The Disease with a Thousand Faces and the Human Microbiome—A Physiopathogenic Intercorrelation in Pediatric Practice
Abstract
:1. Introduction
2. Materials and Methods
3. Epidemiology
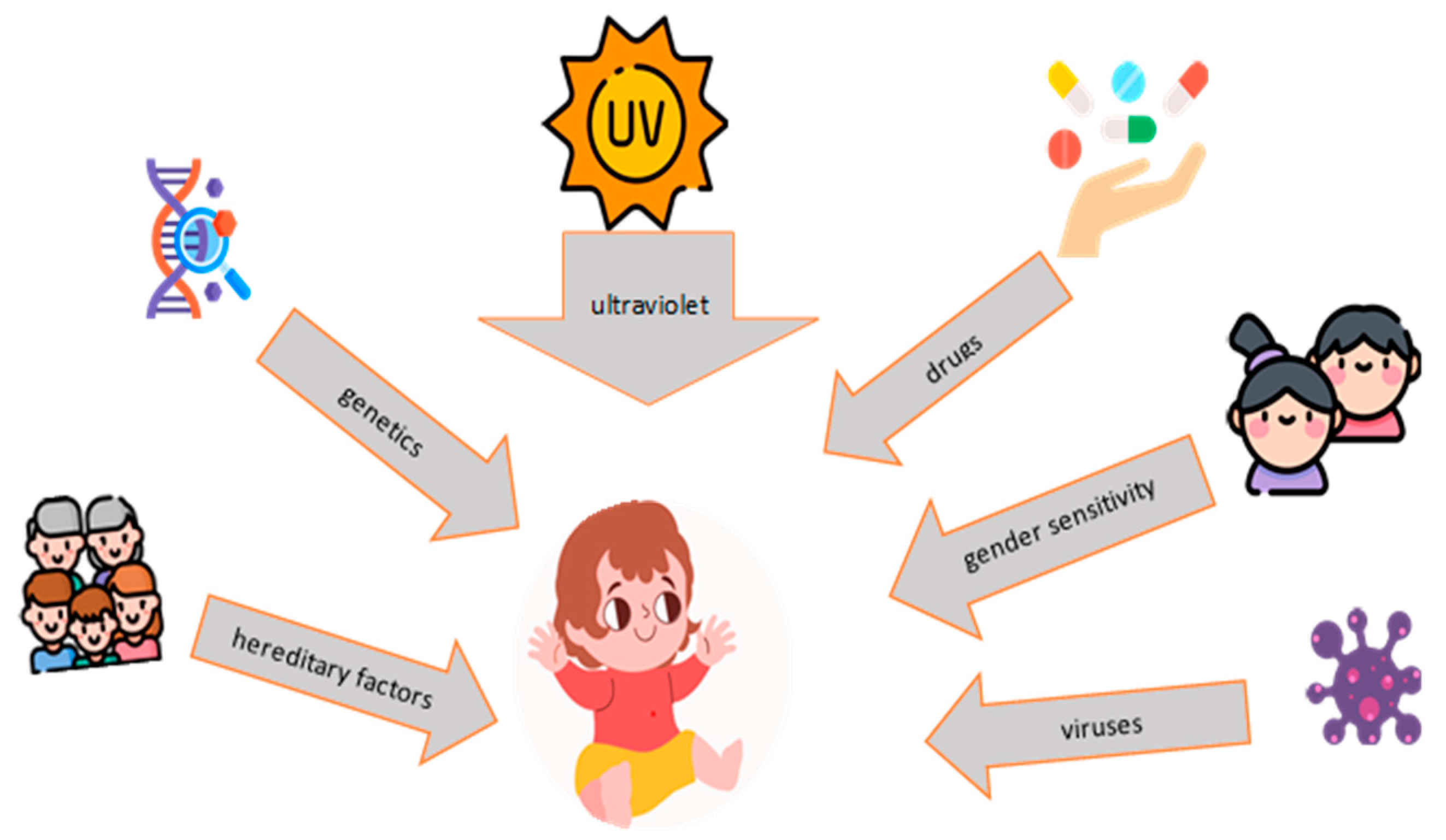
4. Pathogenesis
5. Diagnosis
6. The Role of the Microbiome
6.1. Skin
6.2. Respiratory System
6.3. Genitourinary System
6.4. Gastrointestinal Tract
7. Modulation of Intestinal Microbiota in the Adjuvant Therapy of SLE
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Charras, A.; Smith, E.; Hedrich, C.M. Systemic Lupus Erythematosus in Children and Young People. Curr. Rheumatol. Rep. 2021, 23, 20. [Google Scholar] [CrossRef] [PubMed]
- Lythgoe, H.; Lj, M.; Hedrich, C.M.; Aringer, M. Classification of systemic lupus erythematosus in children and adults. Clin. Immunol. 2022, 234, 108898. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, L.N.; Peterson, M.G.; Harrison, M.J.; Onel, K.B.; Lehman, T.J. Quality of life in children with systemic lupus erythematosus: A review. Lupus 2007, 16, 663–669. [Google Scholar] [CrossRef]
- Aggarwal, A.; Srivastava, P. Childhood onset systemic lupus erythematosus: How is it different from adult SLE? Int. J. Rheum. Dis. 2015, 18, 182–191. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; Godoy-Vitorino, F.; Knight, R.; Blaser, M.J. Role of the microbiome in human development. Gut 2019, 68, 1108–1114. [Google Scholar] [CrossRef] [Green Version]
- Manos, J. The human microbiome in disease and pathology. Apmis 2022, 130, 690–705. [Google Scholar] [CrossRef] [PubMed]
- Integrative HMP (iHMP) Research Network Consortium. The Integrative Human Microbiome Project. Nature 2019, 569, 641–648. [Google Scholar] [CrossRef] [Green Version]
- Hiraki, L.T.; Feldman, C.H.; Liu, J.; Alarcón, G.S.; Fischer, M.A.; Winkelmayer, W.C.; Costenbader, K.H. Prevalence, incidence, and demographics of systemic lupus erythematosus and lupus nephritis from 2000 to 2004 among children in the US Medicaid beneficiary population. Arthritis Rheum. 2012, 64, 2669–2676. [Google Scholar] [CrossRef] [Green Version]
- Concannon, A.; Rudge, S.; Yan, J.; Reed, P. The incidence, diagnostic clinical manifestations and severity of juvenile systemic lupus erythematosus in New Zealand Maori and Pacific Island children: The Starship experience (2000–2010). Lupus 2013, 22, 1156–1161. [Google Scholar] [CrossRef]
- VanEvery, H.; Franzosa, E.A.; Nguyen, L.H.; Huttenhower, C. Microbiome epidemiology and association studies in human health. Nat. Rev. Genet. 2023, 24, 109–124. [Google Scholar] [CrossRef]
- Hayes, W.; Sahu, S. The Human Microbiome: History and Future. J. Pharm. Pharm. Sci. 2020, 23, 404–411. [Google Scholar] [CrossRef]
- Chi, M.; Ma, K.; Wang, J.; Ding, Z.; Li, Y.; Zhu, S.; Liang, X.; Zhang, Q.; Song, L.; Liu, C. The Immunomodulatory Effect of the Gut Microbiota in Kidney Disease. J. Immunol. Res. 2021, 15, 5516035. [Google Scholar] [CrossRef]
- Omarjee, O.; Picard, C.; Frachette, C.; Moreews, M.; Rieux-Laucat, F.; Soulas-Sprauel, P.; Viel, S.; Lega, J.C.; Bader-Meunier, B.; Walzer, T.; et al. Monogenic lupus: Dissecting heterogeneity. Autoimmun. Rev. 2019, 18, 102361. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chang, C.; Lu, Q. The Epigenetics of Lupus Erythematosus. Adv. Exp. Med. Biol. 2020, 1253, 185–207. [Google Scholar] [CrossRef] [PubMed]
- Lo, M.S. Insights Gained from the Study of Pediatric Systemic Lupus Erythematosus. Front. Immunol. 2018, 9, 1278. [Google Scholar] [CrossRef] [Green Version]
