li C-M, Yeh Y-T, Ho H-H. A Three-Arm, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Safety of Lactobacillus salivarius AP-32 and Bifidobacterium animalis CP-9 Used Individually in Healthy Infants. Nutrients. 2023; 15(15):3426. https://doi.org/10.3390/nu15153426
Chicago/Turabian StyleChen, Jui-Fen, Mei-Chen Ou-Yang, Ko-Chiang Hsia, Neonatal Probiotic Use and Safety Research Group, Ching-Min Li, Yao-Tsung Yeh, and Hsieh-Hsun Ho. 2023. "A Three-Arm, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Safety of Lactobacillus salivarius AP-32 and Bifidobacterium animalis CP-9 Used Individually in Healthy Infants" Nutrients 15, no. 15: 3426. https://doi.org/10.3390/nu15153426
APA StyleChen, J.-F., Ou-Yang, M.-C., Hsia, K.-C., Neonatal Probiotic Use and Safety Research Group, Li, C.-M., Yeh, Y.-T., & Ho, H.-H. (2023). A Three-Arm, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Safety of Lactobacillus salivarius AP-32 and Bifidobacterium animalis CP-9 Used Individually in Healthy Infants. Nutrients, 15(15), 3426. https://doi.org/10.3390/nu15153426







