Association between Vitamin A and E Forms and Prostate Cancer Risk in the Singapore Prostate Cancer Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Ethical Considerations
2.3. Serum Measurements
2.4. Questionnaire Measurement
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Cases and Controls
3.2. Serum Vitamin A and E in Cases and Controls
3.3. Associations of Individual Serum Vitamin A and E with Prostate Cancer Risk
3.4. Smoking and Alcohol Consumption Status as Effect Modifiers
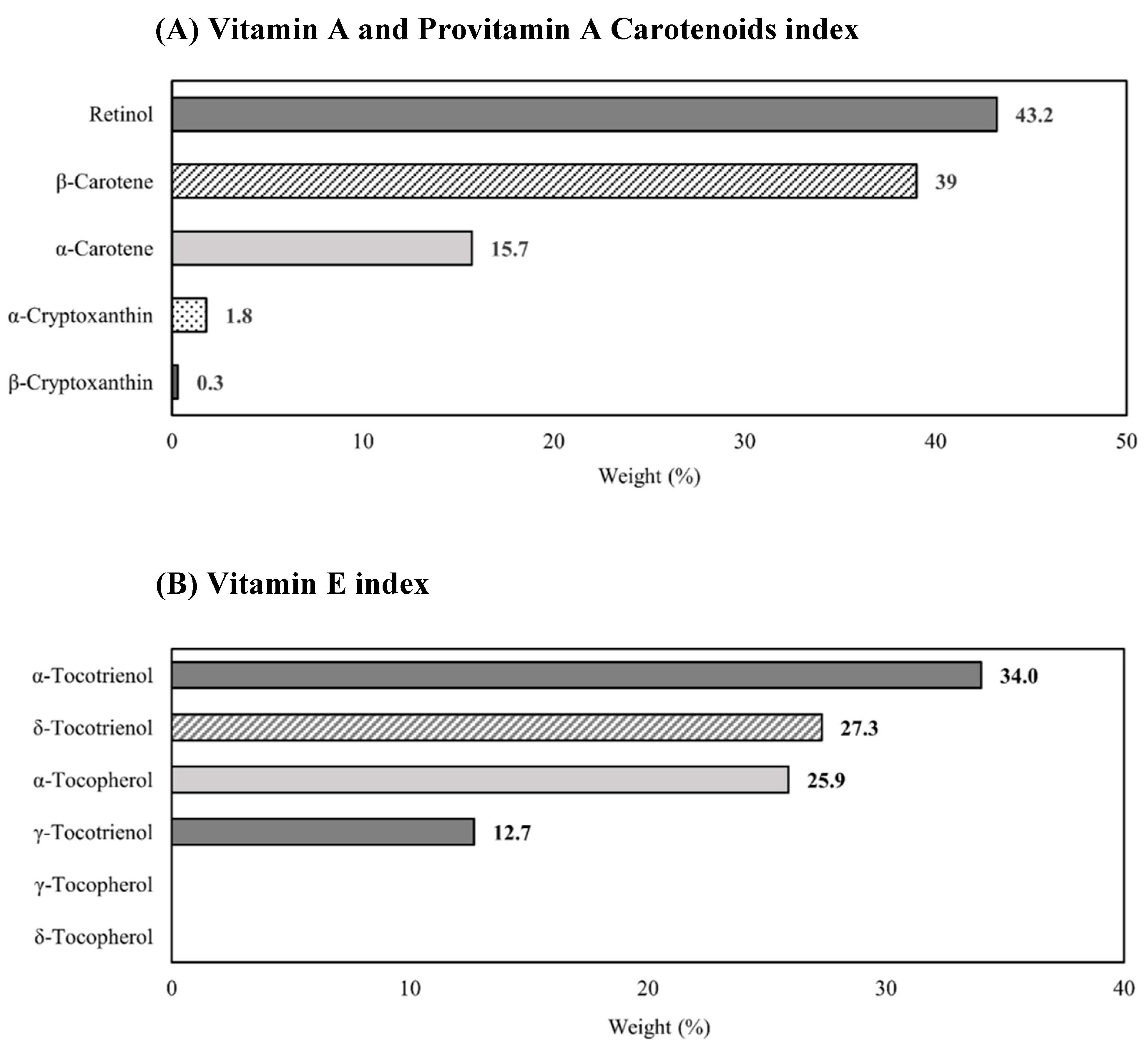
3.5. Mixture Analysis with Multiple Vitamin A and E
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef]
- Attard, G.; Parker, C.; Eeles, R.A.; Schröder, F.; Tomlins, S.A.; Tannock, I.; Drake, C.G.; de Bono, J.S. Prostate cancer. Lancet 2016, 387, 70–82. [Google Scholar] [CrossRef]
- Kimura, T.; Egawa, S. Epidemiology of prostate cancer in Asian countries. Int. J. Neurol. 2018, 25, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Demissie, K.; Lu, S.E.; Rhoads, G.G. Cancer Incidence Among Korean-American Immigrants in the United States and Native Koreans in South Korea. Cancer Control. 2007, 14, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.W.; Ritchey, J.; Devesa, S.S.; Quraishi, S.M.; Zhang, H.; Hsing, A.W. Prostate cancer incidence rates in Africa. Prostate Cancer 2011, 2011, 947870. [Google Scholar] [CrossRef] [PubMed]
- Shenkin, A. Micronutrients in health and disease. Postgrad. Med. J. 2006, 82, 559–567. [Google Scholar] [CrossRef]
- Dawson, M. The Importance of Vitamin A in Nutrition. Curr. Pharm. Des. 2000, 6, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Mamede, A.C.; Tavares, S.D.; Abrantes, A.M.; Trindade, J.; Maia, J.M.; Botelho, M.F. The role of vitamins in cancer: A review. Nutr. Cancer 2011, 63, 479–494. [Google Scholar] [CrossRef] [PubMed]
- Eggersdorfer, M.; Wyss, A. Carotenoids in human nutrition and health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef]
- Willis, M.S.; Wians, F.H. The role of nutrition in preventing prostate cancer: A review of the proposed mechanism of action of various dietary substances. Clin. Chim. Acta 2003, 330, 57–83. [Google Scholar] [CrossRef]
