A Global Overview of Dietary Supplements: Regulation, Market Trends, Usage during the COVID-19 Pandemic, and Health Effects
Abstract
1. Introduction
2. Overview of Dietary Supplements (DS)
2.1. Quantitative Research Literature Analysis
2.2. Characteristics of DS
| Country | Category Name | Definition |
|---|---|---|
| China | Health food (HF) | HF refers to foods that claim to have specific health functions or provide vitamins and minerals. It is specific to certain groups and modifies organic functions in humans, but is not intended to treat disease and does not cause acute, sub-acute, or chronic harm to the human body. |
| USA | Dietary supplements (DS) | DS are dietary supplements that contain one ingredient or multiple ingredients, such as vitamins, minerals, herbs or other botanicals, amino acids, and enzymes, to supplement one’s total dietary intake. They are sold in forms such as tablets, capsules, softgels, gel capsules, powders, and liquids. Unlike medicines, dietary supplements are not intended to treat, diagnose, prevent, or cure disease. |
| EU | Food supplements (FS) | FS are concentrated sources of nutrients or other substances containing a wide range of ingredients, including vitamins, minerals, amino acids, essential fatty acids, fiber, and various plant and herbal extracts, that have a nutritional or physiological effect and are available in specific dosage forms (pills, tablets, capsules, liquids) to supplement one’s normal diet. |
| Canada | Natural health products (NHP) | NHP is a category of naturally derived products such as vitamins, minerals, amino acids, probiotics, herbal and homeopathic medicines, and traditional medicines intended to improve human health (diagnosis; treatment; alleviation or prevention of a disease, disorder, or abnormal physical condition or its symptoms; restoration; modification or correction of organic functions). |
| Australia | Complementary medicine (CM) | CMs are therapeutic products consisting of one or more designated active ingredients, each of which has an established identity and a traditional use that is not of the conventional healthcare practices of a country. |
2.3. Consumer Interest in DS and Uptake
2.4. Safety Issues, Efficacy, and Quality of DS
3. Legislative and Regulatory Issues
3.1. Regulation in the United States of America (USA)
3.2. Regulation in China
3.3. Regulation in European Union (EU)
3.4. Regulation in Algeria
4. Trends in the Use of DS before and during the Emergence of the COVID-19 Pandemic
4.1. Characteristics of the Consumption of DS
4.2. Consumption of Supplements According to Pandemic Status
| Most Used DS (%) | Vit. D | 39.0 | 55.7 | 15.3 | 31.6 | 49.1 | 60.2 | 50.1 | 52.5 | 31.1 | 30 | 22.4 |
| Vit. C | 19.4 | 77.8 | 11.4 | 84.5 | 26.6 | 31.4 | 30.1 | 27.0 | - | - | 23.0 | |
| Vit. B | - | 14.1 | 9.1 | 9.4 | - | - | - | - | - | - | 6.2 | |
| Multivitamin | 27.4 | 21.9 | 16.6 | 17 | 43.9 | 58.3 | 41 | - | - | 14 | 12.6 | |
| Zinc | 15.8 | 42.9 | 5.7 | 8 | 12.4 | 13.4 | 17.8 | 17.4 | 1.8 | 9 | 30.4 | |
| Selenium | - | 19.3 | - | - | - | - | - | 13.1 | - | - | 2.5 | |
| Omega 3 | 81.9 | - | 8.6 | 11.7 | 22.4 | 26.8 | 22.6 | 25.0 | - | - | 25.5 | |
| Probiotic | 22.3 | - | 4 | 4.4 | 11.6 | 22.5 | 12.8 | 4.5 | 20.9 | - | - | |
| Country | Turkey | Middle East | Turkey | UAE | UK | USA | Sweden | Poland | Poland | Tehran | Egypt | |
| Number of Participants | 550 | 2100 | 488 | 2060 | 372,720 | 45,757 | 27,373 | 3274 | 935 | 510 | 400 | |
| Type of Study | Cross-sectional | Cross-sectional (web survey) | Cross-sectional | Cross-sectional inquiry | App-based community survey | Cross-sectional (web survey) | Survey (questionnaire) | Cross-sectional inquiry | Cross-sectisectional | |||
| References | [67] | [6] | [68] | [69] | [46] | [10] | [70] | [71] | [72] | |||
| Nutrient | Health Benefits | Mode of Action against SARS-CoV-2 | References |
|---|---|---|---|
| Vit. D | Support immune system; immunomodulating, anti inflammatory, and anti-infectious role |
| [81,82,83,84,85] |
| Vit. C | Antioxidant, anti-inflammatory, antiviral, immunomodulatory, and anti-thrombotic effects; pleiotropic function |
| [4,9,65,86] |
| Zinc | Immunomodulatory and antiviral properties |
| [53,75,76,77,79,87] |
5. The Market for Dietary Supplements in Producer and Consumer Countries and the Impact of the COVD-19 Pandemic
5.1. Distribution Channel
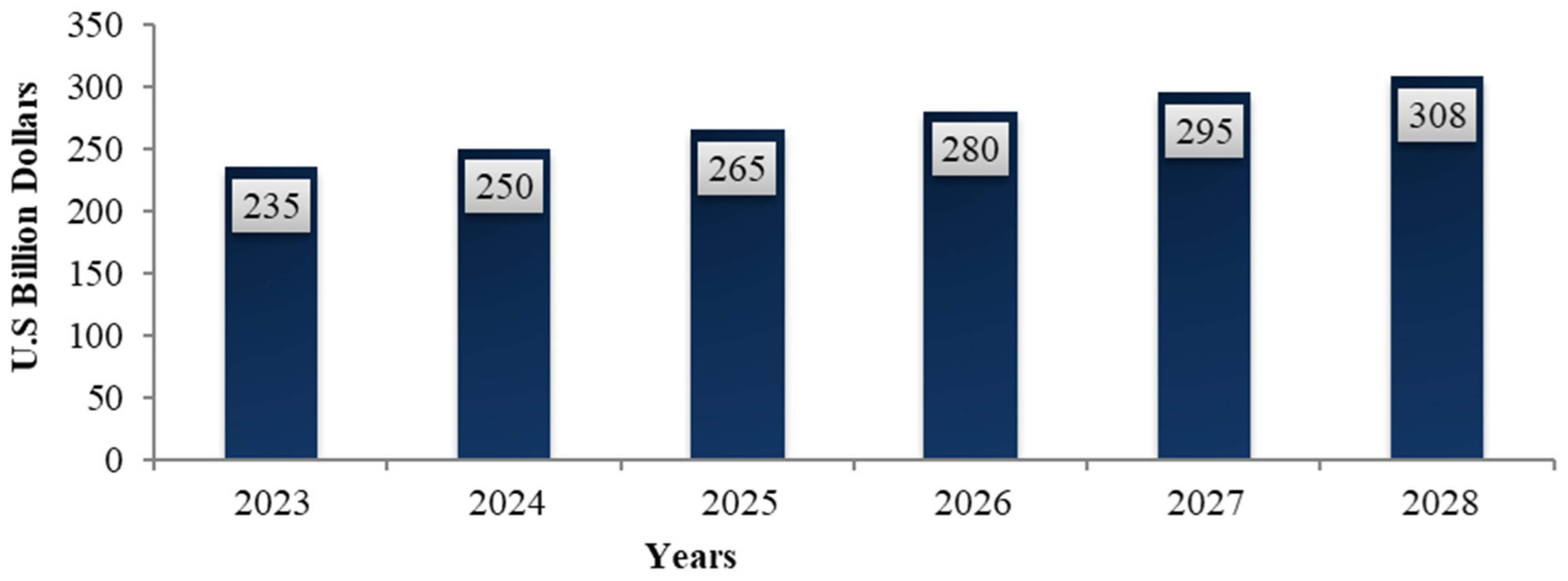
5.2. DS Global Market
5.3. Market Size of DS in USA and EU
5.4. Market Size of DS in the Middle East and Africa
5.5. Economic Impact of DS
6. Consumer Profile of DS
6.1. Pregnant and Lactating Women
6.2. Older Adults
6.3. Children and Infants
6.4. Athletes
6.5. Others
7. Conclusions and Prospects