- Pan, L.; Lu, M.P.; Wang, J.H.; Xu, M.; Yang, S.R. Immunological pathogenesis and treatment of systemic lupus erythematosus. World J. Pediatr. 2020, 16, 19–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Midgley, A.; Watson, L.; Beresford, M.W. New insights into the pathogenesis and management of lupus in children. Arch. Dis. Child. 2014, 99, 563–567. [Google Scholar] [CrossRef]
- Fava, A.; Petri, M. Systemic lupus erythematosus: Diagnosis and clinical management. J. Autoimmun. 2019, 96, 1–13. [Google Scholar] [CrossRef]
- Ogunrinde, E.; Zhou, Z.; Luo, Z.; Alekseyenko, A.; Li, Q.Z.; Macedo, D.; Kamen, D.L.; Oates, J.C.; Gilkeson, G.S.; Jiang, W. A Link Between Plasma Microbial Translocation, Microbiome, and Autoantibody Development in First-Degree Relatives of Systemic Lupus Erythematosus Patients. Arthritis Rheumatol. 2019, 71, 1858–1868. [Google Scholar] [CrossRef]
- Clancy, R.M.; Marion, M.C.; Ainsworth, H.C.; Chang, M.; Howard, T.D.; Izmirly, P.M.; Masson, M.; Buyon, J.P.; Langefeld, C.D. Gut dysbiosis and the clinical spectrum in anti-Ro positive mothers of children with neonatal lupus. Gut Microbes 2022, 14, 2081474. [Google Scholar] [CrossRef]
- Clancy, R.M.; Marion, M.C.; Ainsworth, H.C.; Blaser, M.J.; Chang, M.; Howard, T.D.; Izmirly, P.M.; Lacher, C.; Masson, M.; Robins, K.; et al. Salivary dysbiosis and the clinical spectrum in anti-Ro positive mothers of children with neonatal lupus. J. Autoimmun. 2020, 107, 102354. [Google Scholar] [CrossRef]
- Putri, P.Z.; Hamijoyo, L.; Sahiratmadja, E. The Role of Diet in Influencing the Diversity of Gut Microbiome Related to Lupus Disease Activities: A Systematic Review. Int. J. Microbiol. 2022, 2022, 6908677. [Google Scholar] [CrossRef]
- Levy, D.M.; Kamphuis, S. Systemic lupus erythematosus in children and adolescents. Pediatr. Clin. N. Am. 2012, 59, 345–364. [Google Scholar] [CrossRef] [Green Version]
- Tucker, L.B. Making the diagnosis of systemic lupus erythematosus in children and adolescents. Lupus 2007, 16, 546–549. [Google Scholar] [CrossRef] [PubMed]
- Pistone, D.; Meroni, G.; Panelli, S.; D’Auria, E.; Acunzo, M.; Pasala, A.R.; Zuccotti, G.V.; Bandi, C.; Drago, L. A Journey on the Skin Microbiome: Pitfalls and Opportunities. Int. J. Mol. Sci. 2021, 22, 9846. [Google Scholar] [CrossRef] [PubMed]
- Requena, T.; Velasco, M. The human microbiome in sickness and in health. Rev. Clínica Española 2021, 221, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Lupu, V.V.; Adam Raileanu, A.; Mihai, C.M.; Morariu, I.D.; Lupu, A.; Starcea, I.M.; Frasinariu, O.E.; Mocanu, A.; Dragan, F.; Fotea, S. The Implication of the Gut Microbiome in Heart Failure. Cells 2023, 12, 1158. [Google Scholar] [CrossRef]
- Kim, H.; Sitarik, A.R.; Woodcroft, K.; Johnson, C.C.; Zoratti, E. Birth Mode, Breastfeeding, Pet Exposure, and Antibiotic Use: Associations with the Gut Microbiome and Sensitization in Children. Curr. Allergy Asthma Rep. 2019, 19, 22. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.J.; Langdon, A.E.; Dantas, G. Understanding the impact of antibiotic perturbation on the human microbiome. Genome Med. 2020, 12, 82. [Google Scholar] [CrossRef]
- Patangia, D.V.; Anthony Ryan, C.; Dempsey, E.; Paul Ross, R.; Stanton, C. Impact of antibiotics on the human microbiome and consequences for host health. MicrobiologyOpen 2022, 11, e1260. [Google Scholar] [CrossRef]
- Le Doare, K.; Holder, B.; Bassett, A.; Pannaraj, P.S. Mother’s Milk: A Purposeful Contribution to the Development of the Infant Microbiota and Immunity. Front. Immunol. 2018, 9, 361. [Google Scholar] [CrossRef] [Green Version]
- Lupu, V.V.; Miron, I.C.; Raileanu, A.A.; Starcea, I.M.; Lupu, A.; Tarca, E.; Mocanu, A.; Buga, A.M.L.; Lupu, V.; Fotea, S. Difficulties in Adaptation of the Mother and Newborn via Cesarean Section versus Natural Birth-A Narrative Review. Life 2023, 13, 300. [Google Scholar] [CrossRef] [PubMed]