- Key, T.J.; Appleby, P.N.; Travis, R.C.; Albanes, D.; Alberg, A.J.; Barricarte, A.; Black, A.; Boeing, H.; Bueno-de-Mesquita, H.B.; Chan, J.M.; et al. Carotenoids, retinol, tocopherols, and prostate cancer risk: Pooled analysis of 15 studies. Am. J. Clin. Nutr. 2015, 102, 1142–1157. [Google Scholar] [CrossRef]
- Rowles, J.L., 3rd; Ranard, K.M.; Smith, J.W.; An, R.; Erdman, J.W., Jr. Increased dietary and circulating lycopene are associated with reduced prostate cancer risk: A systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2017, 20, 361–377. [Google Scholar] [CrossRef] [PubMed]
- Cui, R.; Liu, Z.Q.; Xu, Q. Blood alpha-tocopherol, gamma-tocopherol levels and risk of prostate cancer: A meta-analysis of prospective studies. PLoS ONE 2014, 9, e93044. [Google Scholar] [CrossRef]
- Watters, J.L.; Gail, M.H.; Weinstein, S.J.; Virtamo, J.; Albanes, D. Associations between alpha-tocopherol, beta-carotene, and retinol and prostate cancer survival. Cancer Res. 2009, 69, 3833–3841. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.Y.; Alberg, A.J.; Norkus, E.P.; Hoffman, S.C.; Comstock, G.W.; Helzlsouer, K.J. Prospective study of antioxidant micronutrients in the blood and the risk of developing prostate cancer. Am. J. Epidemiol. 2003, 157, 335–344. [Google Scholar] [CrossRef]
- Gill, J.K.; Franke, A.A.; Steven Morris, J.; Cooney, R.V.; Wilkens, L.R.; Le Marchand, L.; Goodman, M.T.; Henderson, B.E.; Kolonel, L.N. Association of selenium, tocopherols, carotenoids, retinol, and 15-isoprostane F(2t) in serum or urine with prostate cancer risk: The multiethnic cohort. Cancer Causes Control. 2009, 20, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Chia, S.E.; Wong, K.Y.; Cheng, C.; Lau, W.; Tan, P.H. Sun exposure and the risk of prostate cancer in the Singapore Prostate Cancer Study: A case-control study. Asian Pac. J. Cancer Prev. 2012, 13, 3179–3185. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.L.; Ong, C.N. Comprehensive high-performance liquid chromatographic method for the measurements of lipophilic antioxidants in human plasma. J. Chromatogr. A 2009, 1216, 3131–3137. [Google Scholar] [CrossRef]
- Energy & Nutrient Composition of Food. Available online: https://focos.hpb.gov.sg/eservices/ENCF/ (accessed on 24 August 2022).
