Adiposity Reduction by Cucumis melo var. gaettongchamoe Extract in High-Fat Diet-Induced Obese Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. CG Collection and Extract Preparation
2.2. Animal Study
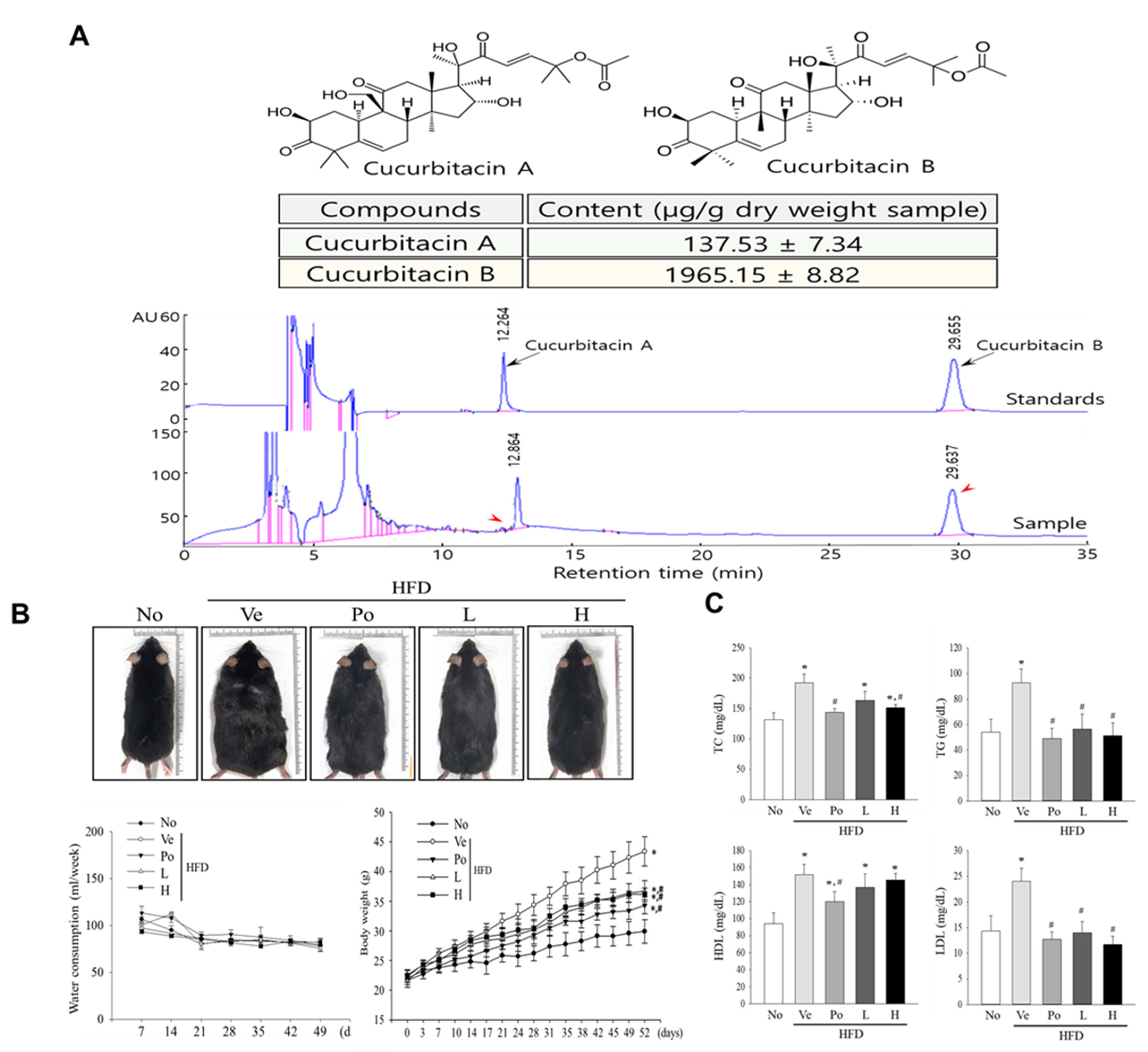
2.3. HPLC Analysis
2.4. Serum Biochemical Analysis
2.5. Histopathological Analysis
2.6. Quantitative Real Time–Polymerase Chain Reaction (qRT-PCR) Analysis
2.7. Western Blotting Analysis
2.8. Statistical Analysis
3. Results
3.1. Effects of CGWE Treatment on Body Weight and Serum Lipid Profiles in HFD-Induced Obese Mice
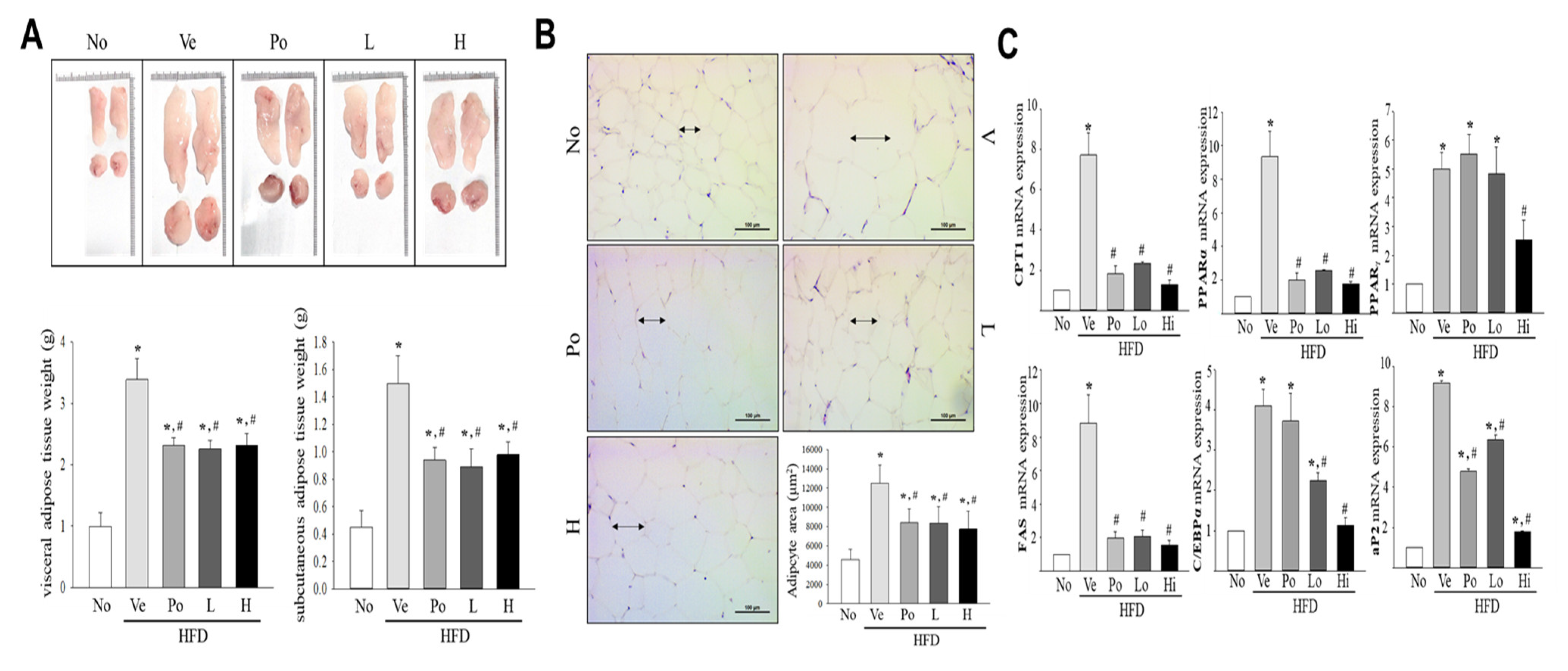
3.2. The Inhibitory Effect of CGWE on Abdominal Fat Accumulation in HFD-Induced Obese Mice
3.3. Effects of CGWE on Fat Accumulation and Lipolysis in Hepatic Tissue of HFD-Induced Obese Mice
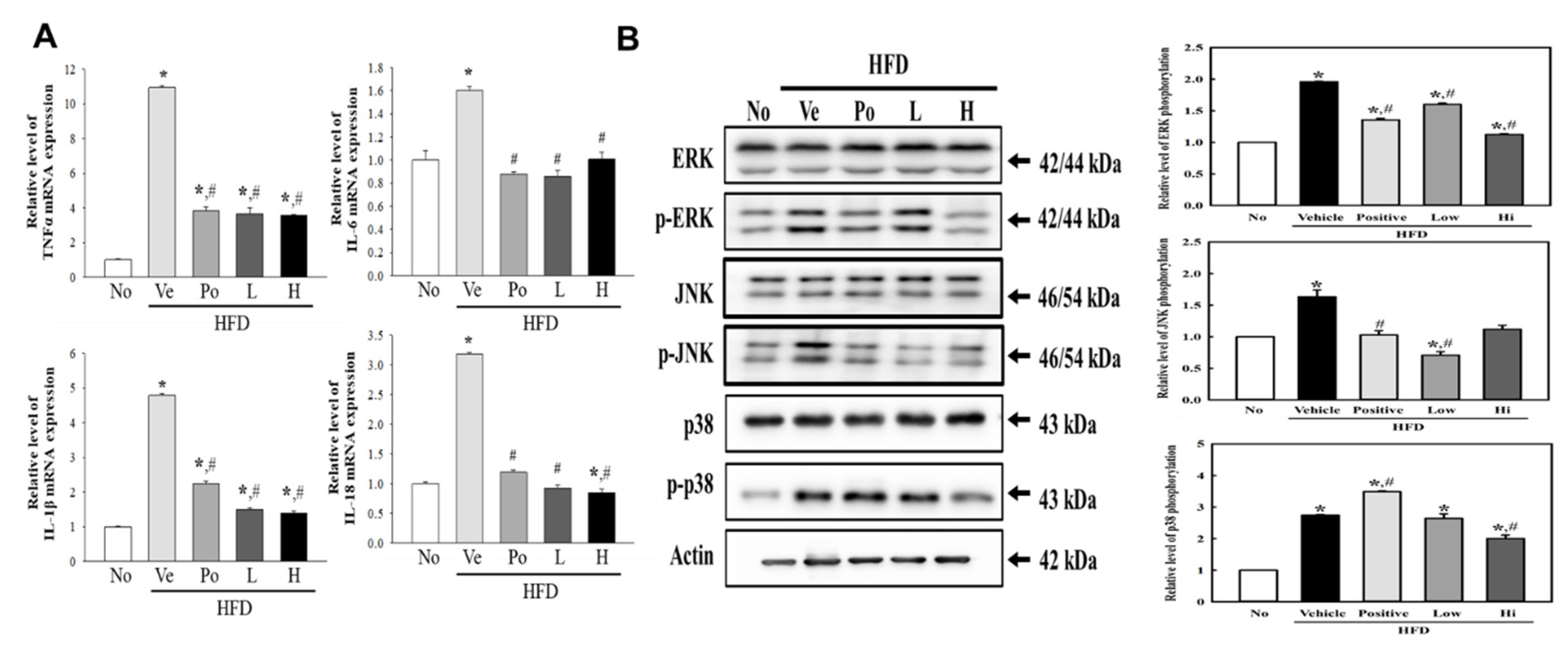
3.4. Attenuation of CGWE on Inflammatory Response Regulation during Hepatic Steatosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aldamarany, W.A.S.; Taocui, H.; Liling, D.; Mei, H.; Yi, Z.; Zhong, G. Perilla, sunflower, and tea seed oils as potential dietary supplements with anti-obesity effects by modulating the gut microbiota composition in mice fed a high-fat diet. Eur. J. Nutr. 2023, 62, 2337–2339. [Google Scholar] [CrossRef] [PubMed]
- Gopal, S.S.; Sukhdeo, S.V.; Vallikannan, B.; Ponesakki, G. Lutein ameliorates high-fat diet-induced obesity, fatty liver, and glucose intolerance in C57BL/6J mice. Phytother. Res. 2023, 37, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Kwon, H.J.; Jung, H.Y.; Lim, S.; Kang, B.; Jo, Y.; Yu, D.; Choi, S.Y.; Hwang, I.K.; Kim, D.W. Extracts from the leaves of Cissus verticillata ameliorate high-fat diet-Induced memory deficits in mice. Plants 2021, 10, 1814. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Ha, M.J.; Shin, M.; Kim, O.Y.; Yoo, E.H.; Song, J.; Chung, J.H. Semen cuscutae administration improves hepatic lipid metabolism and adiposity in high fat diet-induced obese mice. Nutrients 2019, 11, 3035. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Xu, Z.; Li, Y.; Gan, L.; Wu, P.; Wu, R.; Jin, J.; Zheng, X.; Zhang, K.; Ma, H.; et al. Polysaccharides from Callerya speciosa alleviate metabolic disorders and gut microbiota dysbiosis in diet-induced obese C57BL/6 mice. Food Funct. 2022, 13, 8662–8675. [Google Scholar] [CrossRef]
- Yuan, D.; Huang, Q.; Li, C.; Fu, X. A polysaccharide from Sargassum pallidum reduces obesity in high-fat diet-induced obese mice by modulating glycolipid metabolism. Food Funct. 2022, 13, 7181–7191. [Google Scholar] [CrossRef]
- Lan, Y.; Sun, Q.; Ma, Z.; Peng, J.; Zhang, M.; Wang, C.; Zhang, X.; Yan, X.; Chang, L.; Hou, X.; et al. Seabuckthorn polysaccharide ameliorates high-fat diet-induced obesity by gut microbiota-SCFAs-liver axis. Food Funct. 2022, 13, 2925–2937. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Shen, Q.; Wu, J.; Li, J. Oleanolic acid derivative HA-20 inhibits adipogenesis in a manner involving PPARγ-FABP4/aP2 pathway. J. Mol. Endocrinol. 2021, 66, 245–258. [Google Scholar] [CrossRef]
- Han, J.; Kim, M.; Myung, C. Garcinia cambogia improves high-fat diet-induced glucose imbalance by enhancing calcium/CaMKII/AMPK/GLUT4-mediated glucose uptake in skeletal muscle. Mol. Nutr. Food Res. 2022, 66, e2100669. [Google Scholar] [CrossRef]
- Lee, J.; Kim, T.Y.; Kang, H.; Oh, J.; Park, J.W.; Kim, S.; Kim, M.; Apostolidis, E.; Kim, Y.; Kwon, Y. Anti-obesity and anti-adipogenic effects of chitosan oligosaccharide (GO2KA1) in SD rats and in 3T3-L1 preadipocytes models. Molecules 2021, 26, 331. [Google Scholar] [CrossRef]
- Allam, E.A.; Ibrahim, H.F.; Abdulmalek, S.A.; Abdelmeniem, I.M.; Basta, M. Coenzyme Q(10) alleviates testicular endocrine and spermatogenic dysfunction induced by high-fat diet in male Wistar rats: Role of adipokines, oxidative stress and MAPK/ERK/JNK pathway. Andrologia 2022, 54, e14544. [Google Scholar] [CrossRef] [PubMed]