- Derrien, M.; Alvarez, A.S.; de Vos, W.M. The Gut Microbiota in the First Decade of Life. Trends Microbiol. 2019, 27, 997–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carmona-Cruz, S.; Orozco-Covarrubias, L.; Sáez-de-Ocariz, M. The Human Skin Microbiome in Selected Cutaneous Diseases. Front. Cell Infect. Microbiol. 2022, 12, 834135. [Google Scholar] [CrossRef] [PubMed]
- Orlova, E.; Smirnova, L.; Nesvizhsky, Y.; Kosenkov, D.; Zykova, E. Acute urticaria in children: Course of the disease, features of skin microbiome. Postepy Dermatol. Alergol. 2022, 39, 164–170. [Google Scholar] [CrossRef]
- Isler, M.F.; Coates, S.J.; Boos, M.D. Climate change, the cutaneous microbiome and skin disease: Implications for a warming world. Int. J. Dermatol. 2023, 62, 337–345. [Google Scholar] [CrossRef]
- Pantazi, A.C.; Mihai, C.M.; Balasa, A.L.; Chisnoiu, T.; Lupu, A.; Frecus, C.E.; Mihai, L.; Ungureanu, A.; Kassim, M.A.K.; Andrusca, A.; et al. Relationship between Gut Microbiota and Allergies in Children: A Literature Review. Nutrients 2023, 15, 2529. [Google Scholar] [CrossRef]
- Bouslimani, A.; da Silva, R.; Kosciolek, T.; Janssen, S.; Callewaert, C.; Amir, A.; Dorrestein, K.; Melnik, A.V.; Zaramela, L.S.; Kim, J.N.; et al. The impact of skin care products on skin chemistry and microbiome dynamics. BMC Biol. 2019, 17, 47. [Google Scholar] [CrossRef] [Green Version]
- Khadka, V.D.; Key, F.M.; Romo-González, C.; Martínez-Gayosso, A.; Campos-Cabrera, B.L.; Gerónimo-Gallegos, A.; Lynn, T.C.; Durán-McKinster, C.; Coria-Jiménez, R.; Lieberman, T.D.; et al. The Skin Microbiome of Patients with Atopic Dermatitis Normalizes Gradually During Treatment. Front. Cell Infect. Microbiol. 2021, 11, 720674. [Google Scholar] [CrossRef]
- Koh, L.F.; Ong, R.Y.; Common, J.E. Skin microbiome of atopic dermatitis. Allergol. Int. 2022, 71, 31–39. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Cao, N.W.; Guo, B.; Chen, W.J.; Tao, J.H.; Chu, X.J.; Meng, X.; Zhang, T.X.; Li, B.Z. Systemic lupus erythematosus patients have a distinct structural and functional skin microbiota compared with controls. Lupus 2021, 30, 1553–1564. [Google Scholar] [CrossRef] [PubMed]
- Man, W.H.; de Steenhuijsen Piters, W.A.; Bogaert, D. The microbiota of the respiratory tract: Gatekeeper to respiratory health. Nat. Rev. Microbiol. 2017, 15, 259–270. [Google Scholar] [CrossRef] [PubMed]
- de Steenhuijsen Piters, W.A.; Binkowska, J.; Bogaert, D. Early Life Microbiota and Respiratory Tract Infections. Cell Host Microbe 2020, 28, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Sun, Y.; Zhao, N.; Song, J.; Zhang, N.; Liu, L.; Liu, Q. Characteristics of the bacterial microbiota in the upper respiratory tract of children. Eur. Arch. Otorhinolaryngol. 2022, 279, 1081–1089. [Google Scholar] [CrossRef]
- Shah, R.; Bunyavanich, S. The airway microbiome and pediatric asthma. Curr. Opin. Pediatr. 2021, 33, 639–647. [Google Scholar] [CrossRef]
- Merenstein, C.; Liang, G.; Whiteside, S.A.; Cobián-Güemes, A.G.; Merlino, M.S.; Taylor, L.J.; Glascock, A.; Bittinger, K.; Tanes, C.; Graham-Wooten, J.; et al. Signatures of COVID-19 Severity and Immune Response in the Respiratory Tract Microbiome. mBio 2021, 12, e0177721. [Google Scholar] [CrossRef]
- Lupu, A.; Miron, I.C.; Gavrilovici, C.; Raileanu, A.A.; Starcea, I.M.; Ioniuc, I.; Azoicai, A.; Mocanu, A.; Butnariu, L.I.; Dragan, F.; et al. Pediatric Systemic Lupus Erythematous in COVID-19 Era. Viruses 2023, 15, 272. [Google Scholar] [CrossRef]