- Bostwick, D.G.; Burke, H.B.; Djakiew, D.; Euling, S.; Ho, S.M.; Landolph, J.; Morrison, H.; Sonawane, B.; Shifflett, T.; Waters, D.J.; et al. Human prostate cancer risk factors. Cancer 2004, 101, 2371–2490. [Google Scholar] [CrossRef]
- Wheeler, D.C.; Rustom, S.; Carli, M.; Whitehead, T.P.; Ward, M.H.; Metayer, C. Assessment of Grouped Weighted Quantile Sum Regression for Modeling Chemical Mixtures and Cancer Risk. Int. J. Environ. Res. Public Health 2021, 18, 504. [Google Scholar] [CrossRef] [PubMed]
- Hada, M.; Mondul, A.M.; Weinstein, S.J.; Albanes, D. Serum Retinol and Risk of Overall and Site-Specific Cancer in the ATBC Study. Am. J. Epidemiol. 2020, 189, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Mondul, A.M.; Watters, J.L.; Mannisto, S.; Weinstein, S.J.; Snyder, K.; Virtamo, J.; Albanes, D. Serum retinol and risk of prostate cancer. Am. J. Epidemiol. 2011, 173, 813–821. [Google Scholar] [CrossRef]
- Nash, S.H.; Till, C.; Song, X.; Lucia, M.S.; Parnes, H.L.; Thompson, I.M., Jr.; Lippman, S.M.; Platz, E.A.; Schenk, J. Serum Retinol and Carotenoid Concentrations and Prostate Cancer Risk: Results from the Prostate Cancer Prevention Trial. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1507–1515. [Google Scholar] [CrossRef]
- Cao, D.; Meng, Y.; Li, S.; Xin, J.; Ben, S.; Cheng, Y.; Wang, M.; Hua, L.; Cheng, G. Association study between genetic variants in retinol metabolism pathway genes and prostate cancer risk. Cancer Med. 2020, 9, 9462–9470. [Google Scholar] [CrossRef] [PubMed]
- Hartman, T.J.; Albanes, D.; Pietinen, P.; Hartman, A.M.; Rautalahti, M.; Tangrea, J.A.; Taylor, P.R. The Association between Baseline Vitamin E, Selenium, and Prostate cancer in the Alpha-Tocopherol, Beta-carotene Cancer Prevention Study. Cancer Epidemiol. Biomark. Prev. 1998, 7, 335–340. [Google Scholar]
- Weinstein, S.J.; Wright, M.E.; Lawson, K.A.; Snyder, K.; Mannisto, S.; Taylor, P.R.; Virtamo, J.; Albanes, D. Serum and dietary vitamin E in relation to prostate cancer risk. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Cai, Q.; Franke, A.A.; Steinwandel, M.; Wu, J.; Wen, W.; Zheng, W.; Blot, W.J.; Shu, X.O. Associations of subtype and isomeric plasma carotenoids with prostate cancer risk in low-income African and European Americans. Cancer Epidemiol. Biomark. Prev. 2021, 30, 1846–1857. [Google Scholar] [CrossRef]
- Peters, U.; Leitzmann, M.F.; Chatterjee, N.; Wang, Y.; Albanes, D.; Gelmann, E.P.; Friesen, M.D.; Riboli, E.; Hayes, R.B. Serum lycopene, other carotenoids, and prostate cancer risk: A nested case-control study in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol. Biomark. Prev. 2007, 16, 962–968. [Google Scholar] [CrossRef]
- Wu, K.; Erdman, J.W., Jr.; Schwartz, S.J.; Platz, E.A.; Leitzmann, M.; Clinton, S.K.; DeGroff, V.; Willett, W.C.; Giovannucci, E. Plasma and dietary carotenoids, and the risk of prostate cancer: A nested case-control study. Cancer Epidemiol. Biomark. Prev. 2004, 13, 260–269. [Google Scholar] [CrossRef]
