Hydroethanolic Extract of Fritillariae thunbergii Bulbus Alleviates Dextran Sulfate Sodium-Induced Ulcerative Colitis by Enhancing Intestinal Barrier Integrity
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of FTB
2.3. Identification of Phytochemicals Using Ultra-High Performance Liquid Chromatography-Tandem Mass Spectroscopy (UPLC-MS/MS)
2.4. Animal Study
2.4.1. Animals
2.4.2. Animal Group and Administrations
2.4.3. Colitis Severity Evaluation and Histopathological Analysis
2.4.4. Immunohistochemistry (IHC)
2.4.5. Real-Time PCR
2.5. In Vitro Study
2.5.1. Cell Culture and Viability
2.5.2. Measurement of Transepithelial Electrical Resistance (TEER) and Epithelial Paracellular Permeability
2.5.3. Western Blot Analysis
2.6. Statistical Analysis
3. Results
3.1. Nine Phytochemicals of FTB Were Identified
3.2. FTB Improves Clinical Symptoms of DSS-Induced Ulcerative Colitis
3.3. FTB Improves Clinical Symptoms of DSS-Induced Ulcerative Colitis and Reduces Inflammation in the Colon Tissue of DSS-Induced Ulcerative Colitis
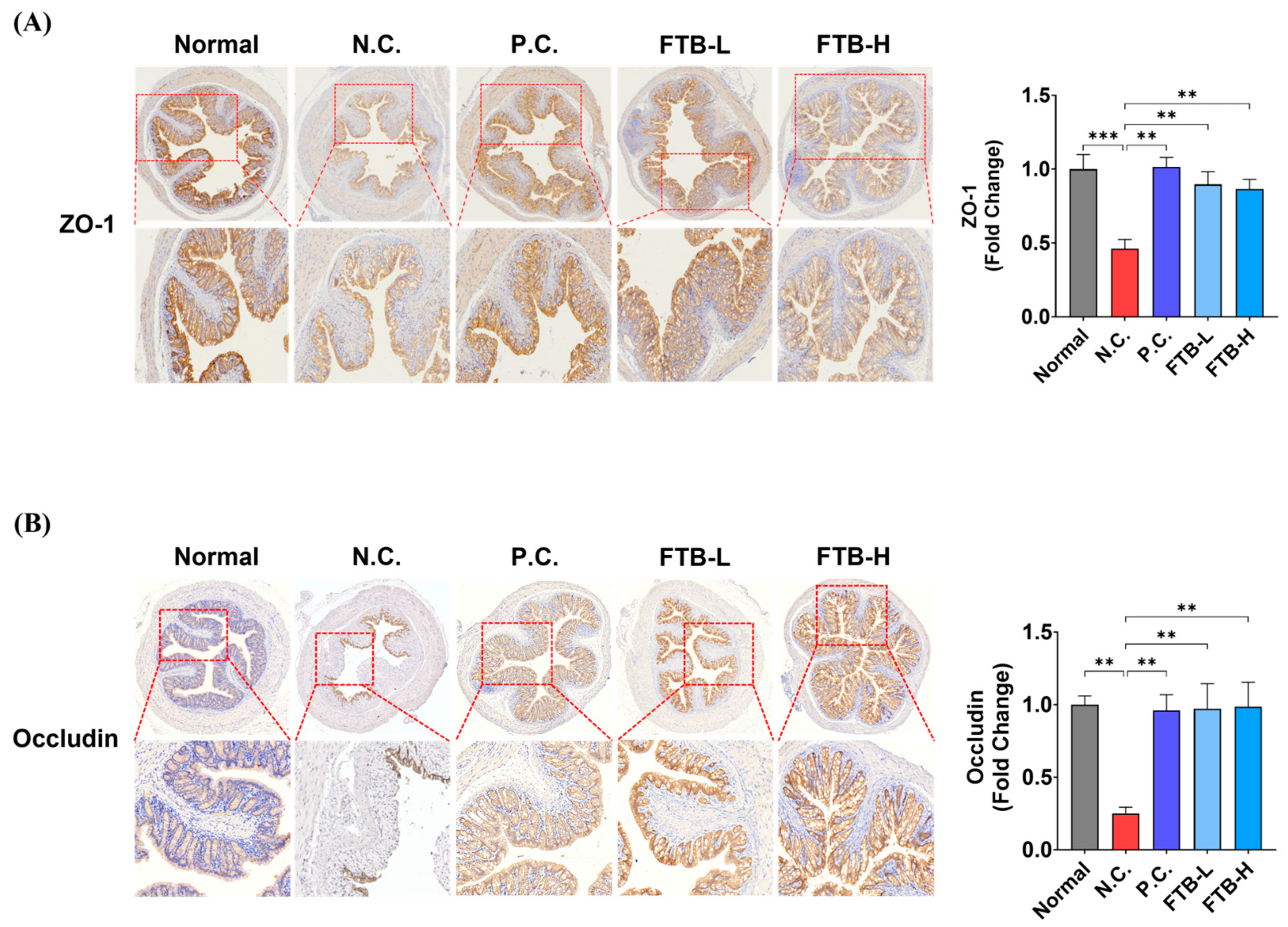
3.4. FTB Prevents the Loss of Tight Junction Protein in the Colon of DSS-Induced Ulcerative Colitis Mice
3.5. FTB Inhibits Fibrosis by Delaying Extracellular Matrix (ECM) Remodeling and the Collapse of Intestinal Mucosal Layers in the Colon Tissue of DSS-Induced Ulcerative Colitis
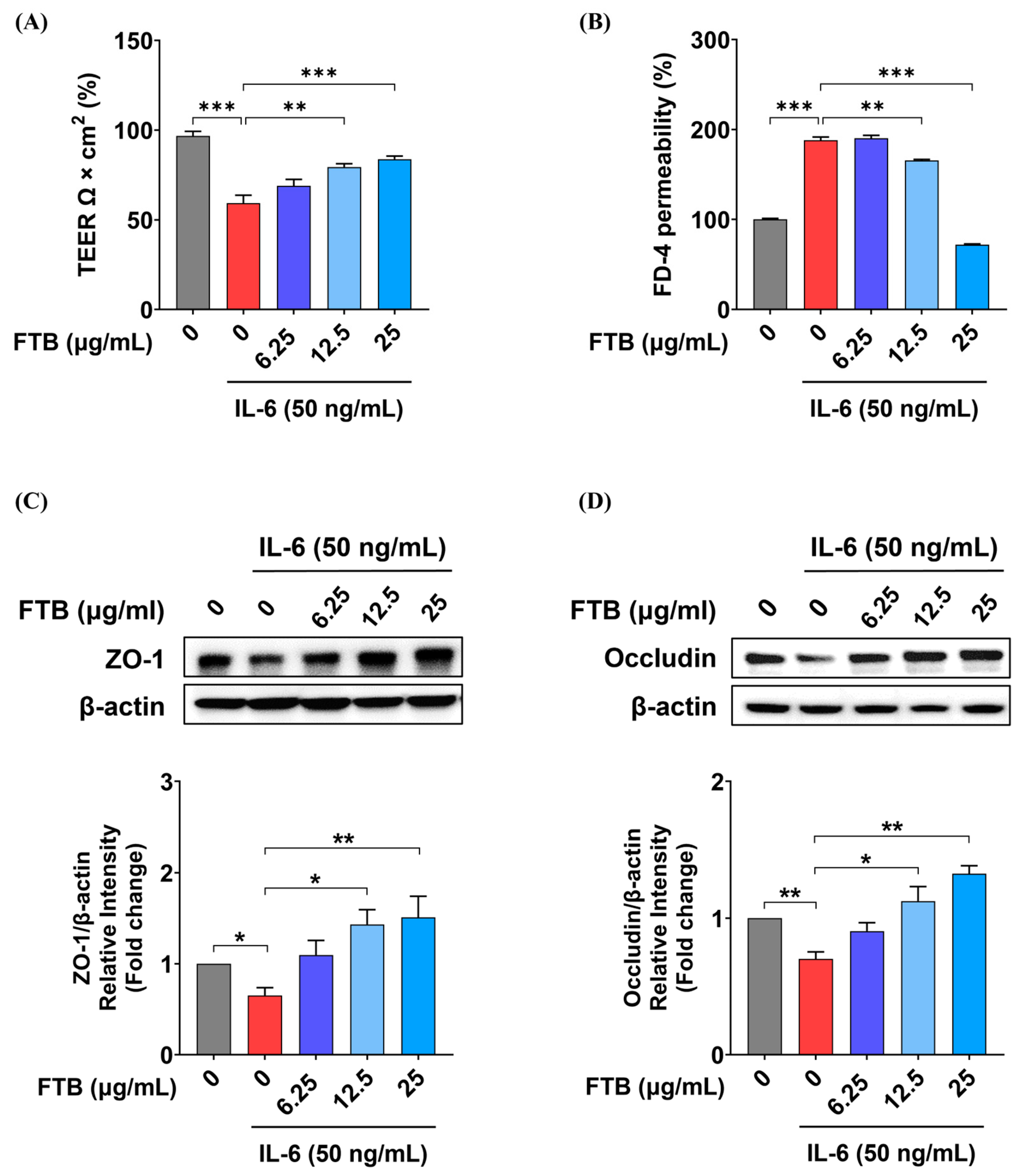
3.6. Impact of FTB on Intestinal Barrier Function in a Barrier Model In Vitro
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Wang, X.; Chen, Q.; Luo, L.; Ma, M.; Xiao, B.; Zeng, L. Camellia sinensis and Litsea coreana Ameliorate Intestinal Inflammation and Modulate Gut Microbiota in Dextran Sulfate Sodium-Induced Colitis Mice. Mol. Nutr. Food Res. 2020, 64, e1900943. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, S.; Li, J. Treatment of Inflammatory Bowel Disease: A Comprehensive Review. Front. Med. 2021, 8, 765474. [Google Scholar] [CrossRef]
- de Souza, H.S.; Fiocchi, C. Immunopathogenesis of IBD: Current state of the art. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 13–27. [Google Scholar] [CrossRef]
- Head, K.A.; Jurenka, J.S. Inflammatory bowel disease Part 1: Ulcerative colitis--pathophysiology and conventional and alternative treatment options. Altern. Med. Rev. 2003, 8, 247–283. [Google Scholar]
- Antoni, L.; Nuding, S.; Wehkamp, J.; Stange, E.F. Intestinal barrier in inflammatory bowel disease. World J. Gastroenterol. 2014, 20, 1165–1179. [Google Scholar] [CrossRef]
