The Association of Carbohydrate Antigen (CA) 19-9 Levels and Low Skeletal Muscle Mass in Healthy Adults
Abstract
:1. Introduction
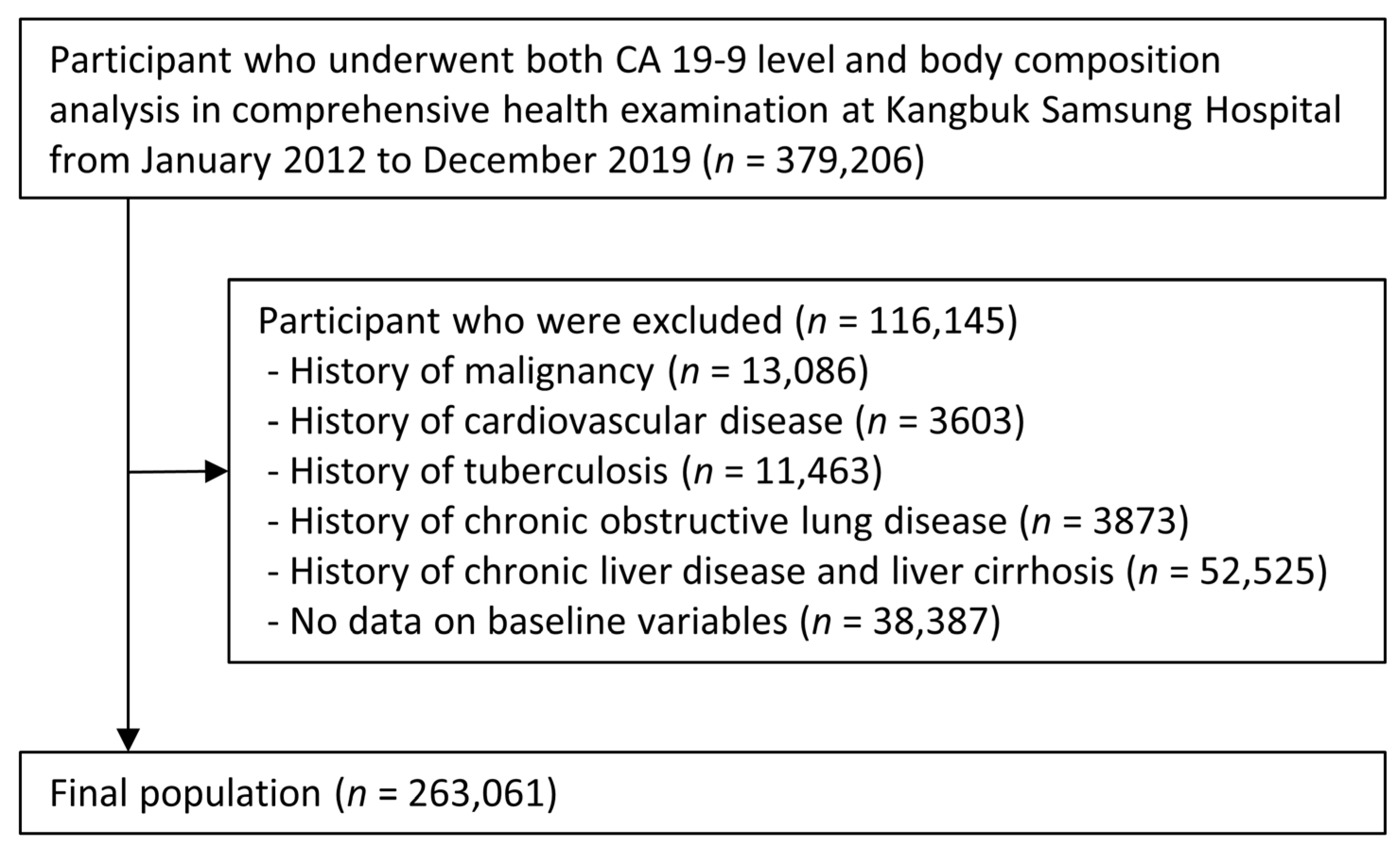
2. Materials and Methods
2.1. Study Population
2.2. Measurements
2.3. Threshold Levels of Skeletal Muscle Mass and CA 19-9
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
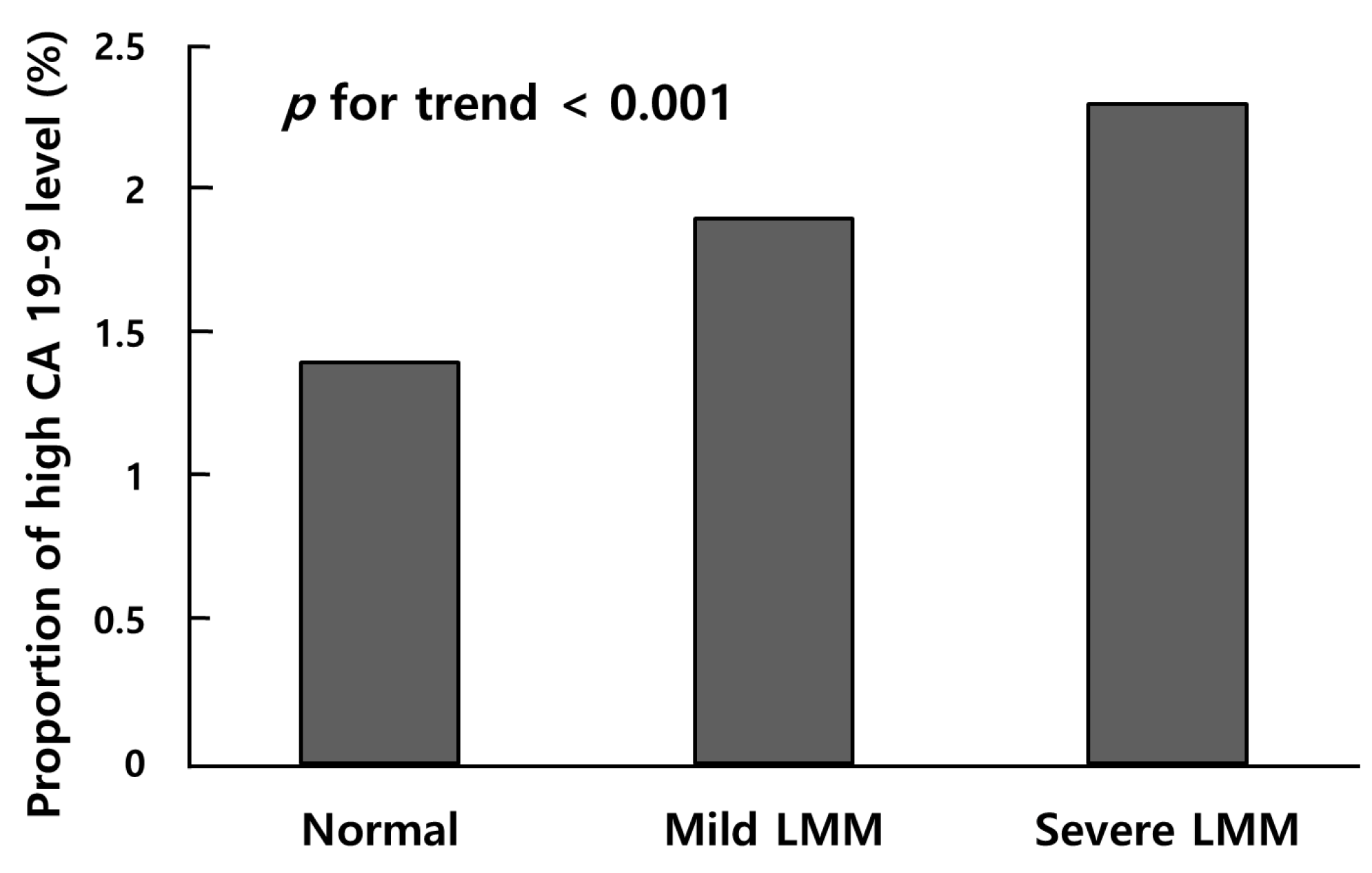
3.2. Comparison of CA 19-9 Level among Groups Classified by a Low Skeletal Muscle Mass
3.3. Association of High CA 19-9 with Low Skeletal Muscle Mass
3.4. Subgroup Analysis by Age and Sex
4. Discussion
5. Limitation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tuttle, C.S.L.; Thang, L.A.N.; Maier, A.B. Markers of inflammation and their association with muscle strength and mass: A systematic review and meta-analysis. Ageing Res. Rev. 2020, 64, 101185. [Google Scholar] [CrossRef] [PubMed]
- Masschelin, P.M.; Cox, A.R.; Chernis, N.; Hartig, S.M. The Impact of Oxidative Stress on Adipose Tissue Energy Balance. Front. Physiol. 2019, 10, 1638. [Google Scholar] [CrossRef]
- Kitamura, A.; Seino, S.; Abe, T.; Nofuji, Y.; Yokoyama, Y.; Amano, H.; Nishi, M.; Taniguchi, Y.; Narita, M.; Fujiwara, Y.; et al. Sarcopenia: Prevalence, associated factors, and the risk of mortality and disability in Japanese older adults. J. Cachexia Sarcopenia Muscle 2021, 12, 30–38. [Google Scholar] [CrossRef]
- Xu, J.; Wan, C.S.; Ktoris, K.; Reijnierse, E.M.; Maier, A.B. Sarcopenia Is Associated with Mortality in Adults: A Systematic Review and Meta-Analysis. Gerontology 2022, 68, 361–376. [Google Scholar] [CrossRef] [PubMed]
- Petermann-Rocha, F.; Gray, S.R.; Pell, J.P.; Celis-Morales, C.; Ho, F.K. Biomarkers Profile of People With Sarcopenia: A Cross-sectional Analysis From UK Biobank. J. Am. Med. Dir. Assoc. 2020, 21, 2017.e2011–2017.e2019. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e302. [Google Scholar] [CrossRef]
- Li, Z.; Tong, X.; Ma, Y.; Bao, T.; Yue, J. Relationship Between Low Skeletal Muscle Mass and Arteriosclerosis in Western China: A Cross-Sectional Study. Front. Cardiovasc. Med. 2021, 8, 735262. [Google Scholar] [CrossRef] [PubMed]
- Scara, S.; Bottoni, P.; Scatena, R. CA 19-9: Biochemical and Clinical Aspects. Adv. Exp. Med. Biol. 2015, 867, 247–260. [Google Scholar] [PubMed]
- Goonetilleke, K.S.; Siriwardena, A.K. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur. J. Surg. Oncol. 2007, 33, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.J.; Sturgeon, C.; Lamerz, R.; Haglund, C.; Holubec, V.L.; Klapdor, R.; Nicolini, A.; Topolcan, O.; Heinemann, V. Tumor markers in pancreatic cancer: A European Group on Tumor Markers (EGTM) status report. Ann. Oncol. 2010, 21, 441–447. [Google Scholar] [CrossRef]
