Epigallocatechin-3-Gallate, an Active Green Tea Component to Support Anti-VEGFA Therapy in Wet Age-Related Macular Degeneration
Abstract
1. Introduction
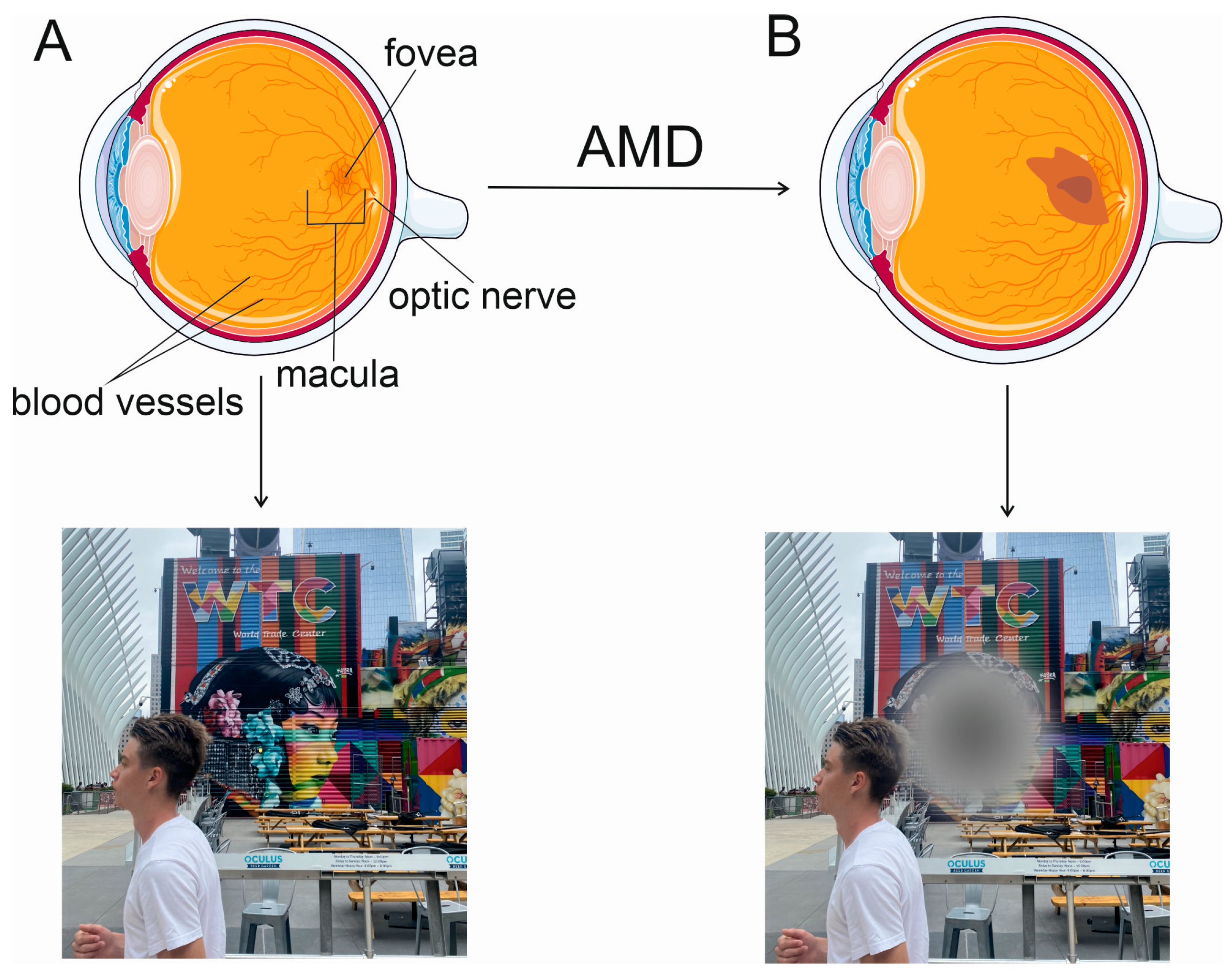
2. Wet AMD and Anti-VEGFA Therapy
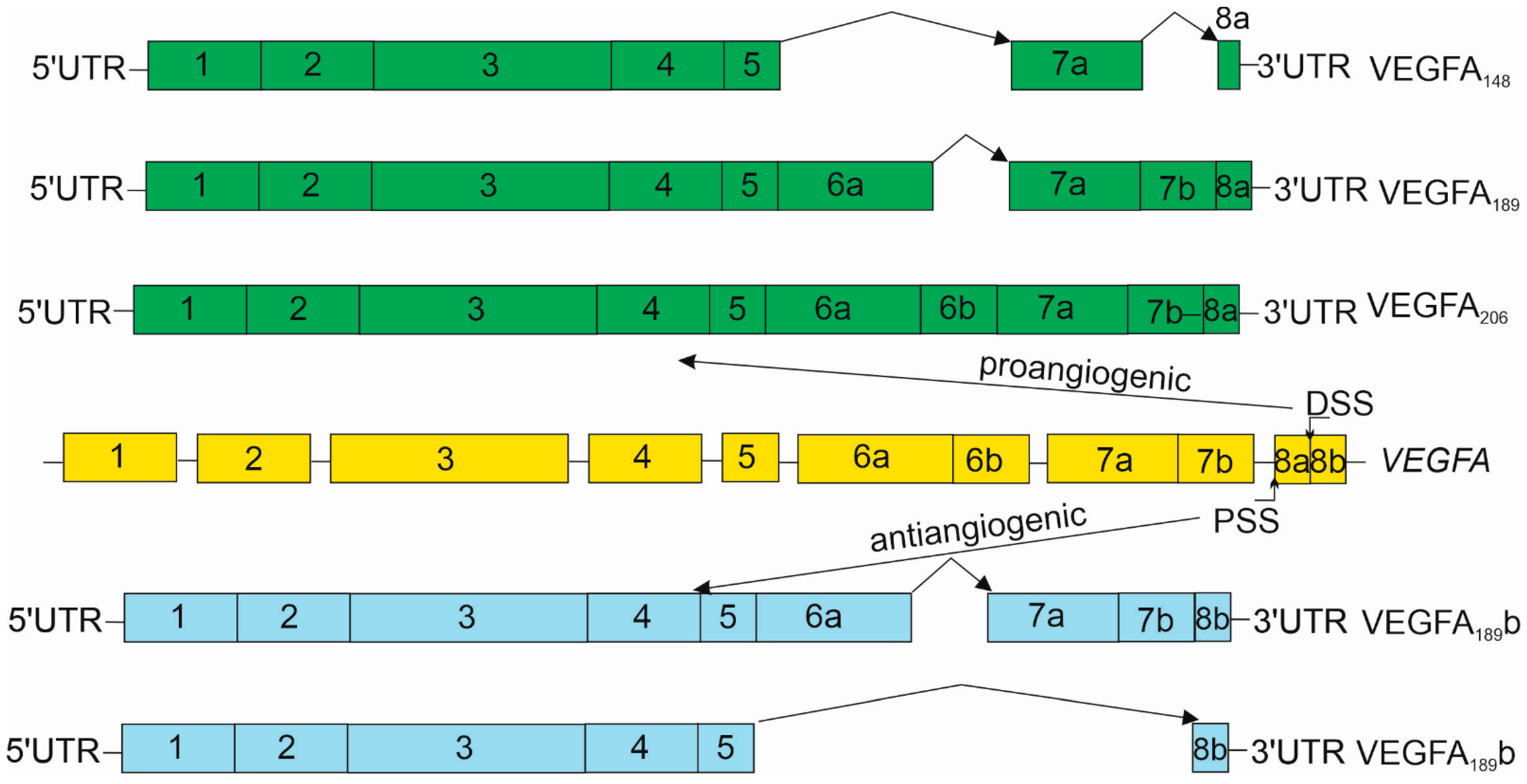
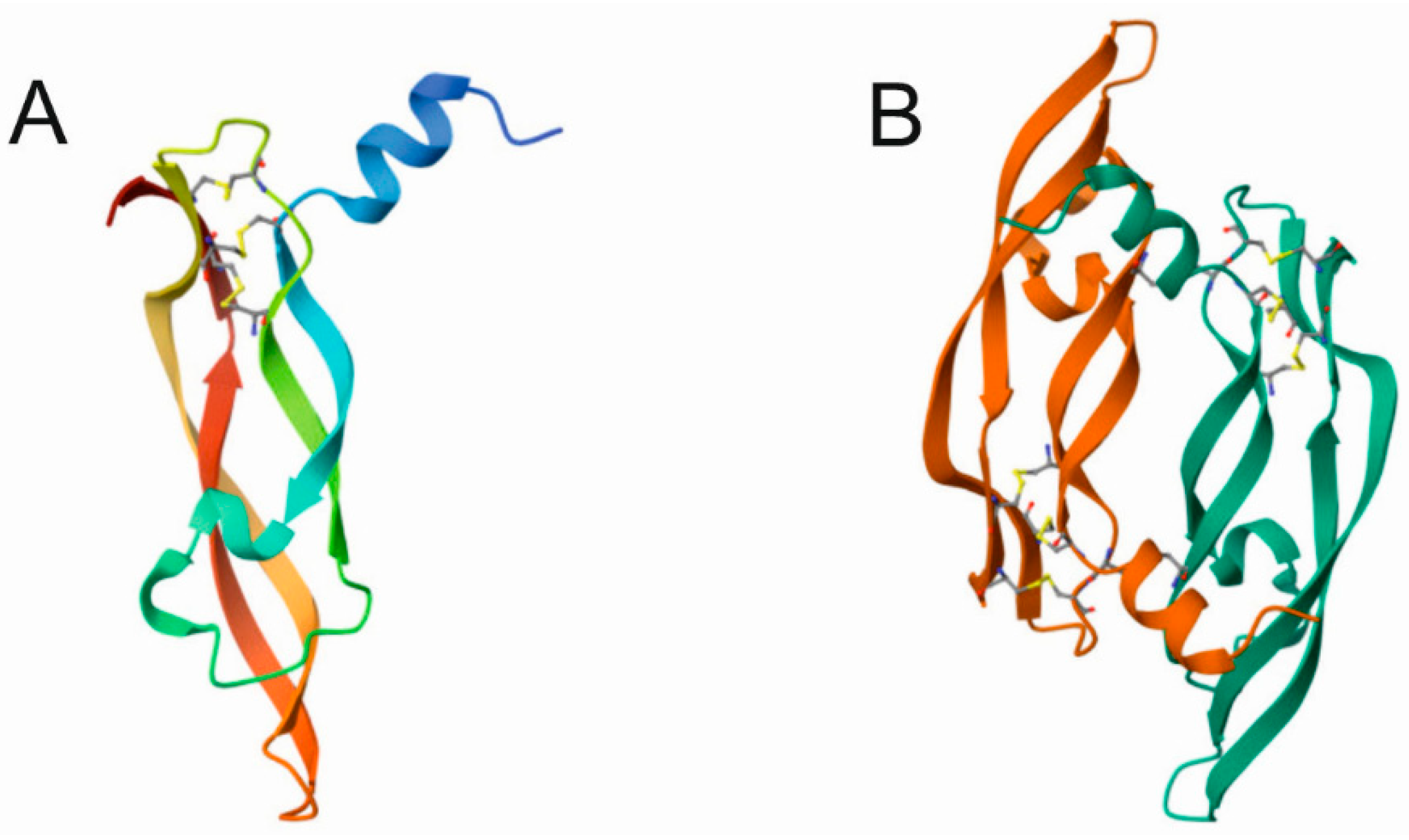
3. Vascular Endothelial Growth Factor A
4. Potential of (-)-Epigallocatechin-3-Gallate to Support Anti-VEGFA Therapy in Wet AMD
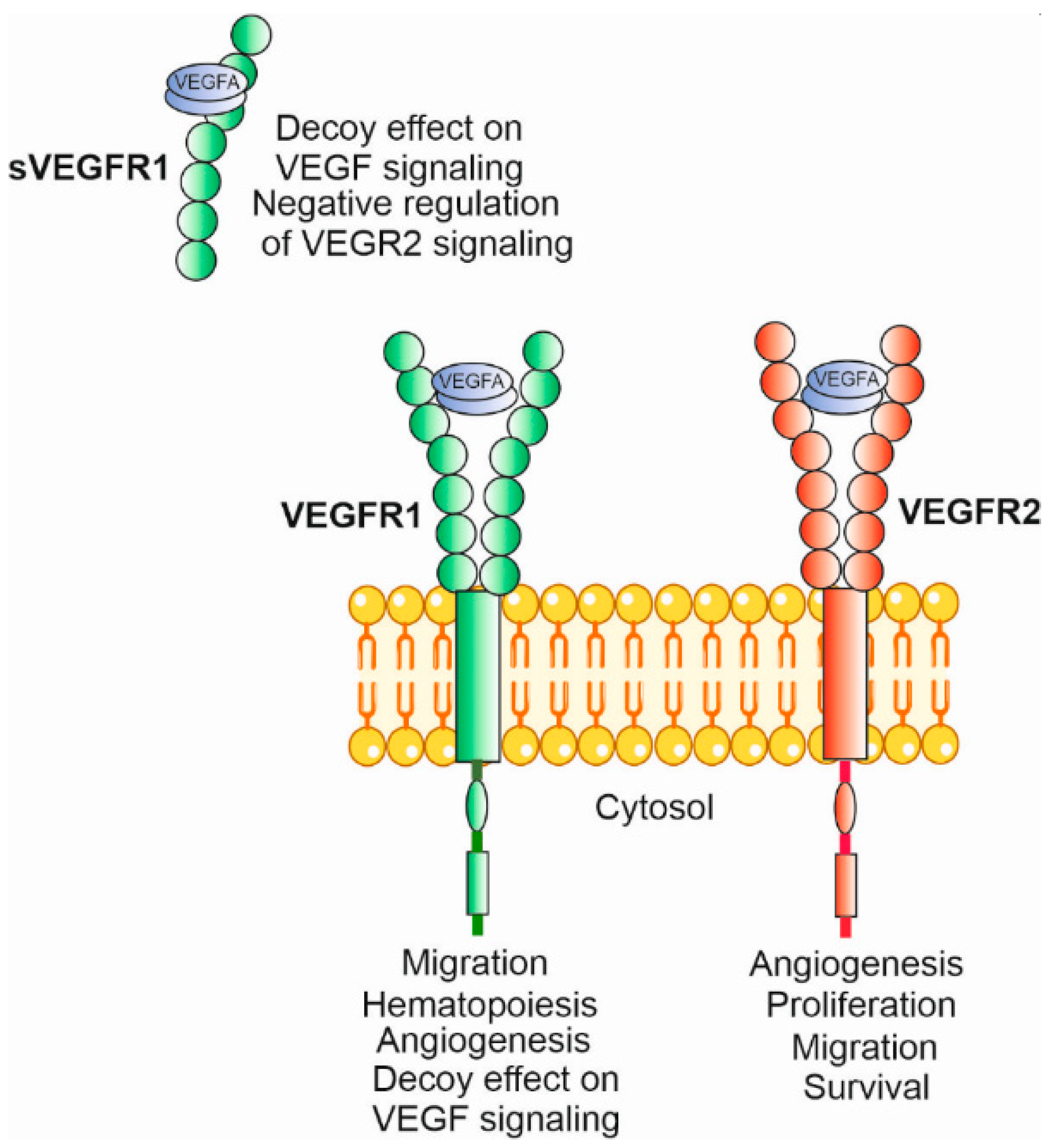
4.1. Angiogenesis, VEGFA and Its Receptors
4.2. The Retinal Pigment Epithelium and the Neuroretina
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gehrs, K.M.; Anderson, D.H.; Johnson, L.V.; Hageman, G.S. Age-related macular degeneration--emerging pathogenetic and therapeutic concepts. Ann. Med. 2006, 38, 450–471. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Donati, S.; Lindsley, K.B.; Krzystolik, M.G.; Virgili, G. Treatment regimens for administration of anti-vascular endothelial growth factor agents for neovascular age-related macular degeneration. Cochrane Database Syst. Rev. 2020, 5, Cd012208. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.T.; Eliott, D.; Sobrin, L. Inflammatory Complications of Intravitreal Anti-VEGF Injections. J. Clin. Med. 2021, 10, 981. [Google Scholar] [CrossRef]
- Mulpuri, L.; Sridhar, J.; Goyal, H.; Tonk, R. The relationship between dietary patterns and ophthalmic disease. Curr. Opin. Ophthalmol. 2023, 34, 189–194. [Google Scholar] [CrossRef]
- Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch. Ophthalmol. 2001, 119, 1417–1436. [Google Scholar] [CrossRef]
- Broadhead, G.K.; Grigg, J.R.; Chang, A.A.; McCluskey, P. Dietary modification and supplementation for the treatment of age-related macular degeneration. Nutr. Rev. 2015, 73, 448–462. [Google Scholar] [CrossRef]
- Chew, E.Y.; Clemons, T.E.; Sangiovanni, J.P.; Danis, R.P.; Ferris, F.L., 3rd; Elman, M.J.; Antoszyk, A.N.; Ruby, A.J.; Orth, D.; Bressler, S.B.; et al. Secondary analyses of the effects of lutein/zeaxanthin on age-related macular degeneration progression: AREDS2 report No. 3. JAMA Ophthalmol. 2014, 132, 142–149. [Google Scholar] [CrossRef]
- Kim, E.K.; Kim, H.; Kwon, O.; Chang, N. Associations between fruits, vegetables, vitamin A, β-carotene and flavonol dietary intake, and age-related macular degeneration in elderly women in Korea: The Fifth Korea National Health and Nutrition Examination Survey. Eur. J. Clin. Nutr. 2018, 72, 161–167. [Google Scholar] [CrossRef]
- Nunes, S.; Alves, D.; Barreto, P.; Raimundo, M.; da Luz Cachulo, M.; Farinha, C.; Laíns, I.; Rodrigues, J.; Almeida, C.; Ribeiro, L.; et al. Adherence to a Mediterranean diet and its association with age-related macular degeneration. The Coimbra Eye Study-Report 4. Nutrition 2018, 51–52, 6–12. [Google Scholar] [CrossRef]
- Wang, J.J.; Rochtchina, E.; Smith, W.; Klein, R.; Klein, B.E.; Joshi, T.; Sivakumaran, T.A.; Iyengar, S.; Mitchell, P. Combined effects of complement factor H genotypes, fish consumption, and inflammatory markers on long-term risk for age-related macular degeneration in a cohort. Am. J. Epidemiol. 2009, 169, 633–641. [Google Scholar] [CrossRef]
- Raiten, D.J. Nutrition and pharmacology: General principles and implications for HIV. Am. J. Clin. Nutr. 2011, 94, 1697s–1702s. [Google Scholar] [CrossRef]
- Hinojosa-Nogueira, D.; Pérez-Burillo, S.; Pastoriza de la Cueva, S.; Rufián-Henares, J. Green and white teas as health-promoting foods. Food Funct. 2021, 12, 3799–3819. [Google Scholar] [CrossRef]
- Heloterä, H.; Kaarniranta, K. A Linkage between Angiogenesis and Inflammation in Neovascular Age-Related Macular Degeneration. Cells 2022, 11, 3453. [Google Scholar] [CrossRef]
- Bakaliou, A.; Georgakopoulos, C.; Tsilimbaris, M.; Farmakakis, N. Posterior Vitreous Detachment and Its Role in the Evolution of Dry to Wet Age Related Macular Degeneration. Clin. Ophthalmol. 2023, 17, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Holz, F.G.; Strauss, E.C.; Schmitz-Valckenberg, S.; van Lookeren Campagne, M. Geographic atrophy: Clinical features and potential therapeutic approaches. Ophthalmology 2014, 121, 1079–1091. [Google Scholar] [CrossRef]