- Kayal, M.; Shah, S. Ulcerative Colitis: Current and Emerging Treatment Strategies. J. Clin. Med. 2019, 9, 94. [Google Scholar] [CrossRef]
- Luissint, A.C.; Parkos, C.A.; Nusrat, A. Inflammation and the Intestinal Barrier: Leukocyte-Epithelial Cell Interactions, Cell Junction Remodeling, and Mucosal Repair. Gastroenterology 2016, 151, 616–632. [Google Scholar] [CrossRef]
- Shah, S.C.; Colombel, J.-F.; Sands, B.E.; Narula, N. Mucosal Healing Is Associated With Improved Long-term Outcomes of Patients With Ulcerative Colitis: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2016, 14, 1245–1255.e1248. [Google Scholar] [CrossRef] [PubMed]
- Odenwald, M.A.; Turner, J.R. The intestinal epithelial barrier: A therapeutic target? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 9–21. [Google Scholar] [CrossRef]
- Li, H.; Hung, A.; Li, M.; Yang, A.W.H. Fritillariae thunbergii Bulbus: Traditional Uses, Phytochemistry, Pharmacodynamics, Pharmacokinetics and Toxicity. Int. J. Mol. Sci. 2019, 20, 1667. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.B.; Chen, J.; Zhang, Q.Y.; Shi, Y.H.; Lin, L.; Zheng, H.Y.; Adams, M.J.; Chen, J.P. A new potyvirus from Thunberg fritillary (Fritillaria thunbergii Miq.) in Zhejiang, China. Arch. Virol. 2005, 150, 1271–1280. [Google Scholar] [CrossRef]
- Tu, S.; Qin, C.; Chen, C. Fritillaria thumbergiia new host of Potato leafroll virus in China. Australas. Plant Dis. Notes 2006, 1, 31–32. [Google Scholar] [CrossRef]
- Nile, S.H.; Su, J.; Wu, D.; Wang, L.; Hu, J.; Sieniawska, E.; Kai, G. Fritillaria thunbergii Miq. (Zhe Beimu): A review on its traditional uses, phytochemical profile and pharmacological properties. Food Chem. Toxicol. 2021, 153, 112289. [Google Scholar] [CrossRef] [PubMed]
- Rashid, I.; Yaqoob, U. Traditional uses, phytochemistry and pharmacology of genus Fritillaria—A review. Bull. Natl. Res. Cent. 2021, 45, 124. [Google Scholar] [CrossRef]
- Orhan, I.; Kartal, M.; Abu-Asaker, M.; Şenol, F.S.; Yilmaz, G.; Şener, B. Free radical scavenging properties and phenolic characterization of some edible plants. Food Chem. 2009, 114, 276–281. [Google Scholar] [CrossRef]
- Kim, E.-Y.; Hong, S.; Kim, J.-H.; Kim, M.; Lee, Y.; Sohn, Y.; Jung, H.-S. Effects of chloroform fraction of Fritillariae thunbergii Bulbus on atopic symptoms in a DNCB-induced atopic dermatitis-like skin lesion model and in vitro models. J. Ethnopharmacol. 2021, 281, 114453. [Google Scholar] [CrossRef]