- Haring, M.P.D.; de Cort, B.A.; Nieuwenhuijs, V.B. Elevated CA19-9 levels; not always cancer. Ned. Tijdschr. Geneeskd. 2021, 164, D4048. [Google Scholar]
- Son, D.H.; Lee, H.S.; Lee, Y.J. Association between serum carbohydrate antigen 19-9 levels and leukoaraiosis in middle-aged and older adults: A cross-sectional study. Atherosclerosis 2020, 292, 188–192. [Google Scholar] [CrossRef]
- Ventrucci, M.; Pozzato, P.; Cipolla, A.; Uomo, G. Persistent elevation of serum CA 19-9 with no evidence of malignant disease. Dig. Liver. Dis. 2009, 41, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Lou, A.; Zhang, H.; Zhu, S.; Yang, M.; Lai, W. Serum KL-6, CA19-9, CA125 and CEA are Diagnostic Biomarkers for Rheumatoid Arthritis-Associated Interstitial Lung Disease in the Chinese Population. Rheumatol. Ther. 2021, 8, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, B.K.; Seo, J.H.; Choi, J.; Choi, J.W.; Lee, C.K.; Chung, J.B.; Park, Y.; Kim, D.W. Carbohydrate antigen 19-9 elevation without evidence of malignant or pancreatobiliary diseases. Sci. Rep. 2020, 10, 8820. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.P.; Sung, I.K.; Kim, J.H.; Lee, S.Y.; Park, H.S.; Shim, C.S. Usefulness of Carbohydrate Antigen 19-9 Test in Healthy People and Necessity of Medical Follow-up in Individuals with Elevated Carbohydrate Antigen 19-9 Level. Korean J. Fam. Med. 2019, 40, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Chang, Y.; Jung, H.-S.; Yun, K.E.; Kwon, M.-J.; Choi, Y.; Kim, C.-W.; Cho, J.; Suh, B.-S.; Cho, Y.K. Relationship of sitting time and physical activity with non-alcoholic fatty liver disease. J. Hepatol. 2015, 63, 1229–1237. [Google Scholar] [CrossRef]
- Lee, W.; Chang, Y.; Shin, H.; Ryu, S. Self-reported and cotinine-verified smoking and increased risk of incident hearing loss. Sci. Rep. 2021, 11, 8103. [Google Scholar] [CrossRef]
- Traversy, G.; Chaput, J.P. Alcohol Consumption and Obesity: An Update. Curr. Obes. Rep. 2015, 4, 122–130. [Google Scholar] [CrossRef] [Green Version]
- Yoo, T.K.; Rhim, H.C.; Lee, Y.T.; Yoon, K.J.; Park, C.H. Relationship between hyperhomocysteinemia and coexisting obesity with low skeletal muscle mass in asymptomatic adult population. Sci. Rep. 2022, 12, 12439. [Google Scholar] [CrossRef]
- Lee, P.H.; Macfarlane, D.J.; Lam, T.H.; Stewart, S.M. Validity of the international physical activity questionnaire short form (IPAQ-SF): A systematic review. