Alzheimer’s Disease Genetic Influences Impact the Associations between Diet and Resting-State Functional Connectivity: A Study from the UK Biobank
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cohort and Participants
2.2. Resting-State fMRI
2.3. Genetic Factors—APOE, TOMM40, and Family History
2.4. Covariates
2.5. Diet Consumption
2.6. Statistical Analyses
3. Results
3.1. Demographics and Data Summaries
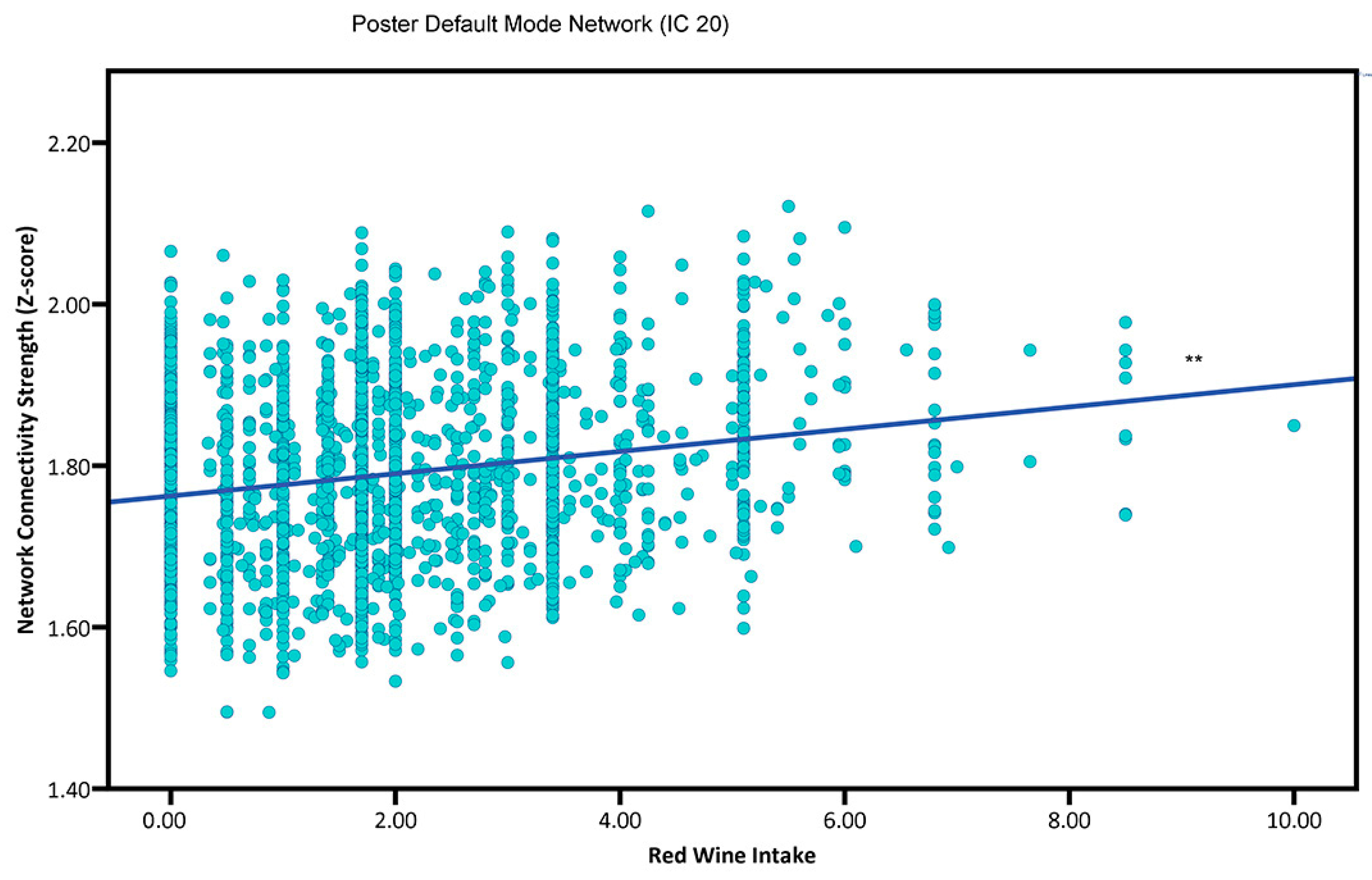
3.2. Main Effects
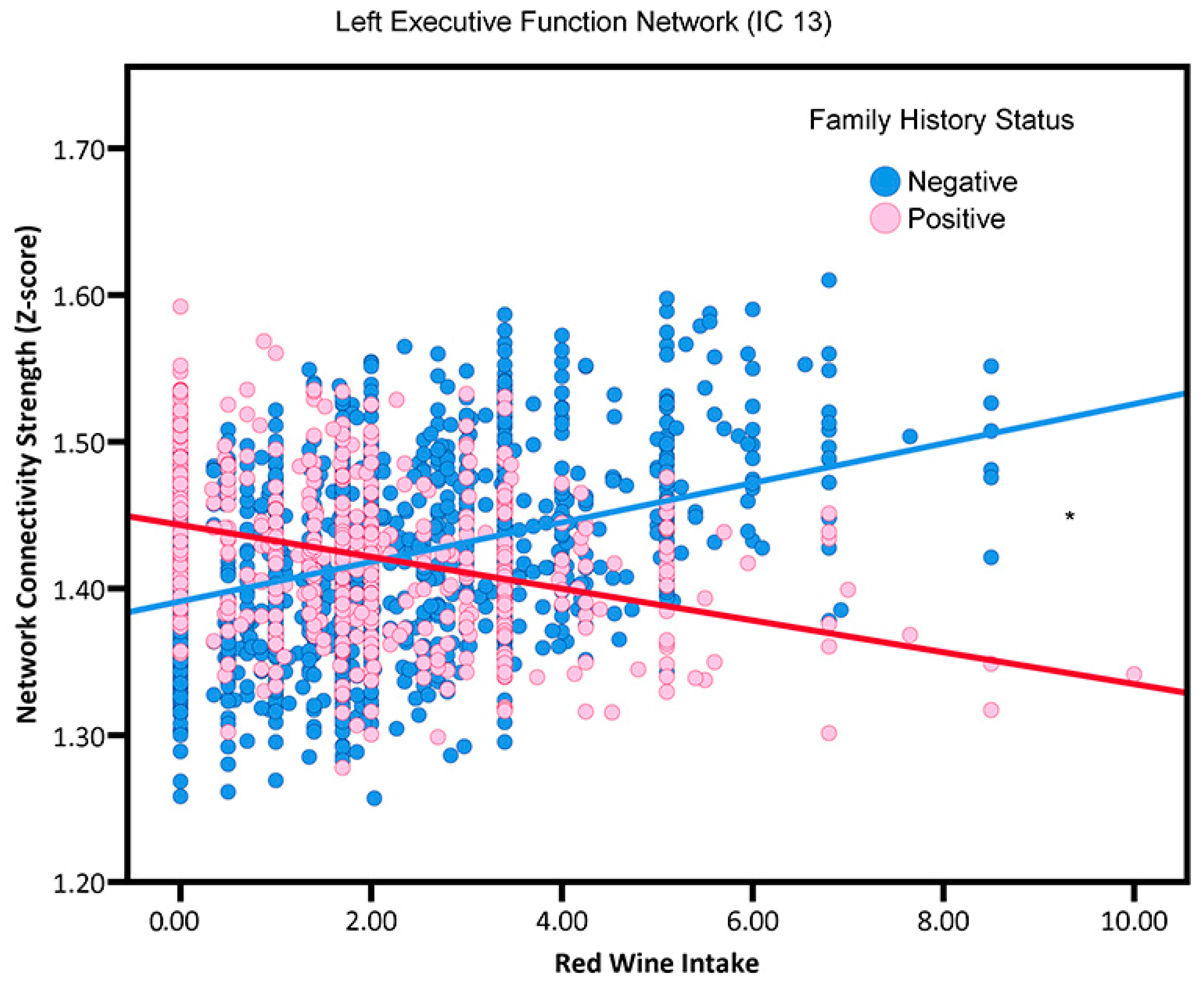
3.3. Red Wine Consumption by Family History Interactions
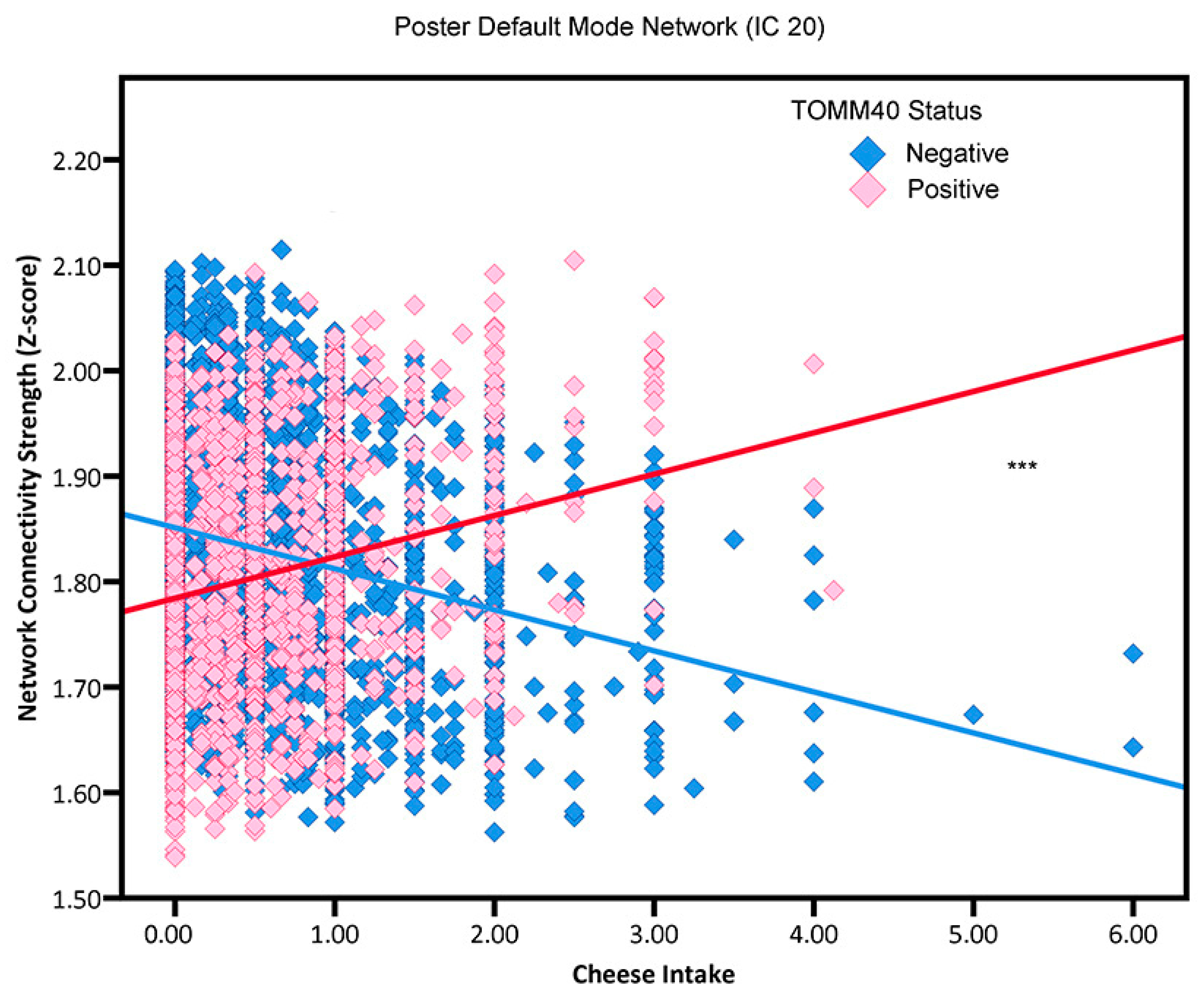
3.4. Cheese Intake by APOE4 Status, TOMM40 Status, and Family History Interactions
4. Discussion
4.1. Red Wine
4.2. Dairy Consumption
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geschwind, M.D. Rapidly progressive dementia. Contin. Lifelong Learn. Neurol. 2016, 22, 510–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nichols, E.; Steinmetz, J.D.; Vollset, S.E.; Fukutaki, K.; Chalek, J.; Abd-Allah, F.; Abdoli, A.; Abualhasan, A.; Abu-Gharbieh, E.; Akram, T.T. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022, 7, e105–e125. [Google Scholar] [CrossRef] [PubMed]
- Garre-Olmo, J. Epidemiology of Alzheimer’s disease and other dementias. Rev. Neurol. 2018, 66, 377–386. [Google Scholar]
- Norton, S.; Matthews, F.E.; Barnes, D.E.; Yaffe, K.; Brayne, C. Potential for primary prevention of Alzheimer’s disease: An analysis of population-based data. Lancet Neurol. 2014, 13, 788–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hooshmand, B.; Mangialasche, F.; Kalpouzos, G.; Solomon, A.; Kåreholt, I.; Smith, A.D.; Refsum, H.; Wang, R.; Mühlmann, M.; Ertl-Wagner, B. Association of vitamin B12, folate, and sulfur amino acids with brain magnetic resonance imaging measures in older adults: A longitudinal population-based study. JAMA Psychiatry 2016, 73, 606–613. [Google Scholar] [CrossRef] [Green Version]
- Virtanen, J.K.; Siscovick, D.S.; Lemaitre, R.N.; Longstreth, W.T., Jr.; Spiegelman, D.; Rimm, E.B.; King, I.B.; Mozaffarian, D. Circulating omega-3 polyunsaturated fatty acids and subclinical brain abnormalities on MRI in older adults: The cardiovascular health study. J. Am. Heart Assoc. 2013, 2, e000305. [Google Scholar] [CrossRef] [Green Version]