- Meng, Y.; Wang, S.; Cai, R.; Jiang, B.; Zhao, W. Discrimination and Content Analysis of Fritillaria Using Near Infrared Spectroscopy. J. Anal. Methods Chem. 2015, 2015, 752162. [Google Scholar] [CrossRef]
- Li, H.-J.; Jiang, Y.; Li, P. Chemistry, bioactivity and geographical diversity of steroidal alkaloids from the Liliaceae family. Nat. Prod. Rep. 2006, 23, 735–752. [Google Scholar] [CrossRef]
- Kim, J.H.; Yang, W.K.; Lee, S.W.; Lyu, Y.R.; Kim, S.H.; Park, Y.C. Experimental Study on Anti-inflammatory, Antitussive, and Expectoration Effects of Friltillariae thunbergii Bulbus. J. Int. Korean Med. 2020, 41, 339–349. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, W.; Pan, L.; Zhang, A.; Chen, Q.; Xu, K.; Lu, H.; Chen, Y. Peimine, a main active ingredient of Fritillaria, exhibits anti-inflammatory and pain suppression properties at the cellular level. Fitoterapia 2016, 111, 1–6. [Google Scholar] [CrossRef]
- Shim, K.S.; Gu, D.R.; Hwang, Y.H.; Yang, H.; Ryuk, J.A.; Ha, H. Water Extract of Fritillariae thunbergii Bulbus Inhibits RANKL-Mediated Osteoclastogenesis and Ovariectomy-Induced Trabecular Bone Loss. Molecules 2021, 27, 169. [Google Scholar] [CrossRef]
- Shi, Y.H.; Huang, Q.W.; Zhu, S.M.; Zhou, Y.M.; Zhang, L.J.; Huang, W.K.; Shao, J.J.; Zhou, J.L.; Zhang, W.T. Chemical profiling of Fritillariae thunbergii Miq prepared by different processing methods reveals two new quality markers: Zhebeininoside and imperialine-3-beta-D-glucoside. J. Ethnopharmacol. 2022, 283, 114670. [Google Scholar] [CrossRef] [PubMed]
- Dieleman, L.A.; Palmen, M.J.; Akol, H.; Bloemena, E.; Peña, A.S.; Meuwissen, S.G.; Van Rees, E.P. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin. Exp. Immunol. 1998, 114, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Cooper, H.S.; Murthy, S.N.; Shah, R.S.; Sedergran, D.J. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab. Investig. 1993, 69, 238–249. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-Y.; Oh, T.-W.; Do, H.J.; Yang, J.-H.; Yang, I.J.; Jeon, Y.H.; Go, Y.-H.; Ahn, S.-C.; Ma, J.-Y.; Park, K.-I. Acer palmatum thumb. Ethanol Extract Alleviates Interleukin-6-Induced Barrier Dysfunction and Dextran Sodium Sulfate-Induced Colitis by Improving Intestinal Barrier Function and Reducing Inflammation. J. Immunol. Res. 2018, 2018, 5718396. [Google Scholar] [CrossRef]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying Small Molecules via High Resolution Mass Spectrometry: Communicating Confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef]