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janssen, I.; Heymsfield, S.B.; Baumgartner, R.N.; Ross, R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J. Appl. Physiol. (1985) 2000, 89, 465–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, A.B.; Kupelian, V.; Visser, M.; Simonsick, E.; Goodpaster, B.; Nevitt, M.; Kritchevsky, S.B.; Tylavsky, F.A.; Rubin, S.M.; Harris, T.B.; et al. Sarcopenia: Alternative definitions and associations with lower extremity function. J. Am. Geriatr. Soc. 2003, 51, 1602–1609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janssen, I.; Heymsfield, S.B.; Ross, R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J. Am. Geriatr. Soc. 2002, 50, 889–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, E.C.; Yang, J.Y.; Lee, K.G.; Oh, S.Y.; Suh, Y.S.; Kong, S.H.; Yang, H.K.; Lee, H.J. The value of postoperative serum carcinoembryonic antigen and carbohydrate antigen 19-9 levels for the early detection of gastric cancer recurrence after curative resection. J. Gastric. Cancer 2014, 14, 221–228. [Google Scholar] [CrossRef] [Green Version]
- Waraya, M.; Yamashita, K.; Katagiri, H.; Ishii, K.; Takahashi, Y.; Furuta, K.; Watanabe, M. Preoperative serum CA19-9 and dissected peripancreatic tissue margin as determiners of long-term survival in pancreatic cancer. Ann. Surg. Oncol. 2009, 16, 1231–1240. [Google Scholar] [CrossRef]
- Kang, J.S.; Hong, S.Y.; Han, Y.; Sohn, H.J.; Lee, M.; Kang, Y.H.; Kim, H.S.; Kim, H.; Kwon, W.; Jang, J.Y. Limits of serum carcinoembryonic antigen and carbohydrate antigen 19-9 as the diagnosis of gallbladder cancer. Ann. Surg. Treat. Res. 2021, 101, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Lee, K.T.; Lee, J.K.; Paik, S.W.; Rhee, J.C.; Choi, K.W. Clinical usefulness of carbohydrate antigen 19-9 as a screening test for pancreatic cancer in an asymptomatic population. J. Gastroenterol. Hepatol. 2004, 19, 182–186. [Google Scholar] [CrossRef]
- Chang, C.Y.; Huang, S.P.; Chiu, H.M.; Lee, Y.C.; Chen, M.F.; Lin, J.T. Low efficacy of serum levels of CA 19-9 in prediction of malignant diseases in asymptomatic population in Taiwan. Hepatogastroenterology 2006, 53, 1–4. [Google Scholar]
- Bombski, G.; Gasiorowska, A.; Orszulak-Michalak, D.; Neneman, B.; Kotynia, J.; Strzelczyk, J.; Janiak, A.; Malecka-Panas, E. Differences in plasma gastrin, CEA, and CA 19-9 concentration in patients with proximal and distal colorectal cancer. Int. J. Gastrointest. Cancer 2002, 31, 155–163. [Google Scholar] [CrossRef]
- Qin, X.L.; Wang, Z.R.; Shi, J.S.; Lu, M.; Wang, L.; He, Q.R. Utility of serum CA19-9 in diagnosis of cholangiocarcinoma: In comparison with CEA. World J. Gastroenterol. 2004, 10, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Galli, C.; Basso, D.; Plebani, M. CA 19-9: Handle with care. Clin. Chem. Lab. Med. 2013, 51, 1369–1383. [Google Scholar] [CrossRef]
- Shimomura, C.; Eguchi, K.; Kawakami, A.; Migita, K.; Nakao, H.; Otsubo, T.; Ueki, Y.; Tezuka, H.; Yamashita, S.; Matsunaga, M.; et al. Elevation of a tumor associated antigen CA 19-9 levels in patients with rheumatic diseases. J. Rheumatol. 1989, 16, 1410–1415. [Google Scholar] [PubMed]