- Ozawa, M.; Ohara, T.; Ninomiya, T.; Hata, J.; Yoshida, D.; Mukai, N.; Nagata, M.; Uchida, K.; Shirota, T.; Kitazono, T. Milk and Dairy Consumption and Risk of Dementia in an Elderly Japanese Population: The Hisayama Study. J. Am. Geriatr. Soc. 2014, 62, 1224–1230. [Google Scholar] [CrossRef]
- Petruski-Ivleva, N.; Kucharska-Newton, A.; Palta, P.; Couper, D.; Meyer, K.; Graff, M.; Haring, B.; Sharrett, R.; Heiss, G. Milk intake at midlife and cognitive decline over 20 years. The Atherosclerosis Risk in Communities (ARIC) Study. Nutrients 2017, 9, 1134. [Google Scholar] [CrossRef] [Green Version]
- Talebi, S.; Asoudeh, F.; Naeini, F.; Sadeghi, E.; Travica, N.; Mohammadi, H. Association between animal protein sources and risk of neurodegenerative diseases: A systematic review and dose-response meta-analysis. Nutr. Rev. 2023. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Garach, A.; Cornejo-Pareja, I.; Martinez-Gonzalez, M.A.; Bullo, M.; Corella, D.; Castaner, O.; Romaguera, D.; Vioque, J.; Alonso-Gomez, A.M.; Warnberg, J.; et al. Milk and Dairy Products Intake Is Related to Cognitive Impairment at Baseline in Predimed Plus Trial. Mol. Nutr. Food Res. 2021, 65, e2000728. [Google Scholar] [CrossRef]
- Lee, J.; Fu, Z.; Chung, M.; Jang, D.J.; Lee, H.J. Role of milk and dairy intake in cognitive function in older adults: A systematic review and meta-analysis. Nutr. J. 2018, 17, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuesta-Triana, F.; Verdejo-Bravo, C.; Fernández-Pérez, C.; Martín-Sánchez, F.J. Effect of milk and other dairy products on the risk of frailty, sarcopenia, and cognitive performance decline in the elderly: A systematic review. Adv. Nutr. 2019, 10, S105–S119. [Google Scholar] [CrossRef] [PubMed]
- Crichton, G.E.; Murphy, K.J.; Bryan, J. Dairy intake and cognitive health in middle-aged South Australians. Asia Pac. J. Clin. Nutr. 2010, 19, 161–171. [Google Scholar] [PubMed]
- de Goeij, L.C.; van de Rest, O.; Feskens, E.J.M.; de Groot, L.; Brouwer-Brolsma, E.M. Associations between the Intake of Different Types of Dairy and Cognitive Performance in Dutch Older Adults: The B-PROOF Study. Nutrients 2020, 12, 468. [Google Scholar] [CrossRef] [Green Version]
- Tessier, A.J.; Presse, N.; Rahme, E.; Ferland, G.; Bherer, L.; Chevalier, S. Milk, Yogurt, and Cheese Intake Is Positively Associated With Cognitive Executive Functions in Older Adults of the Canadian Longitudinal Study on Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 2223–2231. [Google Scholar] [CrossRef]