- Langhorst, J.; Wulfert, H.; Lauche, R.; Klose, P.; Cramer, H.; Dobos, G.J.; Korzenik, J. Systematic Review of Complementary and Alternative Medicine Treatments in Inflammatory Bowel Diseases. J. Crohn’s Colitis 2014, 9, 86–106. [Google Scholar] [CrossRef]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 2014, 104, 15.25.11–25.25.14. [Google Scholar] [CrossRef]
- Li, Y.H.; Zhang, M.; Xiao, H.T.; Fu, H.B.; Ho, A.; Lin, C.Y.; Huang, Y.; Lin, G.; Bian, Z.X. Addition of Berberine to 5-Aminosalicylic Acid for Treatment of Dextran Sulfate Sodium-Induced Chronic Colitis in C57BL/6 Mice. PLoS ONE 2015, 10, e0144101. [Google Scholar] [CrossRef]
- Melgar, S.; Karlsson, A.; Michaëlsson, E. Acute colitis induced by dextran sulfate sodium progresses to chronicity in C57BL/6 but not in BALB/c mice: Correlation between symptoms and inflammation. Am. J. Physiol.-Gastrointest. Liver Physiol. 2005, 288, G1328–G1338. [Google Scholar] [CrossRef]
- Guimbaud, R.; Bertrand, V.; Chauvelot-Moachon, L.; Quartier, G.; Vidon, N.; Giroud, J.P.; Couturier, D.; Chaussade, S. Network of inflammatory cytokines and correlation with disease activity in ulcerative colitis. Am. J. Gastroenterol. 1998, 93, 2397–2404. [Google Scholar] [CrossRef] [PubMed]
- Podolsky, D.K. Inflammatory bowel disease. N. Engl. J. Med. 2002, 347, 417–429. [Google Scholar] [CrossRef]
- Panwar, S.; Sharma, S.; Tripathi, P. Role of Barrier Integrity and Dysfunctions in Maintaining the Healthy Gut and Their Health Outcomes. Front. Physiol. 2021, 12, 5611. [Google Scholar] [CrossRef]
- Ding, L.; Lu, Z.; Lu, Q.; Chen, Y.H. The claudin family of proteins in human malignancy: A clinical perspective. Cancer Manag. Res. 2013, 5, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Tsukita, S.; Furuse, M.; Itoh, M. Multifunctional strands in tight junctions. Nat. Rev. Mol. Cell Biol. 2001, 2, 285–293. [Google Scholar] [CrossRef]
- Liang, L.; Xiong, Q.; Kong, J.; Tian, C.; Miao, L.; Zhang, X.; Du, H. Intraperitoneal supplementation of iron alleviates dextran sodium sulfate-induced colitis by enhancing intestinal barrier function. Biomed. Pharmacother. 2021, 144, 112253. [Google Scholar] [CrossRef]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER Measurement Techniques for In Vitro Barrier Model Systems. Jala-J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Derkacz, A.; Olczyk, P.; Olczyk, K.; Komosinska-Vassev, K. The Role of Extracellular Matrix Components in Inflammatory Bowel Diseases. J. Clin. Med. 2021, 10, 1122. [Google Scholar] [CrossRef] [PubMed]