- Shudo, R.; Saito, T.; Takahashi, K.; Horita, K.; Waku, K.; Honma, I.; Sato, T. Giant hydronephrosis due to a ureteral stone, and elevated serum levels of CA 19-9. Intern. Med. 1999, 38, 887–891. [Google Scholar] [CrossRef] [Green Version]
- Ito, S.; Gejyo, F. Elevation of serum CA19-9 levels in benign diseases. Intern. Med. 1999, 38, 840–841. [Google Scholar] [CrossRef] [Green Version]
- Choi, M.H.; Yoon, S.B.; Lee, K.; Song, M.; Lee, I.S.; Lee, M.A.; Hong, T.H.; Choi, M.G. Preoperative sarcopenia and post-operative accelerated muscle loss negatively impact survival after resection of pancreatic cancer. J. Cachexia Sarcopenia Muscle 2018, 9, 326–334. [Google Scholar] [CrossRef]
- Tu, Y.; Yu, H.; Zhang, P.; Di, J.; Han, X.; Wu, S.; Bao, Y.; Jia, W. Decreased serum CA19-9 is associated with improvement of insulin resistance and metabolic control in patients with obesity and type 2 diabetes after Roux-en-Y gastric bypass. J. Diabetes Investig. 2014, 5, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.J.; Zhu, C.F. Causal relationship between insulin resistance and sarcopenia. Diabetol. Metab. Syndr. 2023, 15, 46. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, Y.; Bi, Y.; Xu, M.; Lu, J.; Wang, T.; Li, M.; Chen, Y.; Liu, Y.; Huang, F.; et al. Relationship between CA 19-9 levels and glucose regulation in a middle-aged and elderly Chinese population. J. Diabetes 2012, 4, 147–152. [Google Scholar] [CrossRef]
- Petit, J.M.; Vaillant, G.; Olsson, N.O.; Guignier, F.; Collignon, S.; Verges, B.; Brun, J.M. Elevated serum CA19-9 levels in poorly controlled diabetic patients. Relationship with Lewis blood group. Gastroenterol. Clin. Biol. 1994, 18, 17–20. [Google Scholar]
- Benhamou, P.Y.; Vuillez, J.P.; Halimi, S.; Meffre, G.; Bachelot, I. Influence of metabolic disturbances of diabetes mellitus on serum CA 19-9 tumor marker. Diabete Metab. 1991, 17, 39–43. [Google Scholar] [PubMed]
- Uygur-Bayramicli, O.; Dabak, R.; Orbay, E.; Dolapcioglu, C.; Sargin, M.; Kilicoglu, G.; Guleryuzlu, Y.; Mayadagli, A. Type 2 diabetes mellitus and CA 19-9 levels. World J. Gastroenterol. 2007, 13, 5357–5359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gul, K.; Nas, S.; Ozdemir, D.; Gumus, M.; Ersoy, R.; Cakir, B. CA 19-9 level in patients with type 2 diabetes mellitus and its relation to the metabolic control and microvascular complications. Am. J. Med. Sci. 2011, 341, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, N.; Aoji, O.; Yoshikawa, T.; Mori, K.; Kajiyama, S.; Kitagawa, Y.; Kanatsuna, T.; Kondo, M. Elevated serum CA19-9 levels in poorly controlled diabetic patients. Jpn. J. Med. 1986, 25, 278–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, H.; Li, R.; Zhang, L.; Chen, H.; Bao, Y.; Jia, W. Serum CA19-9 level associated with metabolic control and pancreatic beta cell function in diabetic patients. Exp. Diabetes Res. 2012, 2012, 745189. [Google Scholar] [CrossRef] [Green Version]