- Inamdar, S.; Joshi, A.; Malik, S.; Boppana, R.; Ghaskadbi, S. Vitexin alleviates non-alcoholic fatty liver disease by activating AMPK in high fat diet fed mice. Biochem. Biophys. Res. Commun. 2019, 519, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liang, C.; Cao, W.; Luo, G.; Zhong, S.; Zeng, Z.; Dai, L.; Song, J. Dietary sinapic acid attenuated high-fat diet-induced lipid metabolism and oxidative stress in male Syrian hamsters. J. Food Biochem. 2022, 46, e14203. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.; Lee, M. Polygonum multiflorum Thunb. hot water extract reverses high-fat diet-induced lipid metabolism of white and brown adipose tissues in obese mice. Plants 2021, 10, 1509. [Google Scholar] [CrossRef]
- Yin, K.; Zhou, X.; Jiang, W.; Wang, L.; Dai, Z.; Tang, B. Jiangzhi ligan decoction inhibits GSDMD-mediated canonical/noncanonical pyroptosis pathways and alleviates high-fat diet-Induced nonalcoholic fatty liver disease. Dis. Markers 2021, 2021, 9963534. [Google Scholar] [CrossRef]
- Sebastian, P.; Schaefer, H.; Telford, I.R.H.; Renner, S.S. Cucumber (Cucumis sativus) and melon (C. melo) have numerous wild relatives in Asia and Australia, and the sister species of melon is from Australia. Proc. Natl. Acad. Sci. USA 2010, 107, 14269–14273. [Google Scholar] [CrossRef]
- Villanueva, M.J.; Tenorio, M.D.; Esteban, M.A.; Mendoza, M.C. Compositional changes during ripening of two cultivars of muskmelon fruits. Food Chem. 2004, 87, 179–185. [Google Scholar] [CrossRef]
- Mallek-Ayadi, S.; Bahloul, N.; Baklouti, S.; Kechaou, N. Bioactive compounds from Cucumis melo L. fruits as potential nutraceutical food ingredients and juice processing using membrane technology. Food Sci. Nutr. 2022, 10, 2922–2934. [Google Scholar] [CrossRef]