- Shimshoni, E.; Yablecovitch, D.; Baram, L.; Dotan, I.; Sagi, I. ECM remodelling in IBD: Innocent bystander or partner in crime? The emerging role of extracellular molecular events in sustaining intestinal inflammation. Gut 2015, 64, 367–372. [Google Scholar] [CrossRef]
- Xiao, Y.; Lian, H.; Zhong, X.S.; Krishnachaitanya, S.S.; Cong, Y.; Dashwood, R.H.; Savidge, T.C.; Powell, D.W.; Liu, X.; Li, Q. Matrix metalloproteinase 7 contributes to intestinal barrier dysfunction by degrading tight junction protein Claudin-7. Front. Immunol. 2022, 13, 1020902. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.E.; Henricks, P.A.J.; Roda, M.A.; Verspaget, H.W.; Wolfkamp, S.C.; te Velde, A.A.; Jones, C.W.; Wolfkamp, S.C.; Velde, A.A.T.; Jones, C.W.; et al. Collagen degradation and neutrophilic infiltration: A vicious circle in inflammatory bowel disease. Gut 2014, 63, 578. [Google Scholar] [CrossRef]
- Rogler, G.; Hausmann, M. Factors Promoting Development of Fibrosis in Crohn’s Disease. Front. Med. 2017, 4, 96. [Google Scholar] [CrossRef]
- van Haaften, W.T.; Blokzijl, T.; Hofker, H.S.; Olinga, P.; Dijkstra, G.; Bank, R.A.; Boersema, M. Intestinal stenosis in Crohn’s disease shows a generalized upregulation of genes involved in collagen metabolism and recognition that could serve as novel anti-fibrotic drug targets. Ther. Adv. Gastroenterol. 2020, 13, 1756284820952578. [Google Scholar] [CrossRef]
- Louis, E.; Ribbens, C.; Godon, A.; Franchimont, D.; De Groote, D.; Hardy, N.; Boniver, J.; Belaiche, J.; Malaise, M. Increased production of matrix metalloproteinase-3 and tissue inhibitor of metalloproteinase-1 by inflamed mucosa in inflammatory bowel disease. Clin. Exp. Immunol. 2001, 120, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Pelaseyed, T.; Bergström, J.H.; Gustafsson, J.K.; Ermund, A.; Birchenough, G.M.; Schütte, A.; van der Post, S.; Svensson, F.; Rodríguez-Piñeiro, A.M.; Nyström, E.E.L.; et al. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol. Rev. 2014, 260, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Taupin, D.; Podolsky, D.K. Trefoil factors: Initiators of mucosal healing. Nat. Rev. Mol. Cell Biol. 2003, 4, 721–732. [Google Scholar] [CrossRef]