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Lam, M.; Khoshkhat, P.; Chamani, M.; Shahsavari, S.; Dorkoosh, F.A.; Rajabi, A.; Maniruzzaman, M.; Nokhodchi, A. In-Depth Multidisciplinary Review of the Usage, Manufacturing, Regulations & Market of Dietary Supplements. J. Drug Deliv. Sci. Technol. 2022, 67, 102985. [Google Scholar] [CrossRef]
- Fahmideh, F.; Marchesi, N.; Barbieri, A.; Govoni, S.; Pascale, A. Non-Drug Interventions in Glaucoma: Putative Roles for Lifestyle, Diet and Nutritional Supplements. Surv. Ophthalmol. 2022, 67, 675–696. [Google Scholar] [CrossRef]
- Skotnicka, M.; Karwowska, K.; Kłobukowski, F.; Wasilewska, E.; Małgorzewicz, S. Dietary Habits before and during the COVID-19 Epidemic in Selected European Countries. Nutrients 2021, 13, 1690. [Google Scholar] [CrossRef]
- Chavda, V.P.; Patel, A.B.; Vihol, D.; Vaghasiya, D.D.; Ahmed, K.M.S.B.; Trivedi, K.U.; Dave, D.J. Herbal Remedies, Nutraceuticals, and Dietary Supplements for COVID-19 Management: An Update. Clin. Complement. Med. Pharmacol. 2022, 2, 100021. [Google Scholar] [CrossRef] [PubMed]
- Augusti, P.R.; Conterato, G.M.M.; Denardin, C.C.; Prazeres, I.D.; Serra, A.T.; Bronze, M.R.; Emanuelli, T. Bioactivity, Bioavailability, and Gut Microbiota Transformations of Dietary Phenolic Compounds: Implications for COVID-19. J. Nutr. Biochem. 2021, 97, 108787. [Google Scholar] [CrossRef]
- Mukattash, T.L.; Alkhalidy, H.; Alzu’bi, B.; Abu-Farha, R.; Itani, R.; Karout, S.; Khojah, H.M.J.; Khdour, M.; El-Dahiyat, F.; Jarab, A. Dietary Supplements Intake during the Second Wave of COVID-19 Pandemic: A Multinational Middle Eastern Study. Eur. J. Integr. Med. 2022, 49, 102102. [Google Scholar] [CrossRef]
- Dietary Supplements Market Size, Share and Trends Analysis Report by Ingredient (Vitamins, Minerals), by from, by Application, by End User, by Distribution Channel, by Region, and Forecasts, 2022–2030. Available online: https://www.grandviewresearch.com/industry-analysis/dietary-supplements-market (accessed on 9 November 2022).
- Hys, K. Identification of the Reasons Why Individual Consumers Purchase Dietary Supplements; Contributions to Management Science; Springer: Cham, Switzerland, 2020; pp. 193–209. [Google Scholar] [CrossRef]
- Lordan, R.; Rando, H.M.; Greene, C.S. Dietary Supplements and Nutraceuticals under Investigation for COVID-19 Prevention and Treatment. mSystems 2021, 6, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Hamulka, J.; Jeruszka-Bielak, M.; Górnicka, M.; Drywień, M.E.; Zielinska-Pukos, M.A. Dietary Supplements during Covid-19 Outbreak. Results of Google Trends Analysis Supported by Plifecovid-19 Online Studies. Nutrients 2021, 13, 54. [Google Scholar] [CrossRef]
- Dwyer, J.T.; Coates, P.M.; Smith, M.J. Dietary Supplements: Regulatory Challenges and Research Resources. Nutrients 2018, 10, 41. [Google Scholar] [CrossRef] [PubMed]
- Binns, C.W.; Lee, M.K.; Lee, A.H. Problems and Prospects: Public Health Regulation of Dietary Supplements. Annu. Rev. Public Health 2018, 39, 403–420. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, S.; Anklam, E.; Xu, A.; Ulberth, F.; Li, J.; Li, B.; Hugas, M.; Sarma, N.; Crerar, S.; Swift, S.; et al. Regulatory Landscape of Dietary Supplements and Herbal Medicines from a Global Perspective. Regul. Toxicol. Pharmacol. 2020, 114, 104647. [Google Scholar] [CrossRef]
- Brown, A.C. An Overview of Herb and Dietary Supplement Efficacy, Safety and Government Regulations in the United States with Suggested Improvements. Part 1 of 5 Series. Food Chem. Toxicol. 2017, 107, 449–471. [Google Scholar] [CrossRef] [PubMed]
- Starr, R.R. Too Little, Too Late: Ineffective Regulation of Dietary Supplements in the United States. Am. J. Public Health 2015, 105, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Wierzejska, R.E. Dietary Supplements—For Whom? The Current State of Knowledge about the Health Effects of Selected Supplement Use. Int. J. Environ. Res. Public Health 2021, 18, 8897. [Google Scholar] [CrossRef] [PubMed]
- Breitweg-lehmann, E.; Liebscher, B. Food Supplements: Definition and Classification. In Drug Discovery and Evaluation: Methods in Clinical Pharmacology; Springer: Cham, Switzerland, 2020; pp. 625–636. ISBN 9783319688640. [Google Scholar]
- Dwyer, J.; Saldanha, L.; Bailen, R.; Durazzo, A.; Le Donne, C.; Piccinelli, R.; Andrews, K.; Pehrsson, P.; Gusev, P.; Calvillo, A.; et al. Commentary: An Impossible Dream? Integrating Dietary Supplement Label Databases: Needs, Challenges, next Steps. J. Food Compos. Anal. 2021, 102, 103882. [Google Scholar] [CrossRef]
- Council for Responsible Nutrition. 2020 CRN Consumer Survey on Dietary Supplements. Available online: https://www.crnusa.org/resources/2020-crn-consumer-survey-dietary-supplements (accessed on 17 October 2022).