- Klinedinst, B.S.; Le, S.T.; Larsen, B.; Pappas, C.; Hoth, N.J.; Pollpeter, A.; Wang, Q.; Wang, Y.; Yu, S.; Wang, L.; et al. Genetic Factors of Alzheimer’s Disease Modulate How Diet is Associated with Long-Term Cognitive Trajectories: A UK Biobank Study. J. Alzheimer’s Dis. 2020, 78, 1245–1257. [Google Scholar] [CrossRef]
- Lu, Y.; Matsuyama, S.; Sugawara, Y.; Sone, T.; Tsuji, I. Dairy intake and incident functional disability among older Japanese adults: The Ohsaki Cohort 2006 Study. Eur. J. Nutr. 2022, 61, 2627–2637. [Google Scholar] [CrossRef]
- Moreno-Arribas, M.V.; Bartolome, B.; Penalvo, J.L.; Perez-Matute, P.; Motilva, M.J. Relationship between Wine Consumption, Diet and Microbiome Modulation in Alzheimer’s Disease. Nutrients 2020, 12, 3082. [Google Scholar] [CrossRef]
- Nooyens, A.C.; Bueno-de-Mesquita, H.B.; van Gelder, B.M.; van Boxtel, M.P.; Verschuren, W.M. Consumption of alcoholic beverages and cognitive decline at middle age: The Doetinchem Cohort Study. Br. J. Nutr. 2014, 111, 715–723. [Google Scholar] [CrossRef] [Green Version]
- Shield, K.D.; Parry, C.; Rehm, J. Chronic diseases and conditions related to alcohol use. Alcohol. Res. 2013, 35, 155–173. [Google Scholar]
- Koch, M.; Fitzpatrick, A.L.; Rapp, S.R.; Nahin, R.L.; Williamson, J.D.; Lopez, O.L.; DeKosky, S.T.; Kuller, L.H.; Mackey, R.H.; Mukamal, K.J.; et al. Alcohol Consumption and Risk of Dementia and Cognitive Decline Among Older Adults with or without Mild Cognitive Impairment. JAMA Netw. Open 2019, 2, e1910319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuccala, G.; Onder, G.; Pedone, C.; Cesari, M.; Landi, F.; Bernabei, R.; Cocchi, A.; Gruppo Italiano di Farmacoepidemiologia nell’Anziano Investigators. Dose-related impact of alcohol consumption on cognitive function in advanced age: Results of a multicenter survey. Alcohol. Clin. Exp. Res. 2001, 25, 1743–1748. [Google Scholar] [CrossRef]
- Seeley, W.W.; Menon, V.; Schatzberg, A.F.; Keller, J.; Glover, G.H.; Kenna, H.; Reiss, A.L.; Greicius, M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007, 27, 2349–2356. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Zhang, Y.; Yan, H.; Bai, L.; Dai, R.; Wei, W.; Zhong, C.; Xue, T.; Wang, H.; Feng, Y.; et al. Altered topological patterns of brain networks in mild cognitive impairment and Alzheimer’s disease: A resting-state fMRI study. Psychiatry Res. 2012, 202, 118–125. [Google Scholar] [CrossRef]