- Katz, J.P.; Perreault, N.; Goldstein, B.G.; Lee, C.S.; Labosky, P.A.; Yang, V.W.; Kaestner, K.H. The zinc-finger transcription factor Klf4 is required for terminal differentiation of goblet cells in the colon. Development 2002, 129, 2619–2628. [Google Scholar] [CrossRef]
- Ghaleb, A.M.; Yang, V.W. Krüppel-like factor 4 (KLF4): What we currently know. Gene 2017, 611, 27–37. [Google Scholar] [CrossRef]
- Michaudel, C.; Danne Agus, A.; Magniez, A.; Aucouturier, A.; Spatz, M.; Lefevre, A.; Kirchgesner, J.; Rolhion, N.; Wang, Y.Z.; Lavelle, A.; et al. Rewiring the altered tryptophan metabolism as a novel therapeutic strategy in inflammatory bowel diseases. Gut 2022, 72, 1296–1307. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, Z.; Zhao, S.; Huang, D.; Wang, X.; Wu, Y.; Pei, C.; Shi, S.; Jia, N.; He, Y.; et al. Sipeimine ameliorates PM2.5-induced lung injury by inhibiting ferroptosis via the PI3K/Akt/Nrf2 pathway: A network pharmacology approach. Ecotoxicol. Environ. Saf. 2022, 239, 113615. [Google Scholar] [CrossRef]
- Huang, D.; Shen, Z.; Zhao, S.; Pei, C.; Jia, N.; Wang, Y.; Wu, Y.; Wang, X.; Shi, S.; He, Y.; et al. Wang, Sipeimine attenuates PM2.5-induced lung toxicity via suppression of NLRP3 inflammasome-mediated pyroptosis through activation of the PI3K/AKT pathway. Chem. -Biol. Interact. 2023, 376, 110448. [Google Scholar] [CrossRef]
- Liu, S.; Yang, T.; Ming, T.W.; Gaun, T.K.W.; Zhou, T.; Wang, S.; Ye, B. Isosteroid alkaloids with different chemical structures from Fritillariae cirrhosae bulbus alleviate LPS-induced inflammatory response in RAW 264.7 cells by MAPK signaling pathway. Int. Immunopharmacol. 2020, 78, 106047. [Google Scholar] [CrossRef]
- Lu, Q.X.; Li, R.; Yang, Y.X.; Zhang, Y.J.; Zhao, Q.; Li, J. Ingredients with anti-inflammatory effect from medicine food homology plants. Food Chem. 2022, 368, 130610. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Jiang, Y.; Wu, K.; Wang, S.; Wang, Y. Evaluation of antitumor property of extracts and steroidal alkaloids from the cultivated Bulbus Fritillariae ussuriensis and preliminary investigation of its mechanism of action. BMC Complement. Altern. Med. 2015, 15, 29. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhu, J.; Wang, S.; Wang, X.; Ou, Y.; Wei, D.; Li, X. Antitussive, expectorant and anti-inflammatory alkaloids from Bulbus Fritillariae cirrhosae. Fitoterapia 2011, 82, 1290–1294. [Google Scholar] [CrossRef]