- Li, M.; Huisingh, C.; Messinger, J.; Dolz-Marco, R.; Ferrara, D.; Freund, K.B.; Curcio, C.A. Histology of geographic atrophy secondary to age-related macular degeneration: A Multilayer Approach. Retina 2018, 38, 1937–1953. [Google Scholar] [CrossRef] [PubMed]
- Kauppinen, A.; Paterno, J.J.; Blasiak, J.; Salminen, A.; Kaarniranta, K. Inflammation and its role in age-related macular degeneration. Cell. Mol. Life Sci. CMLS 2016, 73, 1765–1786. [Google Scholar] [CrossRef]
- Shibuya, M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Feigl, B. Age-related maculopathy in the light of ischaemia. Clin. Exp. Optom. 2007, 90, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, C.M.; Pate, S.; Hiscott, P.; Wong, D.; Pattwell, D.M.; Kent, D. Expression of hypoxia-inducible factor-1alpha and -2alpha in human choroidal neovascular membranes. Graefes Arch. Clin. Exp. Ophthalmol. 2009, 247, 1361–1367. [Google Scholar] [CrossRef]
- Vadlapatla, R.K.; Vadlapudi, A.D.; Pal, D.; Mukherji, M.; Mitra, A.K. Ritonavir inhibits HIF-1α-mediated VEGF expression in retinal pigment epithelial cells in vitro. Eye 2014, 28, 93–101. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, H.; Jiang, Y.; Hartnett, M.E. VEGFA activates erythropoietin receptor and enhances VEGFR2-mediated pathological angiogenesis. Am. J. Pathol. 2014, 184, 1230–1239. [Google Scholar] [CrossRef]
- Schmidt-Erfurth, U.; Chong, V.; Loewenstein, A.; Larsen, M.; Souied, E.; Schlingemann, R.; Eldem, B.; Monés, J.; Richard, G.; Bandello, F. Guidelines for the management of neovascular age-related macular degeneration by the European Society of Retina Specialists (EURETINA). Br. J. Ophthalmol. 2014, 98, 1144–1167. [Google Scholar] [CrossRef] [PubMed]
- Granstam, E.; Aurell, S.; Sjövall, K.; Paul, A. Switching anti-VEGF agent for wet AMD: Evaluation of impact on visual acuity, treatment frequency and retinal morphology in a real-world clinical setting. Graefe’s Arch. Clin. Exp. Ophthalmol. 2021, 259, 2085–2093. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Huang, W.; Zhang, Y.; Huang, X.; Zhang, X.; Ma, H.; Ren, G.; Shi, F.; Kuang, L.; Yan, S.; et al. Factors for Visual Acuity Improvement After Anti-VEGF Treatment of Wet Age-Related Macular Degeneration in China: 12 Months Follow up. Front. Med. 2021, 8, 735318. [Google Scholar] [CrossRef]
- Stover, P.J.; James, W.P.T.; Krook, A.; Garza, C. Emerging concepts on the role of epigenetics in the relationships between nutrition and health. J. Intern. Med. 2018, 284, 37–49. [Google Scholar] [CrossRef]
- Chojnacki, C.; Gąsiorowska, A.; Popławski, T.; Błońska, A.; Konrad, P.; Zajdler, R.; Chojnacki, J.; Blasiak, J. Reduced Intake of Dietary Tryptophan Improves Beneficial Action of Budesonide in Patients with Lymphocytic Colitis and Mood Disorders. Nutrients 2023, 15, 1674. [Google Scholar] [CrossRef]
- Haridas, B. Dietary carbohydrates in the management of epilepsy. Curr. Opin. Clin. Nutr. Metab. Care 2023, 26, 377–381. [Google Scholar] [CrossRef]
- Kohli, A.; Moss, A.C. Personalizing therapy selection in inflammatory bowel disease. Expert Rev. Clin. Immunol. 2023, 19, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Morehead, L.C.; Garg, S.; Wallis, K.F.; Siegel, E.R.; Tackett, A.J.; Miousse, I.R. Increased response to immune checkpoint inhibitors with dietary methionine restriction. bioRxiv 2023. [Google Scholar] [CrossRef]
- Thederan, I.; Pott, A.; Krueger, A.; Chandrasekar, T.; Tennstedt, P.; Knipper, S.; Tilki, D.; Heinzer, H.; Schulz, K.H.; Makarova, N.; et al. Feasibility, acceptability, and behavioral outcomes of a multimodal intervention for prostate cancer patients: Experience from the MARTINI lifestyle program. Prostate 2023, 83, 929–935. [Google Scholar] [CrossRef]