- Haddad, F.; Zaldivar, F.; Cooper, D.M.; Adams, G.R. IL-6-induced skeletal muscle atrophy. J. Appl. Physiol. (1985) 2005, 98, 911–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Moylan, J.S.; Chambers, M.A.; Smith, J.; Reid, M.B. Interleukin-1 stimulates catabolism in C2C12 myotubes. Am. J. Physiol. Cell Physiol. 2009, 297, C706–C714. [Google Scholar] [CrossRef] [Green Version]
- Reid, M.B.; Li, Y.P. Tumor necrosis factor-alpha and muscle wasting: A cellular perspective. Respir. Res. 2001, 2, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Feliciano, E.M.C.; Kroenke, C.H.; Meyerhardt, J.A.; Prado, C.M.; Bradshaw, P.T.; Kwan, M.L.; Xiao, J.; Alexeeff, S.; Corley, D.; Weltzien, E.; et al. Association of Systemic Inflammation and Sarcopenia With Survival in Nonmetastatic Colorectal Cancer: Results From the C SCANS Study. JAMA Oncol. 2017, 3, e172319. [Google Scholar] [CrossRef]
- Murai, J.; Soga, S.; Saito, H.; Otsuki, M.; Kitada, T.; Saisho, Y.; Nakamura, H.; Kasayama, S.; Koga, M. Study on the mechanism causing elevation of serum CA19-9 levels in diabetic patients. Endocr. J. 2013, 60, 885–891. [Google Scholar] [CrossRef] [Green Version]
- Allen, S.L.; Quinlan, J.I.; Dhaliwal, A.; Armstrong, M.J.; Elsharkawy, A.M.; Greig, C.A.; Lord, J.M.; Lavery, G.G.; Breen, L. Sarcopenia in chronic liver disease: Mechanisms and countermeasures. Am. J. Physiol. Gastrointest. Liver. Physiol. 2021, 320, G241–G257. [Google Scholar] [CrossRef] [PubMed]
- Machann, J.; Thamer, C.; Schnoedt, B.; Stefan, N.; Stumvoll, M.; Haring, H.U.; Claussen, C.D.; Fritsche, A.; Schick, F. Age and gender related effects on adipose tissue compartments of subjects with increased risk for type 2 diabetes: A whole body MRI/MRS study. MAGMA 2005, 18, 128–137. [Google Scholar] [CrossRef]
- Garaulet, M.; Perex-Llamas, F.; Fuente, T.; Zamora, S.; Tebar, F.J. Anthropometric, computed tomography and fat cell data in an obese population: Relationship with insulin, leptin, tumor necrosis factor-alpha, sex hormone-binding globulin and sex hormones. Eur. J. Endocrinol. 2000, 143, 657–666. [Google Scholar] [CrossRef] [Green Version]
- Couillard, C.; Bergeron, N.; Prud′homme, D.; Bergeron, J.; Tremblay, A.; Bouchard, C.; Mauriege, P.; Despres, J.P. Gender difference in postprandial lipemia: Importance of visceral adipose tissue accumulation. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 2448–2455. [Google Scholar] [CrossRef] [Green Version]