- Joo, S.H.; Lim, H.K.; Lee, C.U. Three Large-Scale Functional Brain Networks from Resting-State Functional MRI in Subjects with Different Levels of Cognitive Impairment. Psychiatry Investig. 2016, 13, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Pappas, C.; Klinedinst, B.; Pollpeter, A.; Larsen, B.; Hoth, N.; Anton, F.; Wang, Q.; Willette, A.A. Associations between Insulin-Like Growth Factor-1 and Resting-State Functional Connectivity in Cognitively Unimpaired Midlife Adults. J. Alzheimer’s Dis. 2023. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Pappas, C.; Le, S.T.; Wang, Q.; Klinedinst, B.S.; Larsen, B.A.; Pollpeter, A.; Lee, L.Y.; Lutz, M.W.; Gottschalk, W.K.; et al. APOE, TOMM40, and sex interactions on neural network connectivity. Neurobiol. Aging 2022, 109, 158–165. [Google Scholar] [CrossRef]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef] [Green Version]
- Miller, K.L.; Alfaro-Almagro, F.; Bangerter, N.K.; Thomas, D.L.; Yacoub, E.; Xu, J.; Bartsch, A.J.; Jbabdi, S.; Sotiropoulos, S.N.; Andersson, J.L.; et al. Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nat. Neurosci. 2016, 19, 1523–1536. [Google Scholar] [CrossRef] [Green Version]
- Alfaro-Almagro, F.; Jenkinson, M.; Bangerter, N.K.; Andersson, J.L.R.; Griffanti, L.; Douaud, G.; Sotiropoulos, S.N.; Jbabdi, S.; Hernandez-Fernandez, M.; Vallee, E.; et al. Image processing and Quality Control for the first 10,000 brain imaging datasets from UK Biobank. NeuroImage 2018, 166, 400–424. [Google Scholar] [CrossRef]
- Kapogiannis, D.; Reiter, D.A.; Willette, A.A.; Mattson, M.P. Posteromedial cortex glutamate and GABA predict intrinsic functional connectivity of the default mode network. NeuroImage 2013, 64, 112–119. [Google Scholar] [CrossRef] [Green Version]
- Bycroft, C.; Freeman, C.; Petkova, D.; Band, G.; Elliott, L.T.; Sharp, K.; Motyer, A.; Vukcevic, D.; Delaneau, O.; O’Connell, J.; et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 2018, 562, 203–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geijselaers, S.L.C.; Aalten, P.; Ramakers, I.; De Deyn, P.P.; Heijboer, A.C.; Koek, H.L.; OldeRikkert, M.G.M.; Papma, J.M.; Reesink, F.E.; Smits, L.L.; et