- Lee, A.; Yang, H.; Kim, T.; Ha, H.; Hwang, Y.-H. Identification and pharmacokinetics of bioavailable anti-resorptive phytochemicals after oral administration of Psoralea corylifolia L. Biomed. Pharmacother. 2021, 144, 112300. [Google Scholar] [CrossRef]



| No. | Theoretical m/z | Measured m/z | Error (ppm) | Adduct | Rt (min) | Formula | Fragments (m/z) | Identifications |
|---|---|---|---|---|---|---|---|---|
| 1 | 206.0448 | 206.0447 | −0.496 | [M+H]+ | 4.78 | C10H7NO4 | 206, 178 | Xanthurenic Acid * |
| 2 | 444.3108 | 444.3102 | −1.405 | [M+H]+ | 6.48 | C27H41NO4 | 426, 114 | Yibeissine [21] |
| 3 | 594.4000 | 594.3992 | −1.370 | [M+H]+ | 7.08 | C33H55NO8 | 576 | Peiminoside [21] |
| 4 | 592.3844 | 592.3838 | −0.834 | [M+H]+ | 7.17 | C33H53NO8 | 574 | Sipeimine glucoside [22] |
| 5 | 430.3316 | 430.3312 | −0.914 | [M+H]+ | 7.27 | C27H43NO3 | 430 | Sipeimine * |
| 6 | 428.3159 | 428.3155 | −0.931 | [M+H]+ | 7.5 | C27H41NO3 | 428 | Peimisine * |
| 7 | 432.3472 | 432.3466 | −1.320 | [M+H]+ | 7.77 | C27H45NO3 | 432, 414 | Peimine * |
| 8 | 430.3316 | 430.3310 | −1.410 | [M+H]+ | 8.19 | C27H43NO3 | 430, 412 | Peiminine * |
| 9 | 398.3417 | 398.3412 | −1.470 | [M+H]+ | 12.15 | C27H43NO | 398 | Solanidine * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, A.; Chung, Y.C.; Kim, K.-Y.; Jang, C.H.; Song, K.H.; Hwang, Y.-H. Hydroethanolic Extract of Fritillariae thunbergii Bulbus Alleviates Dextran Sulfate Sodium-Induced Ulcerative Colitis by Enhancing Intestinal Barrier Integrity. Nutrients 2023, 15, 2810. https://doi.org/10.3390/nu15122810
Lee A, Chung YC, Kim K-Y, Jang CH, Song KH, Hwang Y-H. Hydroethanolic Extract of Fritillariae thunbergii Bulbus Alleviates Dextran Sulfate Sodium-Induced Ulcerative Colitis by Enhancing Intestinal Barrier Integrity. Nutrients. 2023; 15(12):2810. https://doi.org/10.3390/nu15122810
Chicago/Turabian StyleLee, Ami, You Chul Chung, Kwang-Youn Kim, Chan Ho Jang, Kwang Hoon Song, and Youn-Hwan Hwang. 2023. "Hydroethanolic Extract of Fritillariae thunbergii Bulbus Alleviates Dextran Sulfate Sodium-Induced Ulcerative Colitis by Enhancing Intestinal Barrier Integrity" Nutrients 15, no. 12: 2810. https://doi.org/10.3390/nu15122810
APA StyleLee, A., Chung, Y. C., Kim, K.-Y., Jang, C. H., Song, K. H., & Hwang, Y.-H. (2023). Hydroethanolic Extract of Fritillariae thunbergii Bulbus Alleviates Dextran Sulfate Sodium-Induced Ulcerative Colitis by Enhancing Intestinal Barrier Integrity. Nutrients, 15(12), 2810. https://doi.org/10.3390/nu15122810