- Chakravarthy, U.; Adamis, A.P.; Cunningham, E.T., Jr.; Goldbaum, M.; Guyer, D.R.; Katz, B.; Patel, M. Year 2 efficacy results of 2 randomized controlled clinical trials of pegaptanib for neovascular age-related macular degeneration. Ophthalmology 2006, 113, 1508.e1–1508.e25. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.M.; Michels, M.; Kaiser, P.K.; Heier, J.S.; Sy, J.P.; Ianchulev, T. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: Two-year results of the ANCHOR study. Ophthalmology 2009, 116, 57–65.e55. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, P.J.; Brown, D.M.; Heier, J.S.; Boyer, D.S.; Kaiser, P.K.; Chung, C.Y.; Kim, R.Y. Ranibizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1419–1431. [Google Scholar] [CrossRef] [PubMed]
- Schauwvlieghe, A.M.; Dijkman, G.; Hooymans, J.M.; Verbraak, F.D.; Hoyng, C.B.; Dijkgraaf, M.G.; Peto, T.; Vingerling, J.R.; Schlingemann, R.O. Comparing the Effectiveness of Bevacizumab to Ranibizumab in Patients with Exudative Age-Related Macular Degeneration. The BRAMD Study. PLoS ONE 2016, 11, e0153052. [Google Scholar] [CrossRef] [PubMed]
- Ohr, M.; Kaiser, P.K. Aflibercept in wet age-related macular degeneration: A perspective review. Ther. Adv. Chronic Dis. 2012, 3, 153–161. [Google Scholar] [CrossRef]
- Abedi, F.; Wickremasinghe, S.; Islam, A.F.; Inglis, K.M.; Guymer, R.H. Anti-VEGF treatment in neovascular age-related macular degeneration: A treat-and-extend protocol over 2 years. Retina 2014, 34, 1531–1538. [Google Scholar] [CrossRef]
- Rust, R.; Gantner, C.; Schwab, M.E. Pro- and antiangiogenic therapies: Current status and clinical implications. FASEB J. 2019, 33, 34–48. [Google Scholar] [CrossRef]
- Falavarjani, K.G.; Nguyen, Q.D. Adverse events and complications associated with intravitreal injection of anti-VEGF agents: A review of literature. Eye 2013, 27, 787–794. [Google Scholar] [CrossRef]
- Morioka, M.; Takamura, Y.; Nagai, K.; Yoshida, S.; Mori, J.; Takeuchi, M.; Sawada, T.; Sone, K.; Fukuyama, H.; Kusuhara, S.; et al. Incidence of endophthalmitis after intravitreal injection of an anti-VEGF agent with or without topical antibiotics. Sci. Rep. 2020, 10, 22122. [Google Scholar] [CrossRef]
- Hanna, R.M.; Tran, N.T.; Patel, S.S.; Hou, J.; Jhaveri, K.D.; Parikh, R.; Selamet, U.; Ghobry, L.; Wassef, O.; Barsoum, M.; et al. Thrombotic Microangiopathy and Acute Kidney Injury Induced After Intravitreal Injection of Vascular Endothelial Growth Factor Inhibitors VEGF Blockade-Related TMA after Intravitreal Use. Front. Med. 2020, 7, 579603. [Google Scholar] [CrossRef] [PubMed]
- Hayman, S.R.; Leung, N.; Grande, J.P.; Garovic, V.D. VEGF inhibition, hypertension, and renal toxicity. Curr. Oncol. Rep. 2012, 14, 285–294. [Google Scholar] [CrossRef] [PubMed]
- LaFargue, C.J.; Amero, P.; Noh, K.; Mangala, L.S.; Wen, Y.; Bayraktar, E.; Umamaheswaran, S.; Stur, E.; Dasari, S.K.; Ivan, C.; et al. Overcoming adaptive resistance to anti-VEGF therapy by targeting CD5L. Nat. Commun. 2023, 14, 2407. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhao, J.; Sun, X. Resistance to anti-VEGF therapy in neovascular age-related macular degeneration: A comprehensive review. Drug Des. Devel. Ther. 2016, 10, 1857–1867. [Google Scholar] [CrossRef]
- Binder, S. Loss of reactivity in intravitreal anti-VEGF therapy: Tachyphylaxis or tolerance? Br. J. Ophthalmol. 2012, 96, 1–2. [Google Scholar] [CrossRef][Green Version]
- Senger, D.R.; Galli, S.J.; Dvorak, A.M.; Perruzzi, C.A.; Harvey, V.S.; Dvorak, H.F. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 1983, 219, 983–985. [Google Scholar] [CrossRef]
- Ribatti, D. The crucial role of vascular permeability factor/vascular endothelial growth factor in angiogenesis: A historical review. Br. J. Haematol. 2005, 128, 303–309. [Google Scholar] [CrossRef]
- Ghalehbandi, S.; Yuzugulen, J.; Pranjol, M.Z.I.; Pourgholami, M.H. The role of VEGF in cancer-induced angiogenesis and research progress of drugs targeting VEGF. Eur. J. Pharmacol. 2023, 949, 175586. [Google Scholar] [CrossRef]