al. Association of Cerebrospinal Fluid (CSF) Insulin with Cognitive Performance and CSF Biomarkers of Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 61, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Selvin, S. Statistical Analysis of Epidemiologic Data, 3rd ed.; Oxford University Press: Oxford, UK; New York, NY, USA, 2004; p. xiv. 492p. [Google Scholar]
- Coombs, W.T.; Algina, J.; Oltman, D.O. Univariate and multivariate omnibus hypothesis tests selected to control type I error rates when population variances are not necessarily equal. Rev. Educ. Res. 1996, 66, 137–179. [Google Scholar] [CrossRef]
- Menon, V. Large-scale brain networks and psychopathology: A unifying triple network model. Trends Cogn. Sci. 2011, 15, 483–506. [Google Scholar] [CrossRef]
- Sherman, L.E.; Rudie, J.D.; Pfeifer, J.H.; Masten, C.L.; McNealy, K.; Dapretto, M. Development of the default mode and central executive networks across early adolescence: A longitudinal study. Dev. Cogn. Neurosci. 2014, 10, 148–159. [Google Scholar] [CrossRef] [Green Version]
- Blume, J.; Dhanasekara, C.S.; Kahathuduwa, C.N.; Mastergeorge, A.M. Central Executive and Default Mode Networks: An Appraisal of Executive Function and Social Skill Brain-Behavior Correlates in Youth with Autism Spectrum Disorder. J. Autism Dev. Disord. 2023. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Ridley, N.J.; Draper, B.; Withall, A. Alcohol-related dementia: An update of the evidence. Alzheimer’s Res. Ther. 2013, 5, 3. [Google Scholar] [CrossRef] [Green Version]
- Rehm, J.; Hasan, O.S.M.; Black, S.E.; Shield, K.D.; Schwarzinger, M. Alcohol use and dementia: A systematic scoping review. Alzheimer’s Res. Ther. 2019, 11, 1. [Google Scholar] [CrossRef] [Green Version]
- Schwarzinger, M.; Pollock, B.G.; Hasan, O.S.M.; Dufouil, C.; Rehm, J.; QalyDays Study Group. Contribution of alcohol use disorders to the burden of dementia in France 2008–13: A nationwide retrospective cohort study. Lancet Public Health 2018, 3, e124–e132. [Google Scholar] [CrossRef]
- Zhang, R.; Shen, L.; Miles, T.; Shen, Y.; Cordero, J.; Qi, Y.; Liang, L.; Li, C. Association of Low to Moderate Alcohol Drinking With Cognitive Functions From Middle to Older Age Among US Adults. JAMA Netw Open 2020, 3, e207922. [Google Scholar] [CrossRef] [PubMed]