- Beilby, J.; Ambrosini, G.L.; Rossi, E.; de Klerk, N.H.; Musk, A.W. Serum levels of folate, lycopene, beta-carotene, retinol and vitamin E and prostate cancer risk. Eur. J. Clin. Nutr. 2010, 64, 1235–1238. [Google Scholar] [CrossRef]
- Kagan, V.; Fabisiak, J.P.; Quinn, P.J. Coenzyme Q and vitamin E need each other as antioxidants. Proplasma 2000, 214, 11–18. [Google Scholar] [CrossRef]
- Rodick, T.C.; Seibels, D.R.; Babu, J.R.; Huggins, K.W.; Ren, G.; Mathews, S.T. Potential role of coenzyme Q10 in health and disease conditions. Nutr. Diet. Suppl. 2018, 10, 1–11. [Google Scholar] [CrossRef]
- Chai, W.; Cooney, R.V.; Franke, A.A.; Caberto, C.P.; Wilkens, L.R.; Le Marchand, L.; Goodman, M.T.; Henderson, B.E.; Kolonel, L.N. Plasma coenzyme Q10 levels and prostate cancer risk: The multiethnic cohort study. Cancer Epidemiol. Biomark. Prev. 2011, 20, 708–710. [Google Scholar] [CrossRef]
- Mondul, A.M.; Rohrmann, S.; Menke, A.; Feinleib, M.; Nelson, W.G.; Platz, E.A.; Albanes, D. Association of serum alpha-tocopherol with sex steroid hormones and interactions with smoking: Implications for prostate cancer risk. Cancer Causes Control 2011, 22, 827–836. [Google Scholar] [CrossRef]
- Leo, M.A.; Lieber, C. Alcohol, vitamin A, and beta-carotene: Adverse interactions, including hepatotoxicity and carcinogenicity. Am. J. Clin. Nutr. 1999, 69, 1071–1085. [Google Scholar] [CrossRef]
- Widome, R.; Jacobs, D.R., Jr.; Hozawa, A.; Sijtsma, F.; Gross, M.; Schreiner, P.J.; Iribarren, C. Passive smoke exposure and circulating carotenoids in the CARDIA study. Ann. Nutr. Metab. 2010, 56, 113–118. [Google Scholar] [CrossRef]
- Siems, W.; Sommerburg, O.; Schild, L.; Augustin, W.; Langhans, C.D.; Wiswedel, I. Beta-carotene cleavage products induce oxidative stress in vitro by impairing mitochondrial respiration. FASEB J. 2002, 16, 1289–1291. [Google Scholar] [CrossRef]
- Hemila, H. The effect of beta-carotene on the mortality of male smokers is modified by smoking and by vitamins C and E: Evidence against a uniform effect of nutrient. J. Nutr. Sci. 2020, 9, e11. [Google Scholar] [CrossRef] [PubMed]
- Grune, T.; Lietz, G.; Palou, A.; Ross, A.C.; Stahl, W.; Tang, G.; Thurnham, D.; Yin, S.A.; Biesalski, H.K. Beta-carotene is an important vitamin A source for humans. J. Nutr. 2010, 140, 2268S–2285S. [Google Scholar] [CrossRef] [PubMed]
- Das, B.C.; Thapa, P.; Karki, R.; Das, S.; Mahapatra, S.; Liu, T.C.; Torregroza, I.; Wallace, D.P.; Kambhampati, S.; Van Veldhuizen, P.; et al. Retinoic acid signaling pathways in development and diseases. Bioorg. Med. Chem. 2014, 22, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Schoeff, L. Vitamin A. Am. J. Med. Technol. 1983, 49, 447–452. [Google Scholar]