- Watabe, T.; Takahashi, K.; Pietras, K.; Yoshimatsu, Y. Roles of TGF-β signals in tumor microenvironment via regulation of the formation and plasticity of vascular system. Semin. Cancer Biol. 2023, 92, 130–138. [Google Scholar] [CrossRef]
- Eswarappa, S.M.; Potdar, A.A.; Koch, W.J.; Fan, Y.; Vasu, K.; Lindner, D.; Willard, B.; Graham, L.M.; DiCorleto, P.E.; Fox, P.L. Programmed translational readthrough generates antiangiogenic VEGF-Ax. Cell 2014, 157, 1605–1618. [Google Scholar] [CrossRef]
- Bastide, A.; Karaa, Z.; Bornes, S.; Hieblot, C.; Lacazette, E.; Prats, H.; Touriol, C. An upstream open reading frame within an IRES controls expression of a specific VEGF-A isoform. Nucleic Acids Res. 2008, 36, 2434–2445. [Google Scholar] [CrossRef] [PubMed]
- Arcondéguy, T.; Lacazette, E.; Millevoi, S.; Prats, H.; Touriol, C. VEGF-A mRNA processing, stability and translation: A paradigm for intricate regulation of gene expression at the post-transcriptional level. Nucleic Acids Res. 2013, 41, 7997–8010. [Google Scholar] [CrossRef] [PubMed]
- Prats, H.; Touriol, C. Post-transcriptional Regulation of VEGF-A. In Post-Transcriptional Mechanisms in Endocrine Regulation; Menon, P.K.M.J., Goldstrohm, P.A., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 157–180. [Google Scholar]
- Venkata Subbaiah, K.C.; Hedaya, O.; Wu, J.; Jiang, F.; Yao, P. Mammalian RNA switches: Molecular rheostats in gene regulation, disease, and medicine. Comput. Struct. Biotechnol. J. 2019, 17, 1326–1338. [Google Scholar] [CrossRef] [PubMed]
- Dimitrakopoulos, F.I.; Koliou, G.A.; Kotoula, V.; Papadopoulou, K.; Markou, K.; Vlachtsis, K.; Angouridakis, N.; Karasmanis, I.; Nikolaou, A.; Psyrri, A.; et al. Genetic Variation in the Vascular Endothelial Growth Factor (VEGFA) Gene at rs13207351 Is Associated with Overall Survival of Patients with Head and Neck Cancer. Cancers 2021, 13, 1163. [Google Scholar] [CrossRef]
- Liu, D.; Song, J.; Ji, X.; Liu, Z.; Cong, M.; Hu, B. Association of Genetic Polymorphisms on VEGFA and VEGFR2 with Risk of Coronary Heart Disease. Medicine 2016, 95, e3413. [Google Scholar] [CrossRef]
- Psoma, E.; Koliou, G.A.; Dimitrakopoulos, F.I.; Papadopoulou, K.; Rontogianni, D.; Bobos, M.; Visvikis, A.; Kosmidis, P.A.; Fountzilas, G.; Constantinidis, J.; et al. Genetic Variations of VEGFA Gene Are Associated With Infiltration of Adjacent Tissues and the Clinical Outcome of Patients With Nasopharyngeal Carcinoma. Anticancer. Res. 2020, 40, 677–688. [Google Scholar] [CrossRef]
- Muller, Y.A.; Christinger, H.W.; Keyt, B.A.; de Vos, A.M. The crystal structure of vascular endothelial growth factor (VEGF) refined to 1.93 Å resolution: Multiple copy flexibility and receptor binding. Structure 1997, 5, 1325–1338. [Google Scholar] [CrossRef]
- Muller, Y.A.; Li, B.; Christinger, H.W.; Wells, J.A.; Cunningham, B.C.; de Vos, A.M. Vascular endothelial growth factor: Crystal structure and functional mapping of the kinase domain receptor binding site. Proc. Natl. Acad. Sci. USA 1997, 94, 7192–7197. [Google Scholar] [CrossRef]
- Hiratsuka, S.; Kataoka, Y.; Nakao, K.; Nakamura, K.; Morikawa, S.; Tanaka, S.; Katsuki, M.; Maru, Y.; Shibuya, M. Vascular endothelial growth factor A (VEGF-A) is involved in guidance of VEGF receptor-positive cells to the anterior portion of early embryos. Mol. Cell Biol. 2005, 25, 355–363. [Google Scholar] [CrossRef]
- Shibuya, M. Role of VEGF-flt receptor system in normal and tumor angiogenesis. Adv. Cancer Res. 1995, 67, 281–316. [Google Scholar] [CrossRef]
- Sarabipour, S.; Ballmer-Hofer, K.; Hristova, K. VEGFR-2 conformational switch in response to ligand binding. Elife 2016, 5, e13876. [Google Scholar] [CrossRef]
- Sawano, A.; Takahashi, T.; Yamaguchi, S.; Aonuma, M.; Shibuya, M. Flt-1 but not KDR/Flk-1 tyrosine kinase is a receptor for placenta growth factor, which is related to vascular endothelial growth factor. Cell Growth Differ. 1996, 7, 213–221. [Google Scholar]