- Council for Responsible Nutrition. 2021 CRN Consumer Survey on Dietary Supplements. Available online: https://www.crnusa.org/resources/2021-crn-consumer-survey-dietary-supplements-0 (accessed on 17 October 2022).
- Dwyer, J.T.; Saldanha, L.G.; Bailen, R. Dietary Supplement Databases: Public Health Tools. J. Food Compos. Anal. 2022, 105, 104244. [Google Scholar] [CrossRef]
- Shen, J. Regulatory News—Food & Food Contact Materials—CIRS Group, How Can Health Food (Dietary Supplement) Enter into the Market of China, Japan, Korea, USA, Australia, New Zealand, ASEAN and EU? Available online: https://www.cirs-group.com/en/food/how-can-health-food-dietary-supplement-enter-into-the-market-of-china-japan-korea-usa-australia-new-zealand-asean-and-eu (accessed on 17 October 2022).
- GB 16740-2014; National Food Safety Standard-Health Foods. Ministry of Agriculture: Beijing China, 2014.
- Scheuplein, R.J. History of Food Regulation. In International Food Safety Handbook; Routledge: Abingdon, UK, 1999; pp. 647–659. ISBN 9780203750346. [Google Scholar]
- Wallace, T.C.; MacKay, D.; Al-Mondhiry, R.; Nguyen, H.; Griffiths, J.C. Dietary Supplement Regulation in the United States. In Springer Briefs in Food, Health, and Nutrition; Springer: Cham, Switzerland, 2013; pp. 1–43. ISBN 9783319015026. [Google Scholar]
- Bailey, R.L. Current Regulatory Guidelines and Resources to Support Research of Dietary Supplements in the United States. Crit. Rev. Food Sci. Nutr. 2020, 60, 298–309. [Google Scholar] [CrossRef]
- Information for Consumers on Using Dietary Supplements. Available online: https://www.fda.gov/food/dietary-supplements/information-consumers-using-dietary-supplements (accessed on 15 November 2022).
- Fortin, D.N.D. Food Regulation: Law, Science, Policy, and Practice. Available online: https://books.google.dz/books?hl=fr&lr=&id=TcZ6EAAAQBAJ&oi=fnd&pg=PA23&dq=Fortin,+N.+D.+(2022).+Food+regulation:+law,+science,+policy,+and+practice,+.&ots=OB7B0IbmMM&sig=EtV3RvOgFeCH4yccpUNC7Ki5MPk&redir_esc=y#v=onepage&q=Fortin%2CN.D.(2022).F (accessed on 10 November 2022).
- Bagchi, D. Industry Self- Regulatory Activities Complement FDA’s Dietary Supplement Regulations. In Nutraceutical and Functional Food Regulations in the United States and around the World; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 1–678. ISBN 9780128164679. [Google Scholar]
- ElAmrawy, F.; ElAgouri, G.; Elnoweam, O.; Aboelazayem, S.; Farouk, E.M.; Nounou, M.I. Adulterated and Counterfeit Male Enhancement Nutraceuticals and Dietary Supplements Pose a Real Threat to the Management of Erectile Dysfunction: A Global Perspective. J. Diet. Suppl. 2016, 13, 660–693. [Google Scholar] [CrossRef] [PubMed]
- Denham, B.E. Dietary Supplements in the USA: Problematic Trends. Public Health Nutr. 2021, 24, 2771–2775. [Google Scholar] [CrossRef]
- Hu, C. Change in Raw Materials and Claims of Health Food Regulations in China 2013–17. In Nutraceutical and Functional Food Regulations in the United States and around the World; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 465–495. ISBN 9780128164679. [Google Scholar]
- Roberts, A.; Rogerson, R. Chinese Approach on Regulating Food Additives, Novel Foods, Functional Foods and Dietary Supplements. In Nutraceutical and Functional Food Regulations; R Discovery: New York, NY, USA, 2008; pp. 291–303. ISBN 9780123739018. [Google Scholar]
- Coppens, P.; Da Silva, M.F.; Pettman, S. European Regulations on Nutraceuticals, Dietary Supplements and Functional Foods: A Framework Based on Safety. Toxicology 2006, 221, 59–74. [Google Scholar] [CrossRef]
- Farid, M.; Kodama, K.; Arato, T.; Okazaki, T.; Oda, T.; Ikeda, H.; Sengoku, S. Comparative Study of Functional Food Regulations in Japan and Globally. Glob. J. Health Sci. 2019, 11, 132. [Google Scholar] [CrossRef]
- Silano, V.; Coppens, P.; Larrañaga-Guetaria, A.; Minghetti, P.; Roth-Ehrang, R. Regulations Applicable to Plant Food Supplements and Related Products in the European Union. Food Funct. 2011, 2, 710–719. [Google Scholar] [CrossRef]
- Petkova-Gueorguieva, E.S.; Getov, I.N.; Ivanov, K.V.; Ivanova, S.D.; Gueorguiev, S.R.; Getova, V.I.; Mihaylova, A.A.; Madzharov, V.G.; Staynova, R.A. Regulatory Requirements for Food Supplements in the European Union and Bulgaria. Folia Med. 2019, 61, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Czepielewska, E.; Makarewicz-Wujec, M.; Różewski, F.; Wojtasik, E.; Kozłowska-Wojciechowska, M. Drug Adulteration of Food Supplements: A Threat to Public Health in the European Union? Regul. Toxicol. Pharmacol. 2018, 97, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Rocha, T.; Amaral, J.S.; Oliveira, M.B.P.P. Adulteration of Dietary Supplements by the Illegal Addition of Synthetic Drugs: A Review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 43–62. [Google Scholar] [CrossRef] [PubMed]
- Chermat, S.; Belhadj, N.; Charifi, I. Place Des Compléments Alimentaires à Base de Plantes En Algérie: Évaluation de l’impact Sanitaire et Biosécurité de La Région de Sétif et Bordj Bou Arreridj. Available online: https://www.abebooks.com/9786138431992/Place-Compléments-Alimentaires-base-Plantes-6138431995/plp (accessed on 31 July 2022).