- Kim, H.; Kang, Y. Antioxidant activity of ethanol extracts of non-edible parts (stalk, stem. leaf, seed) from oriental melon. Korean J. Plant Resour. 2010, 23, 451–457. [Google Scholar]
- Silva, M.A.; Albuquerque, T.G.; Alves, R.C.; Oliveira, M.B.P.; Costa, H.S. Melon (Cucumis melo L.) by-products: Potential food ingredients for novel functional foods? Trends Food Sci Technol. 2020, 98, 181–189. [Google Scholar] [CrossRef]
- Guo, L.; Park, S.Y.; Kang, H.M.; Kang, N.J.; Hwang, D.Y.; Choi, Y. Edible Vitalmelon fruit extract inhibits adipogenesis and ameliorates high-fat diet-induced obesity. BioMed Res. Int. 2022, 2022, 369650. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Choi, D.; Cha, A.; Lee, Y.; Baek, N.; Rimal, S.; Sang, J.; Lee, Y.; Lee, S. Critical enzymes for biosynthesis of cucurbitacin derivatives in watermelon and their biological significance. Commun. Biol. 2020, 3, 444. [Google Scholar] [CrossRef] [PubMed]
- Aderiye, B.I.; David, O.M.; Fagbohun, E.D.; Faleye, J.; Olajide, O.M. Immunomodulatory and phytomedicinal properties of watermelon juice and pulp (Citrullus lanatus Linn): A review. GSC Biol. Pharm. Sci. 2020, 11, 153–165. [Google Scholar] [CrossRef]
- Maja, D.; Mavengahama, S.; Mashilo, J. Cucurbitacin biosynthesis in cucurbit crops, their pharmaceutical value and agricultural application for management of biotic and abiotic stress: A review. S. Afr. J. Bot. 2022, 145, 3–12. [Google Scholar] [CrossRef]
- Kim, E.A.; Yang, J.; Byeon, E.; Kim, W.; Kang, D.; Han, J.; Hong, S.; Kim, D.; Park, S.; Huh, J.; et al. Anti-obesity effect of pine needle extract on high-fat diet-induced obese mice. Plants 2021, 10, 837. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, H.; Nam, S.; Chu, J.; Kim, J.; Lim, J.; Kim, S.; Sung, M. Protective effects of high-fat diet against murine colitis in association with leptin signaling and gut microbiome. Life 2022, 12, 972. [Google Scholar] [CrossRef]