- Stockley, C.S. Wine consumption, cognitive function and dementias—A relationship? Nutr. Aging 2015, 3, 125–137. [Google Scholar] [CrossRef]
- Crichton, G.E.; Elias, M.F.; Dore, G.A.; Robbins, M.A. Relation between dairy food intake and cognitive function: The Maine-Syracuse Longitudinal Study. Int. Dairy J. 2012, 22, 15–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eskelinen, M.H.; Ngandu, T.; Helkala, E.L.; Tuomilehto, J.; Nissinen, A.; Soininen, H.; Kivipelto, M. Fat intake at midlife and cognitive impairment later in life: A population-based CAIDE study. Int. J. Geriatr. Psychiatry 2008, 23, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Vercambre, M.N.; Boutron-Ruault, M.C.; Ritchie, K.; Clavel-Chapelon, F.; Berr, C. Long-term association of food and nutrient intakes with cognitive and functional decline: A 13-year follow-up study of elderly French women. Br. J. Nutr. 2009, 102, 419–427. [Google Scholar] [CrossRef] [Green Version]
- Ni, J.; Nishi, S.K.; Babio, N.; Martinez-Gonzalez, M.A.; Corella, D.; Castaner, O.; Martinez, J.A.; Alonso-Gomez, A.M.; Gomez-Gracia, E.; Vioque, J.; et al. Dairy Product Consumption and Changes in Cognitive Performance: Two-Year Analysis of the PREDIMED-Plus Cohort. Mol. Nutr. Food Res. 2022, 66, e2101058. [Google Scholar] [CrossRef]
- Kesse-Guyot, E.; Assmann, K.E.; Andreeva, V.A.; Ferry, M.; Hercberg, S.; Galan, P.; Group, S.V.M.R. Consumption of Dairy Products and Cognitive Functioning: Findings from the SU.VI.MAX 2 Study. J. Nutr. Health Aging 2016, 20, 128–137. [Google Scholar] [CrossRef]
- Ylilauri, M.P.T.; Hantunen, S.; Lonnroos, E.; Salonen, J.T.; Tuomainen, T.P.; Virtanen, J.K. Associations of dairy, meat, and fish intakes with risk of incident dementia and with cognitive performance: The Kuopio Ischaemic Heart Disease Risk Factor Study (KIHD). Eur. J. Nutr. 2022, 61, 2531–2542. [Google Scholar] [CrossRef]
- Lu, Y.; Gwee, X.; Chua, D.Q.; Lee, T.S.; Lim, W.S.; Chong, M.S.; Yap, P.; Yap, K.B.; Rawtaer, I.; Liew, T.M.; et al. Nutritional Status and Risks of Cognitive Decline and Incident Neurocognitive Disorders: Singapore Longitudinal Ageing Studies. J. Nutr. Health Aging 2021, 25, 660–667. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Grieger, J.A.; Hilpert, K.F.; West, S.G. Milk products, dietary patterns and blood pressure management. J. Am. Coll. Nutr. 2009, 28 (Suppl. S1), 103S–119S. [Google Scholar] [CrossRef]