- Hallouch, F.A. Médicament à Base de Plante En Algérie: Entre L’expansion Du Marché et La Réglementation. Rev. Droit Public Algérien Comparé 2021, 7, 31–55. [Google Scholar]
- Un Encadrement s’impose: La Jungle Des Compléments Alimentaires—Actualité. Available online: https://www.lesoirdalgerie.com/actualites/la-jungle-des-complements-alimentaires-62656 (accessed on 30 October 2022).
- Commerce: 20 Compléments Alimentaires Nocifs Interdits de Vente. Available online: https://www.aps.dz/economie/135590-commerce-20-complements-alimentaires-nocifs-interdits-de-vente (accessed on 30 October 2022).
- El-Maouhab, F.; Bedouhene, S.; Bourouba, M.; Lacheheb, S.; Metouri, S.; Zemoul, O. L’avant-Projet: Cadre Réglementaire Régissant Les Compléments Alimentaires En Algérie; Industriel; Fédération Algérienne de Pharmacie Section Pharmacie Industrielle: Paris, France, 2022. [Google Scholar]
- Brahmi, F.; Vejux, A.; Ghzaiel, I.; Ksila, M.; Zarrouk, A.; Ghrairi, T.; Essadek, S.; Mandard, S.; Leoni, V.; Poli, G.; et al. Role of Diet and Nutrients in SARS-CoV-2 Infection: Incidence on Oxidative Stress, Inflammatory Status and Viral Production. Nutrients 2022, 14, 2194. [Google Scholar] [CrossRef]
- Louca, P.; Murray, B.; Klaser, K.; Graham, M.S.; Mazidi, M.; Leeming, E.R.; Thompson, E.; Bowyer, R.; Drew, D.A.; Nguyen, L.H.; et al. Modest Effects of Dietary Supplements during the COVID-19 Pandemic: Insights from 445 850 Users of the COVID-19 Symptom Study App. BMJ Nutr. Prev. Heal. 2021, 4, 149–157. [Google Scholar] [CrossRef]
- Subedi, L.; Tchen, S.; Gaire, B.P.; Hu, B.; Hu, K. Adjunctive Nutraceutical Therapies for COVID-19. Int. J. Mol. Sci. 2021, 22, 1963. [Google Scholar] [CrossRef]
- Mohsen, H.; Yazbeck, N.; Al-Jawaldeh, A.; Chahine, N.B.; Hamieh, H.; Mourad, Y.; Skaiki, F.; Salame, H.; Salameh, P.; Hoteit, M. Knowledge, Attitudes, and Practices Related to Dietary Supplementation, before and during the COVID-19 Pandemic: Findings from a Cross-Sectional Survey in the Lebanese Population. Int. J. Environ. Res. Public Health 2021, 18, 8856. [Google Scholar] [CrossRef]
- Bayazid, A.; Youcef, A.; Mahsar, Y.; Dous, A. Impact of COVID-19 Pandemic on Dietary Supplements Consumption in Algeria. Nutr. Santé 2022, 11, 21–33. [Google Scholar] [CrossRef]
- Alfawaz, H.A.; Khan, N.; Aljumah, G.A.; Hussain, S.D.; Al-Daghri, N.M. Dietary Intake and Supplement Use among Saudi Residents during COVID-19 Lockdown. Int. J. Environ. Res. Public Health 2021, 18, 6435. [Google Scholar] [CrossRef]
- Aldwihi, L.A.; Khan, S.I.; Alamri, F.F.; Alruthia, Y.; Alqahtani, F.; Fantoukh, O.I.; Assiri, A.; Almohammed, O.A. Patients’ Behavior Regarding Dietary or Herbal Supplements before and during COVID-19 in Saudi Arabia. Int. J. Environ. Res. Public Health 2021, 18, 5086. [Google Scholar] [CrossRef]
- Karaçil Ermumcu, M.Ş.; Mengi Çelik, Ö. Evaluation of Using Dietary Supplements, Functional Foods and Herbal Products with Nutritional Habits of Individuals Diagnosed with COVID-19 Before, During, and After Disease. Clin. Sci. Nutr. 2022, 4, 54–60. [Google Scholar] [CrossRef]
- Shakoor, H.; Feehan, J.; Al Dhaheri, A.S.; Ali, H.I.; Platat, C.; Ismail, L.C.; Apostolopoulos, V.; Stojanovska, L. Immune-Boosting Role of Vitamins D, C, E, Zinc, Selenium and Omega-3 Fatty Acids: Could They Help against COVID-19? Maturitas 2021, 143, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Adams, K.K.; Baker, W.L.; Sobieraj, D.M. Myth Busters: Dietary Supplements and COVID-19. Ann. Pharmacother. 2020, 54, 820–826. [Google Scholar] [CrossRef]
- Mrityunjaya, M.; Pavithra, V.; Neelam, R.; Janhavi, P.; Halami, P.M.; Ravindra, P.V. Immune-Boosting, Antioxidant and Anti-Inflammatory Food Supplements Targeting Pathogenesis of COVID-19. Front. Immunol. 2020, 11, 570122. [Google Scholar] [CrossRef]
- Mullin, G.E.; Limektkai, B.; Wang, L.; Hanaway, P.; Marks, L.; Giovannucci, E. Dietary Supplements for COVID-19. Adv. Exp. Med. Biol. 2021, 1318, 499–515. [Google Scholar] [CrossRef]
- Galanakis, C.M.; Aldawoud, T.M.S.; Rizou, M.; Rowan, N.J.; Ibrahim, S.A. Food Ingredients and Active Compounds against the Coronavirus Disease (COVID-19) Pandemic: A Comprehensive Review. Foods 2020, 9, 1701. [Google Scholar] [CrossRef]
- Annweiler, C. Point of View: Should COVID-19 Patients Be Supplemented with Vitamin D? Maturitas 2020, 104, 24–26. [Google Scholar] [CrossRef]
- Cangiano, B.; Fatti, L.M.; Danesi, L.; Gazzano, G.; Croci, M.; Vitale, G.; Gilardini, L.; Bonadonna, S.; Chiodini, I.; Caparello, C.F.; et al. Mortality in an Italian Nursing Home during COVID-19 Pandemic: Correlation with Gender, Age, ADL, Vitamin D Supplementation, and Limitations of the Diagnostic Tests. Aging 2020, 12, 24522–24534. [Google Scholar] [CrossRef]