- Misra, S.; Ikbal, A.M.A.; Bhattacharjee, D.; Hore, M.; Mishra, S.; Karmakar, S.; Ghosh, A.; Srinivas, R.; Das, A.; Agarwal, S.; et al. Validation of antioxidant, antiproliferative, and in vitro anti-rheumatoid arthritis activities of epigallo-catechin-rich bioactive fraction from Camellia sinensis var. assamica, Assam variety white tea, and its comparative evaluation with green tea fraction. J. Food Biochem. 2022, 46, e14487. [Google Scholar] [CrossRef]
- Neyestani, T.R.; Nikooyeh, B. A comprehensive overview on the effects of green tea on anthropometric measures, blood pressure, glycemic and lipidemic status: An umbrella review and meta meta-analysis study. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 2026–2040. [Google Scholar] [CrossRef]
- Kim, J.; Choi, H.; Choi, D.-H.; Park, K.; Kim, H.-J.; Park, M. Application of green tea catechins, polysaccharides, and flavonol prevent fine dust induced bronchial damage by modulating inflammation and airway cilia. Sci. Rep. 2021, 11, 2232. [Google Scholar] [CrossRef]
- Abe, S.K.; Inoue, M. Green tea and cancer and cardiometabolic diseases: A review of the current epidemiological evidence. Eur. J. Clin. Nutr. 2021, 75, 865–876. [Google Scholar] [CrossRef]
- Abunofal, O.; Mohan, C. Salubrious Effects of Green Tea Catechins on Fatty Liver Disease: A Systematic Review. Medicines 2022, 9, 20. [Google Scholar] [CrossRef]
- Asbaghi, O.; Fouladvand, F.; Gonzalez, M.J.; Ashtary-Larky, D.; Choghakhori, R.; Abbasnezhad, A. Effect of green tea on glycemic control in patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Diabetes Metab. Syndr. 2021, 15, 23–31. [Google Scholar] [CrossRef]
- Ma, Y.H.; Wu, J.H.; Xu, W.; Shen, X.N.; Wang, H.F.; Hou, X.H.; Cao, X.P.; Bi, Y.L.; Dong, Q.; Feng, L.; et al. Associations of Green Tea Consumption and Cerebrospinal Fluid Biomarkers of Alzheimer’s Disease Pathology in Cognitively Intact Older Adults: The CABLE Study. J. Alzheimer’s Dis. JAD 2020, 77, 411–421. [Google Scholar] [CrossRef]
- Joo, K.; Mun, Y.S.; Park, S.J.; Park, K.H.; Woo, S.J. Ten-Year Progression From Intermediate to Exudative Age-Related Macular Degeneration and Risk Factors: Bundang AMD Cohort Study Report 1. Am. J. Ophthalmol. 2021, 224, 228–237. [Google Scholar] [CrossRef]
- Sartippour, M.R.; Shao, Z.-M.; Heber, D.; Beatty, P.; Zhang, L.; Liu, C.; Ellis, L.; Liu, W.; Go, V.L.; Brooks, M.N. Green Tea Inhibits Vascular Endothelial Growth Factor (VEGF) Induction in Human Breast Cancer Cells. J. Nutr. 2002, 132, 2307–2311. [Google Scholar] [CrossRef]
- Jung, Y.D.; Kim, M.S.; Shin, B.A.; Chay, K.O.; Ahn, B.W.; Liu, W.; Bucana, C.D.; Gallick, G.E.; Ellis, L.M. EGCG, a major component of green tea, inhibits tumour growth by inhibiting VEGF induction in human colon carcinoma cells. Br. J. Cancer 2001, 84, 844–850. [Google Scholar] [CrossRef]
- Cargnello, M.; Roux, P.P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83. [Google Scholar] [CrossRef]
- Sun, G.; Budde, R.J. Requirement for an additional divalent metal cation to activate protein tyrosine kinases. Biochemistry 1997, 36, 2139–2146. [Google Scholar] [CrossRef]
- Zwolak, I. Epigallocatechin Gallate for Management of Heavy Metal-Induced Oxidative Stress: Mechanisms of Action, Efficacy, and Concerns. Int. J. Mol. Sci. 2021, 22, 4027. [Google Scholar] [CrossRef]
- Neuhaus, T.; Pabst, S.; Stier, S.; Weber, A.-A.; Schrör, K.; Sachinidis, A.; Vetter, H.; Ko, Y.D. Inhibition of the vascular-endothelial growth factor-induced intracellular signaling and mitogenesis of human endothelial cells by epigallocatechin-3 gallate. Eur. J. Pharmacol. 2004, 483, 223–227. [Google Scholar] [CrossRef]
- Conacci-Sorrell, M.; Zhurinsky, J.; Ben-Ze’ev, A. The cadherin-catenin adhesion system in signaling and cancer. J. Clin. Investig. 2002, 109, 987–991. [Google Scholar] [CrossRef]
- Tang, F.Y.; Nguyen, N.; Meydani, M. Green tea catechins inhibit VEGF-induced angiogenesis in vitro through suppression of VE-cadherin phosphorylation and inactivation of Akt molecule. Int. J. Cancer 2003, 106, 871–878. [Google Scholar] [CrossRef]