- Zhu, W.; Wu, Y.; Liu, H.; Jiang, C.; Huo, L. Gut-Lung Axis: Microbial Crosstalk in Pediatric Respiratory Tract Infections. Front. Immunol. 2021, 12, 741233. [Google Scholar] [CrossRef]
- Li, K.L.; Wang, B.Z.; Li, Z.P.; Li, Y.L.; Liang, J.J. Alterations of intestinal flora and the effects of probiotics in children with recurrent respiratory tract infection. World J. Pediatr. 2019, 15, 255–261. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Lu, Y.; Chen, T.; Li, R. The Female Vaginal Microbiome in Health and Bacterial Vaginosis. Front. Cell Infect. Microbiol. 2021, 11, 631972. [Google Scholar] [CrossRef]
- Agostinis, C.; Mangogna, A.; Bossi, F.; Ricci, G.; Kishore, U.; Bulla, R. Uterine Immunity and Microbiota: A Shifting Paradigm. Front. Immunol. 2019, 10, 2387. [Google Scholar] [CrossRef] [Green Version]
- Norenhag, J.; Du, J.; Olovsson, M.; Verstraelen, H.; Engstrand, L.; Brusselaers, N. The vaginal microbiota, human papillomavirus and cervical dysplasia: A systematic review and network meta-analysis. BJOG 2020, 127, 171–180. [Google Scholar] [CrossRef]
- Santella, B.; Schettino, M.T.; Franci, G.; De Franciscis, P.; Colacurci, N.; Schiattarella, A.; Galdiero, M. Microbiota and HPV: The role of viral infection on vaginal microbiota. J. Med. Virol. 2022, 94, 4478–4484. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Fiscella, K.A.; Gill, S.R. Oral microbiome: Possible harbinger for children’s health. Int. J. Oral. Sci. 2020, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.; Cheng, Y.; Gao, J.; Lei, W.; Yan, X.; Hu, X.; Shao, L.; Liu, X.; Kang, R. Alterations of the fecal and vaginal microbiomes in patients with systemic lupus erythematosus and their associations with immunological profiles. Front. Immunol. 2023, 14, 1135861. [Google Scholar] [CrossRef]
- Zuber, A.; Peric, A.; Pluchino, N.; Baud, D.; Stojanov, M. Human Male Genital Tract Microbiota. Int. J. Mol. Sci. 2023, 24, 6939. [Google Scholar] [CrossRef]
- Kawalec, A.; Zwolińska, D. Emerging Role of Microbiome in the Prevention of Urinary Tract Infections in Children. Int. J. Mol. Sci. 2022, 23, 870. [Google Scholar] [CrossRef] [PubMed]
- Lemberger, U.; Quhal, F.; Bruchbacher, A.; Shariat, S.F.; Hiess, M. The microbiome in urinary tract infections in children—An update. Curr. Opin. Urol. 2021, 31, 147–154. [Google Scholar] [CrossRef]
- Miller, A.W.; Penniston, K.L.; Fitzpatrick, K.; Agudelo, J.; Tasian, G.; Lange, D. Mechanisms of the intestinal and urinary microbiome in kidney stone disease. Nat. Rev. Urol. 2022, 19, 695–707. [Google Scholar] [CrossRef]
- Premaraj, T.S.; Vella, R.; Chung, J.; Lin, Q.; Hunter, P.; Underwood, K.; Premaraj, S.; Zhou, Y. Ethnic variation of oral microbiota in children. Sci. Rep. 2020, 10, 14788. [Google Scholar] [CrossRef]
- Pachoński, M.; Koczor-Rozmus, A.; Mocny-Pachońska, K.; Łanowy, P.; Mertas, A.; Jarosz-Chobot, P. Oral microbiota in children with type 1 diabetes mellitus. Pediatr. Endocrinol. Diabetes Metab. 2021, 27, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Lucchese, A.; Cenciarelli, S.; Manuelli, M.; Marcolina, M.; Barzaghi, F.; Calbi, V.; Migliavacca, M.; Bernardo, M.E.; Tucci, F.; Gallo, V.; et al. Wiskott-Aldrich syndrome: Oral findings and microbiota in children and review of the literature. Clin. Exp. Dent. Res. 2022, 8, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Wang, J.; Wang, Y.; Zhang, J.; Zhao, C.; Shen, N.; Yang, J.; Gai, Z.; Zhang, L. Oral microbiota dysbiosis and its association with Henoch-Schönlein Purpura in children. Int. Immunopharmacol. 2018, 65, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Liu, J.; Cetinbas, M.; Sadreyev, R.; Koh, M.; Huang, H.; Adeseye, A.; He, P.; Zhu, J.; Russell, H.; et al. New and Preliminary Evidence on Altered Oral and Gut Microbiota in Individuals with Autism Spectrum Disorder (ASD): Implications for ASD Diagnosis and Subtyping Based on Microbial Biomarkers. Nutrients 2019, 11, 2128. [Google Scholar] [CrossRef] [Green Version]