- D’avolio, A.; Avataneo, V.; Manca, A.; Cusato, J.; De Nicolò, A.; Lucchini, R.; Keller, F.; Cantù, M. 25-Hydroxyvitamin D Concentrations Are Lower in Patients with Positive PCR for SARS-CoV-2. Nutrients 2020, 12, 1359. [Google Scholar] [CrossRef]
- Razdan, K.; Singh, K.; Singh, D. Vitamin D Levels and COVID-19 Susceptibility: Is There Any Correlation? Med. Drug Discov. J. 2020, 7, 100051. [Google Scholar] [CrossRef] [PubMed]
- Sabico, S.; Enani, M.A.; Sheshah, E.; Aljohani, N.J.; Aldisi, D.A.; Alotaibi, N.H.; Alshingetti, N.; Alomar, S.Y.; Alnaami, A.M.; Amer, O.E.; et al. Effects of a 2-Week 5000 Iu versus 1000 Iu Vitamin D3 Supplementation on Recovery of Symptoms in Patients with Mild to Moderate Covid-19: A Randomized Clinical Trial. Nutrients 2021, 13, 2170. [Google Scholar] [CrossRef] [PubMed]
- Sooriyaarachchi, P.; Jeyakumar, D.T.; King, N.; Jayawardena, R. Impact of Vitamin D Deficiency on COVID-19. Clin. Nutr. ESPEN 2021, 44, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Zhou, T.; Heianza, Y.; Qi, L. Habitual Use of Vitamin D Supplements and Risk of Coronavirus Disease 2019 (COVID-19) Infection: A Prospective Study in UK Biobank. Am. J. Clin. Nutr. 2021, 113, 1275–1281. [Google Scholar] [CrossRef]
- Holford, P.; Carr, A.C.; Jovic, T.H.; Ali, S.R.; Whitaker, I.S.; Marik, P.E.; Smith, A.D. Vitamin C—An Adjunctive Therapy for Respiratory Infection, Sepsis and COVID-19. Nutrients 2020, 12, 3760. [Google Scholar] [CrossRef]
- Milani, G.P.; Macchi, M.; Guz-Mark, A. Vitamin c in the Treatment of COVID-19. Nutrients 2021, 13, 1172. [Google Scholar] [CrossRef] [PubMed]
- Kamarli Altun, H.; Karacil Ermumcu, M.S.; Seremet Kurklu, N. Evaluation of Dietary Supplement, Functional Food and Herbal Medicine Use by Dietitians during the COVID-19 Pandemic. Public Health Nutr. 2021, 24, 861–869. [Google Scholar] [CrossRef]
- Ayer, C.; Celep, A.G.S. Assessment of Dietary Habits and Use of Nutritional Supplements in COVID-19: A Cross-Sectional Study. PharmaNutrition 2022, 22, 100309. [Google Scholar] [CrossRef]
- Radwan, H.; Hasan, H.; Jaafar, Z.; Abbas, N.; Rashed Saif, E.; Al Kitbi, M.; Al Hilali, M.; Naja, F. Diets and Dietary Supplements Used during the COVID-19 Pandemic in the United Arab Emirates: A Cross-Sectional Survey. Saudi Pharm. J. 2022, 30, 421–432. [Google Scholar] [CrossRef]
- Puścion-Jakubik, A.; Bielecka, J.; Grabia, M.; Mielech, A.; Markiewicz-żukowska, R.; Mielcarek, K.; Moskwa, J.; Naliwajko, S.K.; Soroczyńska, J.; Gromkowska-Kępka, K.J.; et al. Consumption of Food Supplements during the Three Covid-19 Waves in Poland—Focus on Zinc and Vitamin D. Nutrients 2021, 13, 3361. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, M.; Haghollahi, F.; Shariat, M.; Jafarabadi, M.; Aryamloo, P.; Rezayof, E. Supplement Usage Pattern in a Group of COVID- 19 Patients in Tehran. J. Fam. Reprod. Health 2020, 14, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Taha, S.H.N.; Moawad, A.M.; Ghazy, R.M.; Abdelhalim, W.A. Assessment of Self-Treatment Knowledge, Beliefs and Practice during COVID-19 Pandemic among Egyptian Population: A Cross Sectional Study. Egypt. J. Hosp. Med. 2022, 89, 4516–4525. [Google Scholar] [CrossRef]
- Cerullo, G.; Negro, M.; Parimbelli, M.; Pecoraro, M.; Perna, S.; Liguori, G.; Rondanelli, M.; Cena, H.; D’Antona, G. The Long History of Vitamin C: From Prevention of the Common Cold to Potential Aid in the Treatment of COVID-19. Front. Immunol. 2020, 11, 574029. [Google Scholar] [CrossRef]
- Arvinte, C.; Singh, M.; Marik, P.E. Serum Levels OfVitamin C and Vitamin D in a Cohort of Critically Ill COVID-19 Patients of a North American Community Hospital Intensive Care Unit in May 2020: A Pilot Study. Med. Drug Discov. J. 2020, 8, 100064. [Google Scholar] [CrossRef]
- Costagliola, G.; Spada, E.; Comberiati, P.; Peroni, D.G. Could Nutritional Supplements Act as Therapeutic Adjuvants in COVID-19? Ital. J. Pediatr. 2021, 47, 32. [Google Scholar] [CrossRef]
- Celik, C.; Gencay, A.; Ocsoy, I. Can Food and Food Supplements Be Deployed in the Fight against the COVID 19 Pandemic? BBA Gen. Subj. 2020, 1895, 129801. [Google Scholar] [CrossRef] [PubMed]
- Wessels, I.; Rolles, B.; Rink, L. The Potential Impact of Zinc Supplementation on COVID-19 Pathogenesis. Front. Immunol. 2020, 11, 1712. [Google Scholar] [CrossRef]
- Jothimani, D.; Kailasam, E.; Danielraj, S.; Nallathambi, B.; Ramachandran, H.; Sekar, P.; Manoharan, S.; Ramani, V.; Narasimhan, G.; Kaliamoorthy, I.; et al. COVID-19: Poor Outcomes in Patients with Zinc Deficiency. Int. J. Infect. Dis. 2020, 100, 343–349. [Google Scholar] [CrossRef]