- Kvist, H.; Chowdhury, B.; Grangard, U.; Tylen, U.; Sjostrom, L. Total and visceral adipose-tissue volumes derived from measurements with computed tomography in adult men and women: Predictive equations. Am. J. Clin. Nutr. 1988, 48, 1351–1361. [Google Scholar] [CrossRef]
- Albu, J.B.; Murphy, L.; Frager, D.H.; Johnson, J.A.; Pi-Sunyer, F.X. Visceral fat and race-dependent health risks in obese nondiabetic premenopausal women. Diabetes 1997, 46, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A.; Jablonski, K.A.; Fujimoto, W.Y.; Barrett-Connor, E.; Haffner, S.; Hanson, R.L.; Hill, J.O.; Hubbard, V.; Kriska, A.; Stamm, E.; et al. Relation of central adiposity and body mass index to the development of diabetes in the Diabetes Prevention Program. Am. J. Clin. Nutr. 2008, 87, 1212–1218. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.J.; Kim, H.J.; Lee, K.E.; Kim, D.J.; Kim, S.K.; Ahn, C.W.; Lim, S.K.; Kim, K.R.; Lee, H.C.; Huh, K.B.; et al. Metabolic significance of nonalcoholic fatty liver disease in nonobese, nondiabetic adults. Arch. Intern. Med. 2004, 164, 2169–2175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louet, J.F.; LeMay, C.; Mauvais-Jarvis, F. Antidiabetic actions of estrogen: Insight from human and genetic mouse models. Curr. Atheroscler. Rep. 2004, 6, 180–185. [Google Scholar] [CrossRef]
- Rincon, J.; Holmang, A.; Wahlstrom, E.O.; Lonnroth, P.; Bjorntorp, P.; Zierath, J.R.; Wallberg-Henriksson, H. Mechanisms behind insulin resistance in rat skeletal muscle after oophorectomy and additional testosterone treatment. Diabetes 1996, 45, 615–621. [Google Scholar] [CrossRef]
- Matute, M.L.; Kalkhoff, R.K. Sex steroid influence on hepatic gluconeogenesis and glucogen formation. Endocrinology 1973, 92, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Carter, S.; McKenzie, S.; Mourtzakis, M.; Mahoney, D.J.; Tarnopolsky, M.A. Short-term 17beta-estradiol decreases glucose R(a) but not whole body metabolism during endurance exercise. J. Appl. Physiol. (1985) 2001, 90, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Carter, S.L.; Rennie, C.; Tarnopolsky, M.A. Substrate utilization during endurance exercise in men and women after endurance training. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E898–E907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geer, E.B.; Shen, W. Gender differences in insulin resistance, body composition, and energy balance. Gend. Med. 2009, 6 (Suppl. S1), 60–75. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.; Sit, J.W.H.; Chan, H.Y.L.; Choi, K.C.; Cheung, R.K.Y.; Wong, M.M.H.; Li, F.Y.K.; Lee, T.Y.; Fung, E.S.M.; Tai, K.M.; et al. Sarcopenia risk and associated factors among Chinese community-dwelling older adults living alone. Sci. Rep. 2021, 11, 22219. [Google Scholar] [CrossRef]