- Ano, Y.; Ayabe, T.; Kutsukake, T.; Ohya, R.; Takaichi, Y.; Uchida, S.; Yamada, K.; Uchida, K.; Takashima, A.; Nakayama, H. Novel lactopeptides in fermented dairy products improve memory function and cognitive decline. Neurobiol. Aging 2018, 72, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Rice, B.H.; Cifelli, C.J.; Pikosky, M.A.; Miller, G.D. Dairy components and risk factors for cardiometabolic syndrome: Recent evidence and opportunities for future research. Adv. Nutr. 2011, 2, 396–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ano, Y.; Ozawa, M.; Kutsukake, T.; Sugiyama, S.; Uchida, K.; Yoshida, A.; Nakayama, H. Preventive effects of a fermented dairy product against Alzheimer’s disease and identification of a novel oleamide with enhanced microglial phagocytosis and anti-inflammatory activity. PLoS ONE 2015, 10, e0118512. [Google Scholar] [CrossRef] [Green Version]
- Ohsawa, K.; Nakamura, F.; Uchida, N.; Mizuno, S.; Yokogoshi, H. Lactobacillus helveticus-fermented milk containing lactononadecapeptide (NIPPLTQTPVVVPPFLQPE) improves cognitive function in healthy middle-aged adults: A randomised, double-blind, placebo-controlled trial. Int. J. Food Sci. Nutr. 2018, 69, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.; Arab, L. Nutritional prevention of cognitive decline. Adv. Nutr. 2012, 3, 732–733. [Google Scholar] [CrossRef] [Green Version]

| Characteristic | ||
|---|---|---|
| Baseline Age, mean (SD), y | 55.07 (7.48) | Range: 40–70 |
| Body mass index (BMI), mean (SD), kg/m2 | 26.59 (4.17) | Range: 14.74–56.12 |
| Female, % | 52.54 | |
| APOE ε4 Status, % | 27.68 | |
| TOMM40 ‘650 status, % | 26.57 | |
| Family History of AD, % | 24.26 | |
| Smoking Status, % | ||
| Never | 60.74 | |
| Previous | 32.89 | |
| Current | 6.37 | |
| Alcohol Status, % | ||
| Never | 2.45 | |
| Previous | 1.95 | |
| Current | 95.60 | |
| Milk, mean (SD), glasses/carton//250 mL | 1.48 (1.06) | Range: 0.5–5 |
| Cheese, mean (SD), servings | 0.52 (0.62) | Range: 0–6 |
| Red wine, mean (SD), glasses/carton//250 mL | 1.40 (1.02) | Range: 0.5–5 |
| Component | APOE Status | TOMM40 Status | Family History Status | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| APOE4 Negative | APOE4 Positive | TOMM40 650′ Negative | TOMM40 650′ Positive | Negative | Positive | |||||||