- Razzaque, M.S. COVID-19 Pandemic: Can Zinc Supplementation Provide an Additional Shield against the Infection? Comput. Struct. Biotechnol. J. 2021, 19, 1371–1378. [Google Scholar] [CrossRef]
- Finzi, E. Treatment of SARS-CoV-2 with High Dose Oral Zinc Salts: A Report on Four Patients. Int. J. Infect. Dis. 2020, 99, 307–309. [Google Scholar] [CrossRef]
- Araújo, T.S.S.; Santos, C.S.; Soares, J.K.B.; Freitas, J.C.R. Vitamin D: A Potentially Important Secosteroid for Coping with COVID-19. An. Acad. Bras. Cienc. 2022, 94, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Moscatelli, F.; Sessa, F.; Valenzano, A.; Polito, R.; Monda, V.; Cibelli, G.; Villano, I.; Pisanelli, D.; Perrella, M.; Daniele, A.; et al. Covid-19: Role of Nutrition and Supplementation. Nutrients 2021, 13, 976. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence That Vitamin d Supplementation Could Reduce Risk of Influenza and Covid-19 Infections and Deaths. Nutrients 2020, 12, 988. [Google Scholar] [CrossRef] [PubMed]
- Mohan, M.; Cherian, J.J.; Sharma, A. Exploring Links between Vitamin D Deficiency and Covid-19. PLoS Pathog. 2020, 16, e1008874. [Google Scholar] [CrossRef]
- Mercola, J.; Grant, W.B. Evidence Regarding Vitamin D and Risk of COVID-19 and Its Severity. Nutrients 2020, 12, 3361. [Google Scholar] [CrossRef]
- Bae, M.; Kim, H. The Role of Vitamin C, Vitamin D, and Selenium in Immune System against COVID-19. Molecules 2020, 25, 5346. [Google Scholar] [CrossRef]
- Kumar, A.; Kubota, Y.; Chernov, M.; Kasuya, H. Potential Role of Zinc Supplementation in Prophylaxis and Treatment of COVID-19. Med. Hypotheses 2020, 144, 109848. [Google Scholar] [CrossRef]
- Morgovan, C.; Ghibu, S.; Juncan, A.M.; Rus, L.L.; Butucă, A.; Vonica, L.; Muntean, A.; Moş, L.; Gligor, F.; Olah, N.K. Nutrivigilance: A New Activity in the Field of Dietary Supplements. Farmacia 2019, 67, 537–544. [Google Scholar] [CrossRef]
- Mahdavi-Roshan, M.; Rezazadeh, A.; Joukar, F.; Khorshidi, Y.; Naghipour, M.; Mansour-Ghanaei, F. Dietary Supplements Consumption and Its Association with Socioeconomic Factors, Obesity and Main Non-Communicable Chronic Diseases in the North of Iran: The PERSIAN Guilan Cohort Study (PGCS). BMC Nutr. 2021, 7, 84. [Google Scholar] [CrossRef] [PubMed]
- Dietary Supplements Market—Growth, Trends, Covid-19 Impact, and Forecasts (2022–2027). Available online: https://www.mordorintelligence.com/industry-reports/dietary-supplement-market (accessed on 11 November 2022).
- Hegazy, N.; Sayed, H.A.; Hasan, A.A.; Salem, M.R. Popularity of the Consumption of Dietary Supplements and Its Associated Factors among Students in an Egyptian University: A Cross-Sectional Study. Open Access Maced. J. Med. Sci. 2020, 8, 566–573. [Google Scholar] [CrossRef]
- Altamimi, J.Z. Awareness of the Consumption of Dietary Supplements among Students in a University in Saudi Arabia. J. Nutr. Metab. 2019, 2019, 4641768. [Google Scholar] [CrossRef]
- Lordan, R. Dietary Supplements and Nutraceuticals Market Growth during the Coronavirus Pandemic—Implications for Consumers and Regulatory Oversight. PharmaNutrition 2021, 18, 2020–2022. [Google Scholar] [CrossRef]
- Total Dietary Supplements Market Size Worldwide from 2016 to 2028. Available online: https://www.statista.com/statistics/828514/total-dietary-supplements-market-size-globally/ (accessed on 20 November 2022).
- Europe Dietary Supplements Market Size, Share and COVID-19 Impact Analysis, by Type (Vitamins, Minerals, Enzymes, Fatty Acids, Proteins, and Others) from (Tables, Capsules, Powder, and Liquid) and Regional Forecasts, 2020–2027. 2022. Available online: https://www.fortunebusinessinsights.com/industry-reports/europe-dietary-supplements-market-101918 (accessed on 11 November 2022).
- Alhomoud, F.K.; Basil, M.; Bondarev, A. Knowledge, Attitudes and Practices (KAP) Relating to Dietary Supplements among Health Sciences and Non-Health Sciences Students in One of the Universities of United Arab Emirates (UAE). J. Clin. Diagn. Res. 2016, 10, JC05–JC09. [Google Scholar] [CrossRef]
- El-Dahiyat, F.; Rashrash, M.; Abuhamdah, S.; Abu Farha, R.; Babar, Z.U.D. Herbal Medicines: A Cross-Sectional Study to Evaluate the Prevalence and Predictors of Use among Jordanian Adults. J. Pharm. Policy Pract. 2020, 13, 2. [Google Scholar] [CrossRef]
- Economic Impact of the Dietary Supplement Industry. Available online: https://www.crnusa.org/resources/economic-impact-dietary-supplement-industry (accessed on 5 November 2022).