- Shimojo, N.; Naka, K.; Nakajima, C.; Ishizaki, T.; Okuda, K.; Murai, J.; Yamamoto, M. The effect of non-insulin-dependent diabetes on serum concentrations of tumor-associated carbohydrate antigens of CA19-9, CA-50, and sialyl SSEA-1 in association with the Lewis blood phenotype. Clin. Chim. Acta 1990, 190, 283–289. [Google Scholar] [CrossRef]
- Aoki, Y.; Yanagisawa, Y.; Ohfusa, H.; Kawa, S.; Oguchi, H.; Furuta, S. Elevation of serum CA 19-9 in parallel with HbA1c in a diabetic female with the Lewis(a+b-) blood group. Diabetes Res. Clin. Pract. 1991, 13, 77–81. [Google Scholar] [CrossRef]

| Total | Normal | Mild LMM | Severe LMM | * p Value | |
|---|---|---|---|---|---|
| Number of subjects (n) | 263,061 | 226,759 | 32,532 | 3770 | |
| Age (years) | 41.0 ± 9.5 | 41.0 ± 9.2 | 41.1 ± 10.8 | 44.2 ± 13.5 | <0.001 ‡,# |
| Sex, Men (%) | 50.8 | 49.6 | 56.9 | 73.5 | <0.001 |
| Screening center, Seoul (%) | 48.3 | 48.3 | 48.5 | 47.4 | 0.456 |
| Height (cm) | 167.3 ± 8.6 | 167.6 ± 8.6 | 165.2 ± 8.1 | 164.3 ± 7.8 | <0.001 †,‡,# |
| BMI (kg/m2) | 23.5 ± 3.5 | 24.0 ± 3.4 | 20.7 ± 2.2 | 19.4 ± 2.2 | <0.001 †,‡,# |
| Appendicular skeletal muscle mass (kg) | 20.2 ± 5.0 | 20.7 ± 4.9 | 17.6 ± 4.1 | 16.5 ± 3.4 | <0.001 †,‡,# |
| SMI (kg/m2) | 7.1 ± 1.1 | 7.3 ± 1.1 | 6.4 ± 1.0 | 6.0 ± 0.8 | <0.001 †,‡,# |
| Current smoker (%) | 14.8 | 14.5 | 15.8 | 21.8 | <0.001 |
| Heavy drinking a (%) | 15.8 | 15.9 | 15.0 | 18.1 | <0.001 |
| MET minutes per week (min/wk) | 1584.0 ± 3037.8 | 1616.9 ± 3068.9 | 1374.2 ± 2774.1 | 1415.9 ± 3253.3 | <0.001 †,‡ |
| Systolic blood pressure (mmHg) | 109.6 ± 12.7 | 110.1 ± 12.7 | 106.5 ± 12.0 | 107.6 ± 13.1 | <0.001 †,‡,# |
| Diastolic blood pressure (mmHg) | 70.4 ± 9.7 | 70.5 ± 9.8 | 69.2 ± 9.3 | 70.2 ± 9.4 | <0.001 †,# |
| Hypertension (%) | 8.5 | 8.6 | 7.6 | 10.5 | <0.001 |
| Diabetes mellitus (%) | 2.4 | 2.4 | 2.7 | 5.1 | <0.001 |
| Insulin (uIU/mL) | 7.1 ± 4.8 | 7.3 ± 4.9 | 5.7 ± 3.3 | 5.1 ± 3.1 | <0.001 †,‡,# |
| HOMA-IR | 1.7 ± 1.4 | 1.8 ± 1.4 | 1.4 ± 0.9 | 1.3 ± 0.9 | <0.001 †,‡,# |
| Glucose (mg/dL) | 96.7 ± 15.2 | 96.9 ± 15.1 | 95.4 ± 15.5 | 96.9 ± 20.0 | <0.001 †,# |
| Triglycerides (mg/dL) | 112.9 ± 78.6 | 114.7 ± 80.6 | 101.4 ± 63.0 | 102.4 ± 65.1 | <0.001 †,‡ |
| Total cholesterol (mg/dL) | 190.4 ± 33.9 | 190.4 ± 33.9 | 190.4 ± 33.9 | 190.6 ± 35.6 | 0.931 |
| LDL-C (mg/dL) | 125.6 ± 33.1 | 125.8 ± 33.0 | 124.3 ± 33.2 | 123.8 ± 34.4 | <0.001 †,‡ |
| HDL-C (mg/dL) | 60.8 ± 16.2 | 60.3 ± 16.1 | 63.9 ± 16.3 | 64.1 ± 17.1 | <0.001 †,‡ |
| AST (IU/L) | 22.2 ± 14.7 | 22.2 ± 14.8 | 21.6 ± 13.1 | 23.6 ± 21.1 | <0.001 †,‡,# |
| ALT (IU/L) | 23.6 ± 19.9 | 23.7 ± 20.3 | 20.9 ± 16.8 | 21.8 ± 18.3 | <0.001 †,‡,# |