| Beta | SE | Beta | SE | Beta | SE | Beta | SE | Beta | SE | Beta | SE | |
| IC 1 | −0.0190 | 0.0038 | 0.0320 | 0.0063 | −0.0190 | 0.0038 | 0.0320 | 0.0063 | −0.0244 | 0.0038 * | 0.0553 | 0.0059 * |
| IC 5 | −0.0138 | 0.0025 ** | 0.0632 | 0.0042 ** | −0.0179 | 0.0025 *** | 0.0774 | 0.0044 *** | −0.0104 | 0.0026 * | 0.0616 | 0.0040 * |
| IC 9 | −0.0418 | 0.0034 ** | 0.0695 | 0.0056 ** | −0.0398 | 0.0034 ** | 0.0683 | 0.0057 ** | −0.0213 | 0.0034 | 0.0176 | 0.0055 |
| IC 10 | −0.0340 | 0.0038 ** | 0.0621 | 0.0063 ** | −0.0349 | 0.0037 ** | 0.0685 | 0.0065 ** | −0.0170 | 0.0038 | 0.0198 | 0.0064 |
| IC 13 | −0.0089 | 0.0018 ** | 0.0540 | 0.0030 ** | −0.0029 | 0.0018 | 0.0391 | 0.0031 | −0.0045 | 0.0018 * | 0.0475 | 0.0029 * |
| IC 14 | −0.0141 | 0.0021 * | 0.0234 | 0.0035 * | −0.0148 | 0.0021 * | 0.0265 | 0.0035 * | −0.0165 | 0.0021 ** | 0.0357 | 0.0032 ** |
| IC 20 | −0.0367 | 0.0028 ** | 0.0300 | 0.0047 ** | −0.0389 | 0.0028 *** | 0.0392 | 0.0049 *** | −0.0301 | 0.0029 | 0.0169 | 0.0045 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Willette, A.A.; Wang, Q.; Pollpeter, A.; Larsen, B.A.; Mohammadiarvejeh, P.; Fili, M. Alzheimer’s Disease Genetic Influences Impact the Associations between Diet and Resting-State Functional Connectivity: A Study from the UK Biobank. Nutrients 2023, 15, 3390. https://doi.org/10.3390/nu15153390
Li T, Willette AA, Wang Q, Pollpeter A, Larsen BA, Mohammadiarvejeh P, Fili M. Alzheimer’s Disease Genetic Influences Impact the Associations between Diet and Resting-State Functional Connectivity: A Study from the UK Biobank. Nutrients. 2023; 15(15):3390. https://doi.org/10.3390/nu15153390
Chicago/Turabian StyleLi, Tianqi, Auriel A. Willette, Qian Wang, Amy Pollpeter, Brittany A. Larsen, Parvin Mohammadiarvejeh, and Mohammad Fili. 2023. "Alzheimer’s Disease Genetic Influences Impact the Associations between Diet and Resting-State Functional Connectivity: A Study from the UK Biobank" Nutrients 15, no. 15: 3390. https://doi.org/10.3390/nu15153390
APA StyleLi, T., Willette, A. A., Wang, Q., Pollpeter, A., Larsen, B. A., Mohammadiarvejeh, P., & Fili, M. (2023). Alzheimer’s Disease Genetic Influences Impact the Associations between Diet and Resting-State Functional Connectivity: A Study from the UK Biobank. Nutrients, 15(15), 3390. https://doi.org/10.3390/nu15153390