- Hauguel, V. L’avenir Du Marché Des Probiotiques Dans Le Domaine de La Santé; Université de Borgeaux: Bordeaux, France, 2021. [Google Scholar]
- Sundararaman, A.; Ray, M.; Ravindra, P.V.; Halami, P.M. Role of Probiotics to Combat Viral Infections with Emphasis on COVID-19. Appl. Microbiol. Biotechnol. 2020, 2, 8089–8104. [Google Scholar] [CrossRef]
- Knapik, J.J.; Trone, D.W.; Steelman, R.A.; Farina, E.K.; Lieberman, H.R. Prevalence of and Factors Associated with Dietary Supplement Use in a Stratified, Random Sample of US Military Personnel: The US Military Dietary Supplement Use Study. J. Nutr. 2021, 151, 3495–3506. [Google Scholar] [CrossRef]
- Bardou-Boisnier, S.; Caillaud, K. Les Dispositifs Informationnels Sur Les Compléments Alimentaires: Une Affaire de Sante Publique. Quest. Commun. 2015, 27, 79–104. [Google Scholar] [CrossRef]
- Bernard, D. Recherche Scientifique et Impératif de Croissance: Observations, Questionnements et Pistes de Réflexion. Rev. Interdiscip. D’études Jurid. 2017, 78, 173. [Google Scholar] [CrossRef]
- Industrie de La Production Algérienne Des Compléments Alimentaire: Un Secteur d’avenir Farouchement Attaqué. Available online: https://www.lexpressiondz.com/info-en-continu/industrie-de-la-production-algerienne-des-complements-alimentaire-un-secteur-d-avenir-farouchement-attaque-322749 (accessed on 16 November 2022).
- Jun, S.; Gahche, J.J.; Potischman, N.; Dwyer, J.T.; Guenther, P.M.; Sauder, K.A.; Bailey, R.L. Dietary Supplement Use and Its Micronutrient Contribution During Pregnancy and Lactation in the United States. Obstet. Gynecol. 2020, 135, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.; Wright, C. Safety and Efficacy of Supplements in Pregnancy. Nutr. Rev. 2020, 78, 813–826. [Google Scholar] [CrossRef] [PubMed]
- Nevins, J.E.H.; Donovan, S.M.; Snetselaar, L.; Dewey, K.G.; Novotny, R.; Stang, J.; Taveras, E.M.; Kleinman, R.E.; Bailey, R.L.; Raghavan, R.; et al. Omega-3 Fatty Acid Dietary Supplements Consumed during Pregnancy and Lactation and Child Neurodevelopment: A Systematic Review. J. Nutr. 2021, 151, 3483–3494. [Google Scholar] [CrossRef]
- Tang, L.; Lee, A.H.; Yau, K.K.W.; Hui, Y.V.; Binns, C.W. Consumption of Dietary Supplements by Chinese Women during Pregnancy and Postpartum: A Prospective Cohort Study. Matern. Child Nutr. 2017, 13, e12435. [Google Scholar] [CrossRef]
- Walrand, S. Dietary Supplement Intake among the Elderly: Hazards and Benefits. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 465–470. [Google Scholar] [CrossRef]
- Smolinske, S.C. Dietary Supplements in Children. Pediatr. Clin. N. Am. 2017, 64, 1243–1255. [Google Scholar] [CrossRef] [PubMed]
- Alonso, M.R.; Fernández-García, B. Evolution of the Use of Sports Supplements. PharmaNutrition 2020, 14, 100239. [Google Scholar] [CrossRef]
- Maughan, R.J.; Shirreffs, S.M.; Vernec, A. Making Decisions about Supplement Use. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 212–219. [Google Scholar] [CrossRef]
- Cannataro, R.; Straface, N.; Cione, E. Nutritional Supplements in Combat Sports: What We Know and What We Do. Hum. Nutr. Metab. 2022, 29, 200155. [Google Scholar] [CrossRef]
- Vitale, K.; Getzin, A. Nutrition and Supplement Update for the Endurance Athlete: Review and Recommendations. Nutrients 2019, 11, 1289. [Google Scholar] [CrossRef]
- Dini, I.; Laneri, S. Nutricosmetics: A Brief Overview. Phyther. Res. 2019, 33, 3054–3063. [Google Scholar] [CrossRef] [PubMed]


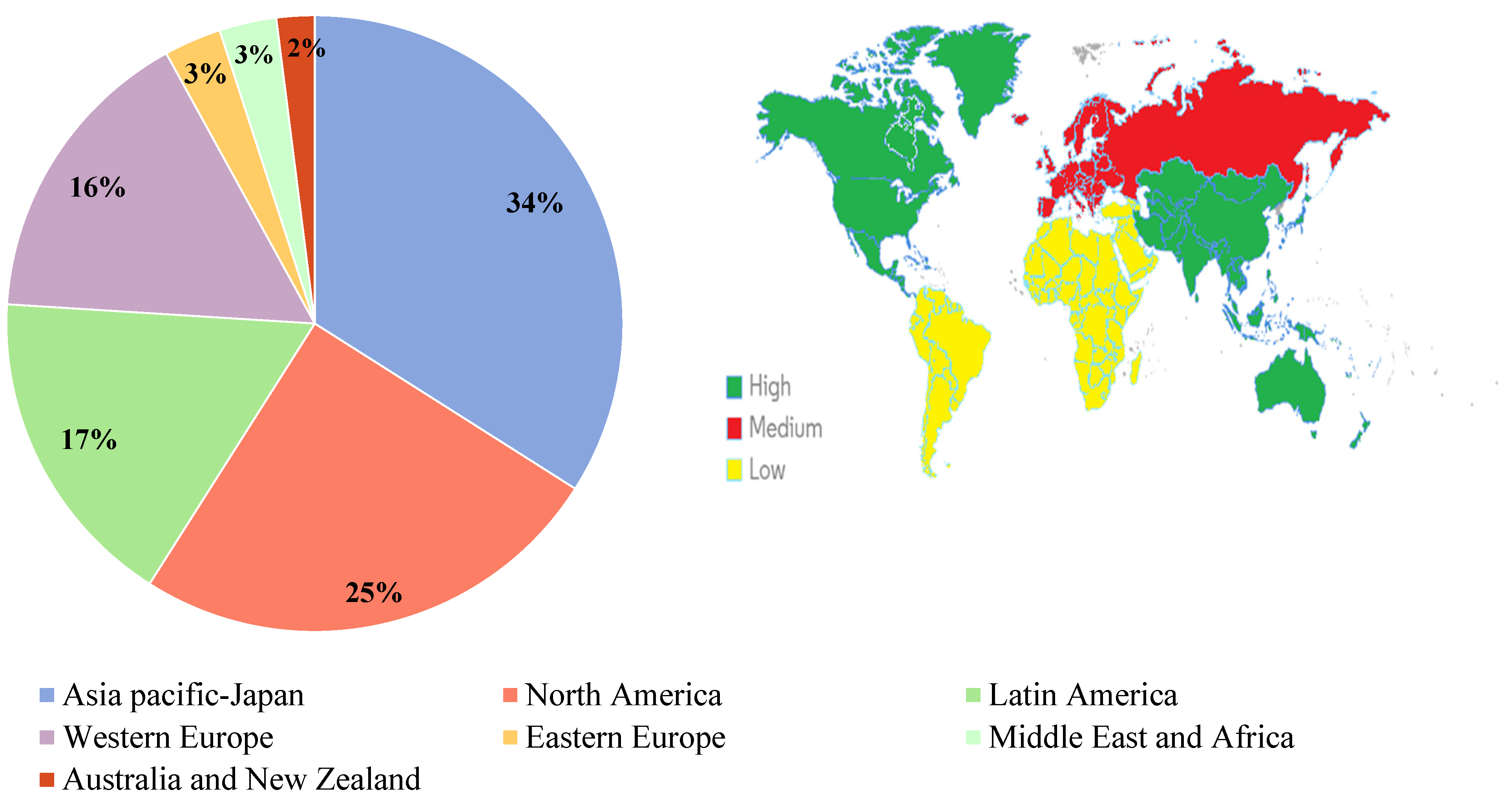

| Contributor | Publication Count (% of Total) | |
|---|---|---|
| Country/Territory | ||
| United States | 12,797 (26.3) | |
| China | 6383 (13.1) | |
| United Kingdom | 3571 (7.3) | |
| Italy | 3152 (6.5) | |
| Canada | 2579 (5.3) | |
| Journal | ||
| Nutrients | 2769 (5.7) | |