| Total bilirubin (mg/dL) | 0.78 ± 0.36 | 0.78 ± 0.36 | 0.80 ± 0.37 | 0.83 ± 0.38 | <0.001 †,‡,# |
| γ-GTP (IU/L) | 30.7 ± 39.5 | 30.8 ± 38.2 | 29.6 ± 41.6 | 37.2 ± 77.8 | <0.001 †,‡,# |
| Alk-phosphatase (IU/L) | 59.4 ± 17.1 | 59.1 ± 17.0 | 60.9 ± 17.5 | 66.5 ± 21.1 | <0.001 †,‡,# |
| Creatinine (mg/dL) | 0.82 ± 0.22 | 0.82 ± 0.22 | 0.81 ± 0.19 | 0.83 ± 0.24 | <0.001†,‡,# |
| CRP (mg/dL) | 0.11 ± 0.30 | 0.12 ± 0.29 | 0.10 ± 0.32 | 0.13 ± 0.43 | <0.001 †,‡,# |
| CA 19-9 (U/mL) | 9.2 ± 10.2 | 9.2 ± 10.0 | 9.6 ± 8.6 | 10.4 ± 24.3 | <0.001 †,‡,# |
| Mild LMM, OR (95% CI) | Severe LMM, OR (95% CI) | |
|---|---|---|
| Model 1 | ||
| Normal (<30 U/mL) | [1] (reference) | [1] (reference) |
| High CA 19-9 level (≥30 U/mL) | 1.531 (1.403–1.672) | 2.280 (1.835–2.835) |
| Model 2 | ||
| Normal (<30 U/mL) | [1] (reference) | [1] (reference) |
| High CA 19-9 level (≥30 U/mL) | 1.530 (1.402–1.671) | 2.258 (1.816–2.807) |
| Model 3 | ||
| Normal (<30 U/mL) | [1] (reference) | [1] (reference) |
| High CA 19-9 level (≥30 U/mL) | 1.677 (1.533–1.834) | 2.651 (2.126–3.306) |
| Mild LMM, OR (95% CI) | Severe LMM, OR (95% CI) | p for Interaction | |
|---|---|---|---|
| Age | 0.869 | ||
| <40 (n = 134,353) | 1.439 (1.274–1.626) | 2.056 (1.463–2.890) | |
| 40~59 (n = 117,388) | 1.753 (1.506–2.041) | 2.397 (1.580–3.635) | |
| ≥60 (n = 11,320) | 1.415 (1.066–1.880) | 1.998 (1.284–3.111) | |
| Sex | 0.003 | ||
| Men (n = 133,685) | 1.939 (1.590–2.364) | 3.418 (2.452–4.764) | |
| Women (n = 129,376) | 1.583 (1.431–1.752) | 2.117 (1.564–2.866) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.H.; Kim, D.-K.; Lee, M.-Y.; Lim, H.-S.; Kwon, M.-J.; Lee, Y.-T.; Yoon, K.J.; Park, C.-H. The Association of Carbohydrate Antigen (CA) 19-9 Levels and Low Skeletal Muscle Mass in Healthy Adults. Nutrients 2023, 15, 3394. https://doi.org/10.3390/nu15153394
Lee JH, Kim D-K, Lee M-Y, Lim H-S, Kwon M-J, Lee Y-T, Yoon KJ, Park C-H. The Association of Carbohydrate Antigen (CA) 19-9 Levels and Low Skeletal Muscle Mass in Healthy Adults. Nutrients. 2023; 15(15):3394. https://doi.org/10.3390/nu15153394
Chicago/Turabian StyleLee, Jae Hyun, Dong-Kun Kim, Mi-Yeon Lee, Han-Sol Lim, Min-Jung Kwon, Yong-Taek Lee, Kyung Jae Yoon, and Chul-Hyun Park. 2023. "The Association of Carbohydrate Antigen (CA) 19-9 Levels and Low Skeletal Muscle Mass in Healthy Adults" Nutrients 15, no. 15: 3394. https://doi.org/10.3390/nu15153394
APA StyleLee, J. H., Kim, D.-K., Lee, M.-Y., Lim, H.-S., Kwon, M.-J., Lee, Y.-T., Yoon, K. J., & Park, C.-H. (2023). The Association of Carbohydrate Antigen (CA) 19-9 Levels and Low Skeletal Muscle Mass in Healthy Adults. Nutrients, 15(15), 3394. https://doi.org/10.3390/nu15153394