- Lupu, V.V.; Trandafir, L.M.; Raileanu, A.A.; Mihai, C.M.; Morariu, I.D.; Starcea, I.M.; Mocanu, A.; Butnariu, L.I.; Stoleriu, G.; Salaru, D.L.; et al. Advances in Understanding the Human Gut Microbiota and Its Implication in Pediatric Celiac Disease—A Narrative Review. Nutrients 2023, 15, 2499. [Google Scholar] [CrossRef]
- Lupu, V.V.; Ghiciuc, C.M.; Stefanescu, G.; Mihai, C.M.; Popp, A.; Sasaran, M.O.; Bozomitu, L.; Starcea, I.M.; Adam Raileanu, A.; Lupu, A. Emerging role of the gut microbiome in post-infectious irritable bowel syndrome: A literature review. World J. Gastroenterol. 2023, 29, 3241–3256. [Google Scholar] [CrossRef]
- Xiao, E.; Mattos, M.; Vieira, G.H.A.; Chen, S.; Corrêa, J.D.; Wu, Y.; Albiero, M.L.; Bittinger, K.; Graves, D.T. Diabetes Enhances IL-17 Expression and Alters the Oral Microbiome to Increase Its Pathogenicity. Cell Host Microbe. 2017, 22, 120–128.e4. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.J.; Li, Z.L. Characteristics of gastric microbiota in children with Helicobacter pylori infection family history. J. Peking Univ. Health Sci. 2021, 53, 1115–1121. (In Chinese) [Google Scholar] [CrossRef]
- Peng, R.; Liu, S.; You, W.; Huang, Y.; Hu, C.; Gao, Y.; Jia, X.; Li, G.; Xu, Z.; Chen, Y. Gastric Microbiome Alterations Are Associated with Decreased CD8+ Tissue-Resident Memory T Cells in the Tumor Microenvironment of Gastric Cancer. Cancer Immunol. Res. 2022, 10, 1224–1240. [Google Scholar] [CrossRef]
- Bozomitu, L.; Miron, I.; Adam Raileanu, A.; Lupu, A.; Paduraru, G.; Marcu, F.M.; Buga, A.M.L.; Rusu, D.C.; Dragan, F.; Lupu, V.V. The Gut Microbiome and Its Implication in the Mucosal Digestive Disorders. Biomedicines 2022, 10, 3117. [Google Scholar] [CrossRef]
- Zheng, W.; Peng, K.R.; Li, F.B.; Zhao, H.; Jiang, L.Q.; Chen, F.B.; Jiang, M.Z. Characteristics of gastric mucosa microbiota in children with chronic gastritis and duodenal ulcer. Chin. J. Pediatr. 2021, 59, 551–556. (In Chinese) [Google Scholar] [CrossRef]
- Noto, J.M.; Piazuelo, M.B.; Shah, S.C.; Romero-Gallo, J.; Hart, J.L.; Di, C.; Carmichael, J.D.; Delgado, A.G.; Halvorson, A.E.; Greevy, R.A.; et al. Iron deficiency linked to altered bile acid metabolism promotes Helicobacter pylori-induced inflammation-driven gastric carcinogenesis. J. Clin. Investig. 2022, 132, e147822. [Google Scholar] [CrossRef] [PubMed]
- Dargenio, C.; Dargenio, V.N.; Bizzoco, F.; Indrio, F.; Francavilla, R.; Cristofori, F. Limosilactobacillus reuteri Strains as Adjuvants in the Management of Helicobacter pylori Infection. Medicina 2021, 57, 733. [Google Scholar] [CrossRef] [PubMed]
- Gebrayel, P.; Nicco, C.; Al Khodor, S.; Bilinski, J.; Caselli, E.; Comelli, E.M.; Egert, M.; Giaroni, C.; Karpinski, T.M.; Loniewski, I.; et al. Microbiota medicine: Towards clinical revolution. J. Transl. Med. 2022, 20, 111. [Google Scholar] [CrossRef] [PubMed]
- Christovich, A.; Luo, X.M. Gut Microbiota, Leaky Gut, and Autoimmune Diseases. Front. Immunol. 2022, 13, 946248. [Google Scholar] [CrossRef]
- Xu, Q.; Ni, J.J.; Han, B.X.; Yan, S.S.; Wei, X.T.; Feng, G.J.; Zhang, H.; Zhang, L.; Li, B.; Pei, Y.F. Causal Relationship Between Gut Microbiota and Autoimmune Diseases: A Two-Sample Mendelian Randomization Study. Front. Immunol. 2022, 12, 746998. [Google Scholar] [CrossRef] [PubMed]