| Poultry Science | 1120 (2.3) | |
| Journal of Animal Science | 1012 (2) | |
| PLOS One | 909 (1.9) | |
| Journal of Dairy Science | 753 (1.5) | |
| Scopus category | ||
| Medicine | 25,160 (51.8) | |
| Agricultural and Biological Sciences | 14,558 (29.9) | |
| Biochemistry, Genetics Molecular Biology | 12,776 (26.3) | |
| Nursing | 11,617 (23.9) | |
| Chemistry | 3892 (8) | |
| USA | EU | China | Algeria | ||
|---|---|---|---|---|---|
| Regulatory agency | Food and Drug Administration (FDA) |
|
| NA | |
| Regulation and law |
|
|
| / | |
| Compliance process |
| Notification/registration |
| / | |
| Category | Foods | + | + | + | + |
| Medicines | − | − | − | − | |
| Manufacturer Registration | − | + (Limited) | + | − | |
| Presence of a positive list | − | + | + | NA | |
| Good Manufacturing Practice (GMP) | + | + (HACCP) | + | NA | |
| Clinical trials of individual products | − | + (New ingredients) | + (New ingredients) | NA | |
| Obligated to display the usage and dosage | + | + | + | NA | |
| Serious adverse event reporting | + | + | − | NA | |
| Labeling and packaging | + | + | + | NA | |
| Shape description | + | + | Often | NA | |
| Advertising requirements | + | + | + | NA | |
| Health claims | − | − | − | NA | |
| Before the Pandemic (%) | During the Pandemic (%) | Type of Study | Country | References | |
|---|---|---|---|---|---|
| Vitamins | 27.7 | 58.0 | Cross-sectional (online questionnaire) | Algeria | [49] |
| Vit. D | 7.1 | 22.4 | |||
| Vit. C | 19.8 | 53.1 | |||
| Minerals | 18.4 | 50.0 | |||
| Zinc | 4.6 | 44.9 | |||
| Magnesium | 11.5 | 18.9 | |||
| Selenium | 0.8 | 6.1 | |||
| Others | 9.0 | 12.6 | |||
| Omega 3 | 4.2 | 9.7 | |||
| Vitamins | Cross-sectional (questionnaire-based) | Saudi Arabia | [51] | ||
| Vit. D | 34.6 | 35.1 | |||
| Vit. C | 48.8 | 68.4 | |||
| Vitamins | Cross-sectional (online survey) | Saudi Arabia | [50] | ||
| Vit. D | 20.6 | 18.7 | |||
| Vit. C | 12.5 | 14.9 | |||
| Multivitamin | 24.6 | 44.9 | |||
| Minerals | |||||
| Zinc | 1.3 | 4.6 | |||
| Vitamins | Cross-sectional (online survey) | Lebanon | [48] | ||
| Vit. D | 35.5 | 41.0 | |||
| Vit. C | 35.3 | 42.1 | |||
| Vit. E | 15.2 | 17.5 | |||
| Minerals | |||||
| Zinc | 18.8 | 29.3 | |||
| Vitamins | Cross-sectional | Turkey | [52] | ||
| Vit. D | 10.7 | 5.5 | |||
| Vit. C | 14.2 | 41.1 | |||
| Minerals | |||||
| Calcium | 0.2 | 0.2 | |||
| Zinc | 0.2 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djaoudene, O.; Romano, A.; Bradai, Y.D.; Zebiri, F.; Ouchene, A.; Yousfi, Y.; Amrane-Abider, M.; Sahraoui-Remini, Y.; Madani, K. A Global Overview of Dietary Supplements: Regulation, Market Trends, Usage during the COVID-19 Pandemic, and Health Effects. Nutrients 2023, 15, 3320. https://doi.org/10.3390/nu15153320
Djaoudene O, Romano A, Bradai YD, Zebiri F, Ouchene A, Yousfi Y, Amrane-Abider M, Sahraoui-Remini Y, Madani K. A Global Overview of Dietary Supplements: Regulation, Market Trends, Usage during the COVID-19 Pandemic, and Health Effects. Nutrients. 2023; 15(15):3320. https://doi.org/10.3390/nu15153320
Chicago/Turabian StyleDjaoudene, Ouarda, Anabela Romano, Yasmine Djedjiga Bradai, Feriel Zebiri, Amina Ouchene, Yasmine Yousfi, Meriem Amrane-Abider, Yasmine Sahraoui-Remini, and Khodir Madani. 2023. "A Global Overview of Dietary Supplements: Regulation, Market Trends, Usage during the COVID-19 Pandemic, and Health Effects" Nutrients 15, no. 15: 3320. https://doi.org/10.3390/nu15153320
APA StyleDjaoudene, O., Romano, A., Bradai, Y. D., Zebiri, F., Ouchene, A., Yousfi, Y., Amrane-Abider, M., Sahraoui-Remini, Y., & Madani, K. (2023). A Global Overview of Dietary Supplements: Regulation, Market Trends, Usage during the COVID-19 Pandemic, and Health Effects. Nutrients, 15(15), 3320. https://doi.org/10.3390/nu15153320