- Lindfors, S.; Polianskyte-Prause, Z.; Bouslama, R.; Lehtonen, E.; Mannerla, M.; Nisen, H.; Tienari, J.; Salmenkari, H.; Forsgård, R.; Mirtti, T.; et al. Adiponectin receptor agonist AdipoRon ameliorates renal inflammation in diet-induced obese mice and endotoxin-treated human glomeruli ex vivo. Diabetologia 2021, 64, 1866–1879. [Google Scholar] [CrossRef]
- Saber, S.; Abd El-Fattah, E.E.; Yahya, G.; Gobba, N.A.; Maghmomeh, A.O.; Khodir, A.E.; Mourad, A.A.E.; Saad, A.S.; Mohammed, H.G.; Nouh, N.A.; et al. A novel combination therapy using rosuvastatin and lactobacillus combats dextran sodium sulfate-induced colitis in high-fat diet-fed rats by targeting the TXNIP/NLRP3 interaction and influencing gut microbiome composition. Pharmaceuticals 2021, 14, 341. [Google Scholar] [CrossRef]
- Zhang, X.; Nie, Q.; Zhang, Z.; Zhao, J.; Zhang, F.; Wang, C.; Wang, X.; Song, G. Resveratrol affects the expression of uric acid transporter by improving inflammation. Mol. Med. Rep. 2021, 24, 564. [Google Scholar] [CrossRef]
- Ge, C.; Xu, M.; Qin, Y.; Gu, T.; Lou, D.; Li, Q.; Hu, L.; Nie, X.; Wang, M.; Tan, J. Fisetin supplementation prevents high fat diet-induced diabetic nephropathy by repressing insulin resistance and RIP3-regulated inflammation. Food Funct. 2019, 10, 2970–2985. [Google Scholar] [CrossRef]

| Gene | Forward Primer (5′→3′) | Reverse Primer (3′→5′) |
|---|---|---|
| CPT1 | GGCAGAGCAGAGGTTCAAGCT | GCCAGCGCCCGWETCAT |
| PPARα | TGGCAAAAGGCAAGGAGAAG | CCCTCTACATAGAACTGCAAGGTT T |
| PPARγ | GAGTTCATGCTTGTGAAGGATGCAAGG | CATACTCTGTGATCTCTTGCACGWE |
| FAS | GATCCTGGAACGWEAGAACACGWEATCTGG | AGACTGTGGAACACGWEGTGGTGGAACC |
| C/EBPα | GTGGACAAGAACAGCAACGAGTAC | GGAATCTCCTAGTCCTGGCTTGC |
| aP2 | GAACCTGGAAGCTTGTCTCCAGTG | GATGCTCTTCACCTTCCTGTCGTCTGC |
| TNF-α | CCTGTAGCCCACGTCGTA | TTGACCTCAGCGWECTGACTTG |
| IL-1β | AGGCTTCCTTGTGCAAGTGT | TGAGTGACACTGCCTTCCTG |