- De Luca, F.; Shoenfeld, Y. The microbiome in autoimmune diseases. Clin. Exp. Immunol. 2019, 195, 74–85. [Google Scholar] [CrossRef] [Green Version]
- Rosser, E.C.; Mauri, C. A clinical update on the significance of the gut microbiota in systemic autoimmunity. J. Autoimmun. 2016, 74, 85–93. [Google Scholar] [CrossRef]
- Xiang, K.; Wang, P.; Xu, Z.; Hu, Y.Q.; He, Y.S.; Chen, Y.; Feng, Y.T.; Yin, K.J.; Huang, J.X.; Wang, J.; et al. Causal Effects of Gut Microbiome on Systemic Lupus Erythematosus: A Two-Sample Mendelian Randomization Study. Front. Immunol. 2021, 12, 667097. [Google Scholar] [CrossRef]
- Xiang, S.; Qu, Y.; Qian, S.; Wang, R.; Wang, Y.; Jin, Y.; Li, J.; Ding, X. Association between systemic lupus erythematosus and disruption of gut microbiota: A meta-analysis. Lupus Sci. Med. 2022, 9, e000599. [Google Scholar] [CrossRef]
- Chen, B.D.; Jia, X.M.; Xu, J.Y.; Zhao, L.D.; Ji, J.Y.; Wu, B.X.; Ma, Y.; Li, H.; Zuo, X.X.; Pan, W.Y.; et al. An Autoimmunogenic and Proinflammatory Profile Defined by the Gut Microbiota of Patients with Untreated Systemic Lupus Erythematosus. Arthritis Rheumatol. 2021, 73, 232–243. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, J.; Xiao, L.; Zhang, X.; Zhao, L.; Wang, M.; Li, L. Gut microbiota in systemic lupus erythematosus: A fuse and a solution. J. Autoimmun. 2022, 132, 102867. [Google Scholar] [CrossRef] [PubMed]
- Wen, M.; Liu, T.; Zhao, M.; Dang, X.; Feng, S.; Ding, X.; Xu, Z.; Huang, X.; Lin, Q.; Xiang, W.; et al. Correlation Analysis between Gut Microbiota and Metabolites in Children with Systemic Lupus Erythematosus. J. Immunol. Res. 2021, 2021, 5579608. [Google Scholar] [CrossRef]
- Silverman, G.J.; Azzouz, D.F.; Alekseyenko, A.V. Systemic Lupus Erythematosus and dysbiosis in the microbiome: Cause or effect or both? Curr. Opin. Immunol. 2019, 61, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Cong, Y. Gut microbiota-derived metabolites in the regulation of host immune responses and immune-related inflammatory diseases. Cell Mol. Immunol. 2021, 18, 866–877. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Qing, P.; Yang, H.; Wu, Y.; Liu, Y.; Luo, Y. Gut Microbiome and Metabolites in Systemic Lupus Erythematosus: Link, Mechanisms and Intervention. Front. Immunol. 2021, 12, 686501. [Google Scholar] [CrossRef]
- He, J.; Chan, T.; Hong, X.; Zheng, F.; Zhu, C.; Yin, L.; Dai, W.; Tang, D.; Liu, D.; Dai, Y. Microbiome and Metabolome Analyses Reveal the Disruption of Lipid Metabolism in Systemic Lupus Erythematosus. Front. Immunol. 2020, 11, 1703. [Google Scholar] [CrossRef]
- Liu, F.; Ren, T.; Li, X.; Zhai, Q.; Xu, X.; Zhang, N.; Jiang, P.; Niu, Y.; Lv, L.; Shi, G.; et al. Distinct Microbiomes of Gut and Saliva in Patients with Systemic Lupus Erythematous and Clinical Associations. Front. Immunol. 2021, 12, 626217. [Google Scholar] [CrossRef]
- Jiao, Y.; Wu, L.; Huntington, N.D.; Zhang, X. Crosstalk Between Gut Microbiota and Innate Immunity and Its Implication in Autoimmune Diseases. Front. Immunol. 2020, 11, 282. [Google Scholar] [CrossRef] [Green Version]
- Dürholz, K.; Schmid, E.; Frech, M.; Azizov, V.; Otterbein, N.; Lucas, S.; Rauh, M.; Schett, G.; Bruns, H.; Zaiss, M.M. Microbiota-Derived Propionate Modulates Megakaryopoiesis and Platelet Function. Front. Immunol. 2022, 13, 908174. [Google Scholar] [CrossRef]
- Vieira, J.R.P.; Rezende, A.T.O.; Fernandes, M.R.; da Silva, N.A. Intestinal microbiota and active systemic lupus erythematosus: A systematic review. Adv. Rheumatol. 2021, 61, 42. [Google Scholar] [CrossRef]