- Li, F.; Wang, Y.; Li, D.; Chen, Y.; Qiao, X.; Fardous, R.; Lewandowski, A.; Liu, J.; Chan, T.H.; Dou, Q.P. Perspectives on the recent developments with green tea polyphenols in drug discovery. Expert Opin. Drug Discov. 2018, 13, 643–660. [Google Scholar] [CrossRef]
- Yang, H.; Sun, D.K.; Chen, D.; Cui, Q.C.; Gu, Y.Y.; Jiang, T.; Chen, W.; Wan, S.B.; Dou, Q.P. Antitumor activity of novel fluoro-substituted (–)-epigallocatechin-3-gallate analogs. Cancer Lett. 2010, 292, 48–53. [Google Scholar] [CrossRef]
- Xu, J.; Tu, Y.; Wang, Y.; Xu, X.; Sun, X.; Xie, L.; Zhao, Q.; Guo, Y.; Gu, Y.; Du, J.; et al. Prodrug of epigallocatechin-3-gallate alleviates choroidal neovascularization via down-regulating HIF-1α/VEGF/VEGFR2 pathway and M1 type macrophage/microglia polarization. Biomed. Pharmacother. 2020, 121, 109606. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Safa, R.; Rusciano, D.; Osborne, N.N. Epigallocatechin gallate, an active ingredient from green tea, attenuates damaging influences to the retina caused by ischemia/reperfusion. Brain Res. 2007, 1159, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Osborne, N.N. Oxidative-induced retinal degeneration is attenuated by epigallocatechin gallate. Brain Res. 2006, 1124, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.V.; Mappes, T.; Schaupp, P.; Lappe, C.; Wahl, S. Ultraviolet radiation oxidative stress affects eye health. J. Biophotonics 2018, 11, e201700377. [Google Scholar] [CrossRef]
- Delcourt, C.; Cougnard-Grégoire, A.; Boniol, M.; Carrière, I.; Doré, J.-F.; Delyfer, M.-N.; Rougier, M.-B.; Le Goff, M.; Dartigues, J.-F.; Barberger-Gateau, P.; et al. Lifetime Exposure to Ambient Ultraviolet Radiation and the Risk for Cataract Extraction and Age-Related Macular Degeneration: The Alienor Study. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7619–7627. [Google Scholar] [CrossRef]
- de Jager, T.L.; Cockrell, A.E.; Du Plessis, S.S. Ultraviolet Light Induced Generation of Reactive Oxygen Species. Adv. Exp. Med. Biol. 2017, 996, 15–23. [Google Scholar] [CrossRef]
- Chan, C.M.; Huang, J.H.; Lin, H.H.; Chiang, H.S.; Chen, B.H.; Hong, J.Y.; Hung, C.F. Protective effects of (-)-epigallocatechin gallate on UVA-induced damage in ARPE19 cells. Mol. Vis. 2008, 14, 2528–2534. [Google Scholar]
- Kokkinopoulos, I.; Shahabi, G.; Colman, A.; Jeffery, G. Mature peripheral RPE cells have an intrinsic capacity to proliferate; a potential regulatory mechanism for age-related cell loss. PLoS ONE 2011, 6, e18921. [Google Scholar] [CrossRef]
- Miller, H.; Miller, B.; Ryan, S.J. The role of retinal pigment epithelium in the involution of subretinal neovascularization. Investig. Ophthalmol. Vis. Sci. 1986, 27, 1644–1652. [Google Scholar]
- Ball, S.G.; Shuttleworth, C.A.; Kielty, C.M. Mesenchymal stem cells and neovascularization: Role of platelet-derived growth factor receptors. J. Cell Mol. Med. 2007, 11, 1012–1030. [Google Scholar] [CrossRef]
- Chan, C.M.; Huang, J.H.; Chiang, H.S.; Wu, W.B.; Lin, H.H.; Hong, J.Y.; Hung, C.F. Effects of (-)-epigallocatechin gallate on RPE cell migration and adhesion. Mol. Vis. 2010, 16, 586–595. [Google Scholar]
- Campochiaro, P.A. Pathogenic mechanisms in proliferative vitreoretinopathy. Arch. Ophthalmol. 1997, 115, 237–241. [Google Scholar] [CrossRef]
- Yu, J.; Peng, R.; Chen, H.; Cui, C.; Ba, J. Elucidation of the pathogenic mechanism of rhegmatogenous retinal detachment with proliferative vitreoretinopathy by proteomic analysis. Investig. Ophthalmol. Vis. Sci. 2012, 53, 8146–8153. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Blasiak, J.; Liton, P.; Boulton, M.; Klionsky, D.J.; Sinha, D. Autophagy in age-related macular degeneration. Autophagy 2022, 19, 388–400. [Google Scholar] [CrossRef]
- Blasiak, J.; Kaarniranta, K. Secretory autophagy: A turn key for understanding AMD pathology and developing new therapeutic targets? Expert Opin. Ther. Targets 2022, 26, 883–895. [Google Scholar] [CrossRef]