| IL-6 | CTCTCTGCAAGAGAGTTCCATCCAG | GCTATGGTACTCCAGAAGACCAGAGG |
| IL-18 | GTACAAAGACAGTGAAGTAAGAGGACTG | CTCCATCTTGTTGTGTCCTGGAACACGWE |
| β-actin | ACGWEGCCAGGTCATCACTATT | CAAGAAGGAAGGCTGGAAAAGA |
| Food Intake (g·day−1) | |||||||
|---|---|---|---|---|---|---|---|
| Group | 7 | 14 | 21 | 28 | 35 | 42 | 49 |
| No | 64.3 ± 5.8 | 63.2 ± 4.7 | 59.7 ± 3.8 | 68.5 ± 5.1 | 60.7 ± 5.0 | 67.2 ± 1.8 | 63.0 ± 2.3 |
| Ve | 63.0 ± 5.4 | 55.2 ± 6.1 | 63.0 ±4.3 | 57.8 ± 2.2 | 57.8 ± 1.2 | 56.5 ± 2.2 | 58.2 ± 1.7 |
| Po | 68.8 ± 5.5 | 62.2 ± 5.2 | 63.0 ± 3.8 | 59.7 ± 4.6 | 59.8 ± 4.5 | 63.5 ± 2.4 | 61.3 ± 3.0 |
| L | 63.3 ± 3.1 | 51.7 ± 3.9 | 5608 ± 3.0 | 57.3 ± 3.4 | 54.7 ± 2.8 | 50.5 ± 3.7 | 48.2 ± 3.4 |
| H | 62.8 ± 2.8 | 50.5 ± 2.0 | 60.7 ± 3.4 | 54.4 ± 2.9 | 53.5 ± 2.6 | 53.0 ± 4.4 | 52.3 ± 3.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.Y.; Kim, J.E.; Kang, H.M.; Song, H.J.; Kang, N.J.; Hwang, D.Y.; Choi, Y.-W. Adiposity Reduction by Cucumis melo var. gaettongchamoe Extract in High-Fat Diet-Induced Obese Mice. Nutrients 2023, 15, 3292. https://doi.org/10.3390/nu15153292
Park SY, Kim JE, Kang HM, Song HJ, Kang NJ, Hwang DY, Choi Y-W. Adiposity Reduction by Cucumis melo var. gaettongchamoe Extract in High-Fat Diet-Induced Obese Mice. Nutrients. 2023; 15(15):3292. https://doi.org/10.3390/nu15153292
Chicago/Turabian StylePark, Sun Young, Ji Eun Kim, He Mi Kang, Hee Jin Song, Nam Jun Kang, Dae Youn Hwang, and Young-Whan Choi. 2023. "Adiposity Reduction by Cucumis melo var. gaettongchamoe Extract in High-Fat Diet-Induced Obese Mice" Nutrients 15, no. 15: 3292. https://doi.org/10.3390/nu15153292
APA StylePark, S. Y., Kim, J. E., Kang, H. M., Song, H. J., Kang, N. J., Hwang, D. Y., & Choi, Y.-W. (2023). Adiposity Reduction by Cucumis melo var. gaettongchamoe Extract in High-Fat Diet-Induced Obese Mice. Nutrients, 15(15), 3292. https://doi.org/10.3390/nu15153292