- de la Visitación, N.; Robles-Vera, I.; Moleón, J.; González-Correa, C.; Aguilera-Sánchez, N.; Toral, M.; Gómez-Guzmán, M.; Sánchez, M.; Jiménez, R.; Martin-Morales, N.; et al. Gut Microbiota Has a Crucial Role in the Development of Hypertension and Vascular Dysfunction in Toll-like Receptor 7-Driven Lupus Autoimmunity. Antioxidants 2021, 10, 1426. [Google Scholar] [CrossRef]
- Mohd, R.; Chin, S.F.; Shaharir, S.S.; Cham, Q.S. Involvement of Gut Microbiota in SLE and Lupus Nephritis. Biomedicines 2023, 11, 653. [Google Scholar] [CrossRef]
- Monticolo, M.; Mucha, K.; Foroncewicz, B. Lupus Nephritis and Dysbiosis. Biomedicines 2023, 11, 1165. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, E.A.; Jørgensen, T.N. Relationships Between Vitamin D, Gut Microbiome, and Systemic Autoimmunity. Front. Immunol. 2020, 10, 3141. [Google Scholar] [CrossRef] [PubMed]
- Katrib, M.; Haddad, R.; Hamdan, Z.; Rida, M.A. The dynamic relationship of gut microbiota with sex hormones in systemic lupus erythematosus. Reumatologia 2023, 61, 130–136. [Google Scholar] [CrossRef]
- Silverman, G.J.; Deng, J.; Azzouz, D.F. Sex-dependent Lupus Blautia (Ruminococcus) gnavus strain induction of zonulin-mediated intestinal permeability and autoimmunity. Front. Immunol. 2022, 13, 897971. [Google Scholar] [CrossRef]
- Chalhoub, N.E.; Wenderfer, S.E.; Levy, D.M.; Rouster-Stevens, K.; Aggarwal, A.; Savani, S.I.; Ruth, N.M.; Arkachaisri, T.; Qiu, T.; Merritt, A.; et al. Childhood Arthritis and Rheumatology Research Alliance Lupus Nephritis Work Group and the Pediatric Rheumatology European Society Lupus Working Party. International Consensus for the Dosing of Corticosteroids in Childhood-Onset Systemic Lupus Erythematosus with Proliferative Lupus Nephritis. Arthritis Rheumatol. 2022, 74, 263–273. [Google Scholar] [CrossRef]
- Groot, N.; de Graeff, N.; Avcin, T.; Bader-Meunier, B.; Brogan, P.; Dolezalova, P.; Feldman, B.; Kone-Paut, I.; Lahdenne, P.; Marks, S.D.; et al. European evidence-based recommendations for diagnosis and treatment of childhood-onset systemic lupus erythematosus: The SHARE initiative. Ann. Rheum. Dis. 2017, 76, 1788–1796. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, A.L.; Eichhorst, A.; Hentze, C.; Kraemer, A.N.; Amend, A.; Sprenger, D.T.L.; Fluhr, C.; Finzel, S.; Daniel, C.; Salzer, U.; et al. Low Dietary Fiber Intake Links Development of Obesity and Lupus Pathogenesis. Front. Immunol. 2021, 12, 696810. [Google Scholar] [CrossRef]
- Yaigoub, H.; Fath, N.; Tirichen, H.; Wu, C.; Li, R.; Li, Y. Bidirectional crosstalk between dysbiotic gut microbiota and systemic lupus erythematosus: What is new in therapeutic approaches? Clin. Immunol. 2022, 244, 109109. [Google Scholar] [CrossRef]
- Wang, X.; Shu, Q.; Song, L.; Liu, Q.; Qu, X.; Li, M. Gut Microbiota in Systemic Lupus Erythematosus and Correlation with Diet and Clinical Manifestations. Front. Med. 2022, 9, 915179. [Google Scholar] [CrossRef]
- Mu, Q.; Zhang, H.; Liao, X.; Lin, K.; Liu, H.; Edwards, M.R.; Ahmed, S.A.; Yuan, R.; Li, L.; Cecere, T.E.; et al. Control of lupus nephritis by changes of gut microbiota. Microbiome 2017, 5, 73. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Pan, Y.; Xia, X.; Liang, J.; Liu, F.; Dou, H.; Hou, Y. Bacteroides fragilis alleviates the symptoms of lupus nephritis via regulating CD1d and CD86 expressions in B cells. Eur. J. Pharmacol. 2020, 884, 173421. [Google Scholar] [CrossRef] [PubMed]
- de la Visitación, N.; Robles-Vera, I.; Toral, M.; Duarte, J. Protective Effects of Probiotic Consumption in Cardiovascular Disease in Systemic Lupus Erythematosus. Nutrients 2019, 11, 2676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esmaeili, S.A.; Mahmoudi, M.; Momtazi, A.A.; Sahebkar, A.; Doulabi, H.; Rastin, M. Tolerogenic probiotics: Potential immunoregulators in Systemic Lupus Erythematosus. J. Cell Physiol. 2017, 232, 1994–2007. [Google Scholar] [CrossRef] [PubMed]