- Li, C.P.; Yao, J.; Tao, Z.F.; Li, X.M.; Jiang, Q.; Yan, B. Epigallocatechin-gallate (EGCG) regulates autophagy in human retinal pigment epithelial cells: A potential role for reducing UVB light-induced retinal damage. Biochem. Biophys. Res. Commun. 2013, 438, 739–745. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Abdel-Aziz, A.K.; Abdelfatah, S.; Abdellatif, M.; Abdoli, A.; Abel, S.; Abeliovich, H.; Abildgaard, M.H.; Abudu, Y.P.; Acevedo-Arozena, A.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition). Autophagy 2021, 17, 1–382. [Google Scholar] [CrossRef]
- Dunlop, E.A.; Tee, A.R. mTOR and autophagy: A dynamic relationship governed by nutrients and energy. Semin. Cell Dev. Biol. 2014, 36, 121–129. [Google Scholar] [CrossRef]
- Wang, X.; Proud, C.G. The mTOR Pathway in the Control of Protein Synthesis. Physiology 2006, 21, 362–369. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, C.; Jiang, D.; An, G.; Jin, F.; Zhang, J.; Han, G.; Cui, C.; Jiang, P. New insights into the interplay between autophagy and oxidative and endoplasmic reticulum stress in neuronal cell death and survival. Front. Cell Dev. Biol. 2022, 10, 994037. [Google Scholar] [CrossRef]
- Holczer, M.; Besze, B.; Zámbó, V.; Csala, M.; Bánhegyi, G.; Kapuy, O. Epigallocatechin-3-Gallate (EGCG) Promotes Autophagy-Dependent Survival via Influencing the Balance of mTOR-AMPK Pathways upon Endoplasmic Reticulum Stress. Oxidative Med. Cell. Longev. 2018, 2018, 6721530. [Google Scholar] [CrossRef]
- Lee, J.H.; Moon, J.H.; Kim, S.W.; Jeong, J.K.; Nazim, U.M.; Lee, Y.J.; Seol, J.W.; Park, S.Y. EGCG-mediated autophagy flux has a neuroprotection effect via a class III histone deacetylase in primary neuron cells. Oncotarget 2015, 6, 9701–9717. [Google Scholar] [CrossRef]
- Wang, L.; Sun, X.; Zhu, M.; Du, J.; Xu, J.; Qin, X.; Xu, X.; Song, E. Epigallocatechin-3-gallate stimulates autophagy and reduces apoptosis levels in retinal Müller cells under high-glucose conditions. Exp. Cell Res. 2019, 380, 149–158. [Google Scholar] [CrossRef]
- Heng, L.Z.; Comyn, O.; Peto, T.; Tadros, C.; Ng, E.; Sivaprasad, S.; Hykin, P.G. Diabetic retinopathy: Pathogenesis, clinical grading, management and future developments. Diabet. Med. 2013, 30, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Lu, W.; Zeng, Q.; Li, D.; Ding, L.; Wu, J. High glucose-induced excessive reactive oxygen species promote apoptosis through mitochondrial damage in rat cartilage endplate cells. J. Orthop. Res. 2018, 36, 2476–2483. [Google Scholar] [CrossRef] [PubMed]
- Kaarniranta, K.; Uusitalo, H.; Blasiak, J.; Felszeghy, S.; Kannan, R.; Kauppinen, A.; Salminen, A.; Sinha, D.; Ferrington, D. Mechanisms of mitochondrial dysfunction and their impact on age-related macular degeneration. Prog. Retin. Eye Res. 2020, 79, 100858. [Google Scholar] [CrossRef]
- Sahel, J.A.; Marazova, K.; Audo, I. Clinical characteristics and current therapies for inherited retinal degenerations. Cold Spring Harb. Perspect. Med. 2014, 5, a017111. [Google Scholar] [CrossRef]
- Perdices, L.; Fuentes-Broto, L.; Segura, F.; Cavero, A.; Orduna-Hospital, E.; Insa-Sánchez, G.; Sánchez-Cano, A.I.; Fernández-Sánchez, L.; Cuenca, N.; Pinilla, I. Systemic epigallocatechin gallate protects against retinal degeneration and hepatic oxidative stress in the P23H-1 rat. Neural Regen. Res. 2022, 17, 625–631. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Y.; Huang, G. Preparation and antioxidant activities of important traditional plant polysaccharides. Int. J. Biol. Macromol. 2018, 111, 780–786. [Google Scholar] [CrossRef]
- Yang, K.; Gao, Z.Y.; Li, T.Q.; Song, W.; Xiao, W.; Zheng, J.; Chen, H.; Chen, G.H.; Zou, H.Y. Anti-tumor activity and the mechanism of a green tea (Camellia sinensis) polysaccharide on prostate cancer. Int. J. Biol. Macromol. 2019, 122, 95–103. [Google Scholar] [CrossRef]
- Yan, Y.; Ren, Y.; Li, X.; Zhang, X.; Guo, H.; Han, Y.; Hu, J. A polysaccharide from green tea (Camellia sinensis L.) protects human retinal endothelial cells against hydrogen peroxide-induced oxidative injury and apoptosis. Int. J. Biol. Macromol. 2018, 115, 600–607. [Google Scholar] [CrossRef]