- Widhani, A.; Djauzi, S.; Suyatna, F.D.; Dewi, B.E. Changes in Gut Microbiota and Systemic Inflammation after Synbiotic Supplementation in Patients with Systemic Lupus Erythematosus: A Randomized, Double-Blind, Placebo-Controlled Trial. Cells 2022, 11, 3419. [Google Scholar] [CrossRef]
- Belvoncikova, P.; Maronek, M.; Gardlik, R. Gut Dysbiosis and Fecal Microbiota Transplantation in Autoimmune Diseases. Int. J. Mol. Sci. 2022, 23, 10729. [Google Scholar] [CrossRef]
- Lupu, V.V.; Jechel, E.; Mihai, C.M.; Mitrofan, E.C.; Lupu, A.; Starcea, I.M.; Fotea, S.; Mocanu, A.; Ghica, D.C.; Mitrofan, C.; et al. Connection between Celiac Disease and Systemic Lupus Erythematosus in Children-A Development Model of Autoimmune Diseases Starting from What We Inherit to What We Eat. Nutrients 2023, 15, 2535. [Google Scholar] [CrossRef]
- Mounsey, A.; Lacy Smith, K.; Reddy, V.C.; Nickolich, S. Clostridioides difficile Infection: Update on Management. Am. Fam. Physician. 2020, 101, 168–175. [Google Scholar]
- Huang, C.; Yi, P.; Zhu, M.; Zhou, W.; Zhang, B.; Yi, X.; Long, H.; Zhang, G.; Wu, H.; Tsokos, G.C.; et al. Safety and efficacy of fecal microbiota transplantation for treatment of systemic lupus erythematosus: An EXPLORER trial. J. Autoimmun. 2022, 130, 102844. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Guo, F.; Huang, Y.; Li, A.; Chen, S.; Chen, J.; Liu, H.F.; Pan, Q. Gut Microbiota Dysbiosis in Systemic Lupus Erythematosus: Novel Insights into Mechanisms and Promising Therapeutic Strategies. Front. Immunol. 2021, 12, 799788. [Google Scholar] [CrossRef] [PubMed]
| Systemic Lupus Erythematosus | Dysbiosis | |
|---|---|---|
| Sampling methods | ||
| Clinical exam |
|
|
| Clinical investigations |
| |
| Diagnosis | ACR (1997)
| |
| Remarks | ||
Differential diagnosis in childhood:
| ||
| Microbial Site | Affections Found in Dysbiosis |
|---|---|
| Skin |
|
| Respiratory system piratory system |
|
| Genitourinary system |
|
| Gastrointestinal system |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lupu, V.V.; Butnariu, L.I.; Fotea, S.; Morariu, I.D.; Badescu, M.C.; Starcea, I.M.; Salaru, D.L.; Popp, A.; Dragan, F.; Lupu, A.; et al. The Disease with a Thousand Faces and the Human Microbiome—A Physiopathogenic Intercorrelation in Pediatric Practice. Nutrients 2023, 15, 3359. https://doi.org/10.3390/nu15153359
Lupu VV, Butnariu LI, Fotea S, Morariu ID, Badescu MC, Starcea IM, Salaru DL, Popp A, Dragan F, Lupu A, et al. The Disease with a Thousand Faces and the Human Microbiome—A Physiopathogenic Intercorrelation in Pediatric Practice. Nutrients. 2023; 15(15):3359. https://doi.org/10.3390/nu15153359
Chicago/Turabian StyleLupu, Vasile Valeriu, Lacramioara Ionela Butnariu, Silvia Fotea, Ionela Daniela Morariu, Minerva Codruta Badescu, Iuliana Magdalena Starcea, Delia Lidia Salaru, Alina Popp, Felicia Dragan, Ancuta Lupu, and et al. 2023. "The Disease with a Thousand Faces and the Human Microbiome—A Physiopathogenic Intercorrelation in Pediatric Practice" Nutrients 15, no. 15: 3359. https://doi.org/10.3390/nu15153359
APA StyleLupu, V. V., Butnariu, L. I., Fotea, S., Morariu, I. D., Badescu, M. C., Starcea, I. M., Salaru, D. L., Popp, A., Dragan, F., Lupu, A., Mocanu, A., Chisnoiu, T., Pantazi, A. C., & Jechel, E. (2023). The Disease with a Thousand Faces and the Human Microbiome—A Physiopathogenic Intercorrelation in Pediatric Practice. Nutrients, 15(15), 3359. https://doi.org/10.3390/nu15153359