- Chu, K.O.; Chan, K.P.; Yang, Y.P.; Qin, Y.J.; Li, W.Y.; Chan, S.O.; Wang, C.C.; Pang, C.P. Effects of EGCG content in green tea extract on pharmacokinetics, oxidative status and expression of inflammatory and apoptotic genes in the rat ocular tissues. J. Nutr. Biochem. 2015, 26, 1357–1367. [Google Scholar] [CrossRef] [PubMed]
- Varano, M.; Eter, N.; Winyard, S.; Wittrup-Jensen, K.U.; Navarro, R.; Heraghty, J. The emotional and physical impact of wet age-related macular degeneration: Findings from the wAMD Patient and Caregiver Survey. Clin. Ophthalmol. 2016, 10, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Blasiak, J.; Watala, C.; Tuuminen, R.; Kivinen, N.; Koskela, A.; Uusitalo-Järvinen, H.; Tuulonen, A.; Winiarczyk, M.; Mackiewicz, J.; Zmorzyński, S.; et al. Expression of VEGFA-regulating miRNAs and mortality in wet AMD. J. Cell Mol. Med. 2019, 23, 8464–8471. [Google Scholar] [CrossRef] [PubMed]

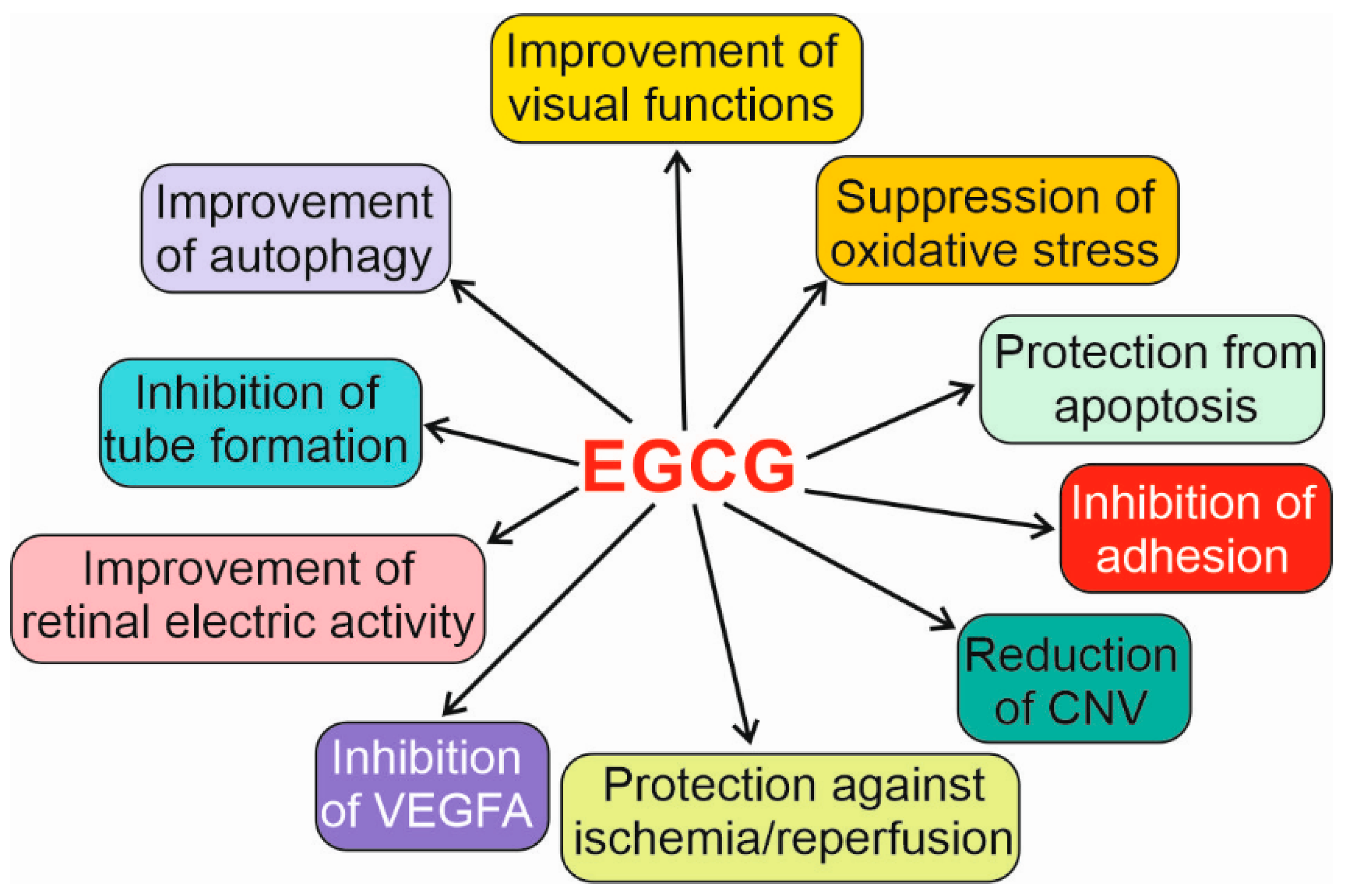
| System | Effect | Reference |
|---|---|---|
| MDA-MB231 breast cancer cells and HUVECs 1 | Decreased VEGFA secreted into conditioned media | [72] |
| Colon cancer cell line HT29 | Inhibited activation of mitogen-activated protein kinases 1 and 3 (MAPK1 and 3), increased apoptosis, and decreased angiogenesis and proliferation | [73] |
| HUVECs | Inhibition of VEGFA-induced DNA synthesis, cell proliferation, the autophosphorylation of VEGFR1 and VEGFR2, the phosphorylation of MAPK1 and MAPK3, the mRNA expression of EGR1, VEGFA-induced intracellular signaling, and mitogenesis | [77] |
| Human microvascular endothelial cells | Inhibited tube formation | [79] |
| Mice with laser-induced CNV, mouse b-End3 cells | Inhibited tube formation, reduced CNV area | [82] |
| Rats, retinal RGC-5 ganglion cells | Protection against oxidative stress and ischemia/reperfusion | [83] |
| Rats | Protection of the neural retina against oxidative stress | [84] |
| Human retinal pigment epithelium ARPE-19 cells | Suppression of UVA-induced cell death, inhibition of MAPK1, MAPK8, and p38 | [88] |
| Inhibition of migration and adhesion to fibronectin | [91,92] | |
| Repression of UVB-mediated autophagy underlined by the interaction with MTOR | [97] | |
| Derivative of human embryonic kidney HEK293T cells | Promotion of autophagy-dependent survival in ER stress, dependent on MTOR and ULK1 | [102] |
| Müller retinal cells | Autophagy activation and protection from apoptosis induced by high glucose concentration mediated by changes in SQSTM1 and BECN1 | [104] |
| Rat model of RP | Improvement in visual functions, retinal electric activity, and antioxidant defense | [109] |
| Rats | Increased oxidative stress at high EGCG concentrations | [113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blasiak, J.; Chojnacki, J.; Szczepanska, J.; Fila, M.; Chojnacki, C.; Kaarniranta, K.; Pawlowska, E. Epigallocatechin-3-Gallate, an Active Green Tea Component to Support Anti-VEGFA Therapy in Wet Age-Related Macular Degeneration. Nutrients 2023, 15, 3358. https://doi.org/10.3390/nu15153358
Blasiak J, Chojnacki J, Szczepanska J, Fila M, Chojnacki C, Kaarniranta K, Pawlowska E. Epigallocatechin-3-Gallate, an Active Green Tea Component to Support Anti-VEGFA Therapy in Wet Age-Related Macular Degeneration. Nutrients. 2023; 15(15):3358. https://doi.org/10.3390/nu15153358
Chicago/Turabian StyleBlasiak, Janusz, Jan Chojnacki, Joanna Szczepanska, Michal Fila, Cezary Chojnacki, Kai Kaarniranta, and Elzbieta Pawlowska. 2023. "Epigallocatechin-3-Gallate, an Active Green Tea Component to Support Anti-VEGFA Therapy in Wet Age-Related Macular Degeneration" Nutrients 15, no. 15: 3358. https://doi.org/10.3390/nu15153358
APA StyleBlasiak, J., Chojnacki, J., Szczepanska, J., Fila, M., Chojnacki, C., Kaarniranta, K., & Pawlowska, E. (2023). Epigallocatechin-3-Gallate, an Active Green Tea Component to Support Anti-VEGFA Therapy in Wet Age-Related Macular Degeneration. Nutrients, 15(15), 3358. https://doi.org/10.3390/nu15153358