- Sen, C.K.; Khanna, S.; Roy, S. Tocotrienols: Vitamin E Beyond Tocopherols. Life Sci. 2006, 78, 2088–2098. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, H.; Ahad, A.; Iqbal, J.; Siddiqui, W.A. Pharmacological potential of tootrienols: A review. Nutr. Metab. 2014, 11, 52. [Google Scholar] [CrossRef]
- Abdulla, K.A.; Um, C.Y.; Gross, M.D.; Bostick, R.M. Circulating gamma-Tocopherol Concentrations Are Inversely Associated with Antioxidant Exposures and Directly Associated with Systemic Oxidative Stress and Inflammation in Adults. J. Nutr. 2018, 148, 1453–1461. [Google Scholar] [CrossRef]
- Cooney, R.V.; Franke, A.A.; Wilkens, L.R.; Gill, J.; Kolonel, L.N. Elevated plasma gamma-tocopherol and decreased alpha-tocopherol in men are associated with inflammatory markers and decreased plasma 25-OH vitamin D. Nutr. Cancer 2008, 60 (Suppl. 1), 21–29. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q. Natural forms of vitamin E: Metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic. Biol. Med. 2014, 72, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Carrico, C.; Gennings, C.; Wheeler, D.C.; Factor-Litvak, P. Characterization of Weighted Quantile Sum Regression for Highly Correlated Data in a Risk Analysis Setting. J. Agric. Biol. Environ. Stat. 2015, 20, 100–120. [Google Scholar] [CrossRef]
- Howe, C.G.; Li, Z.; Zens, M.S.; Palys, T.; Chen, Y.; Channon, J.Y.; Karagas, M.R.; Farzan, S.F. Dietary B Vitamin Intake is Associated with Lower Urinary Monomethyl Arsenic and Oxidative Stress Marker 15-F2t-Isoprostane among New Hampshire Adults. J. Nutr. 2017, 147, 2289–2296. [Google Scholar] [CrossRef]
- Jenab, M.; Slimani, N.; Bictash, M.; Ferrari, P.; Bingham, S.A. Biomarkers in nutritional epidemiology: Applications, needs and new horizons. Hum. Genet. 2009, 125, 507–525. [Google Scholar] [CrossRef]
- Shvetsov, Y.B.; Hernandez, B.Y.; Wong, S.H.; Wilkens, L.R.; Franke, A.A.; Goodman, M.T. Intraindividual variability in serum micronutrients: Effects on reliability of estimated parameters. Epidemiology 2009, 20, 36–43. [Google Scholar] [CrossRef] [PubMed]
- McNaughton, S.A.; Marks, G.C.; Gaffney, P.; Williams, G.; Green, A. Validation of a food-frequency questionnaire assessment of carotenoid and vitamin E intake using weighed food records and plasma biomarkers: The method of triads model. Eur. J. Clin. Nutr. 2005, 59, 211–218. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Cases (n = 156) | Controls (n = 118) | p Value a |
|---|---|---|---|
| Age | 1.1 × 10−5 | ||
| 50–59 years old | 33 (21.2) | 57 (48.3) | |
| 60–69 years old | 80 (51.3) | 37 (31.4) | |
| 70 years and above | 43 (27.6) | 24 (20.3) | |
| Ethnicity | 0.18 | ||
| Chinese | 140 (89.7) | 99 (81.4) | |
| Malay | 6 (3.8) | 9 (6.8) | |
| Indian | 8 (5.1) | 14 (11.0) | |
| Others | 2 (1.3) | 2 (0.8) | |
| Education | 0.0011 | ||
| Never | 3 (1.9) | 5 (4.2) | |
| 1–6 years | 28 (17.9) | 33 (28.0) | |
| 7–10 years | 55 (35.3) | 54 (45.8) | |
| >10 years | 70 (44.9) | 26 (26.0) | |
| Marital Status | 0.13 | ||
| Currently married | 147 (94.2) | 104 (88.1) | |
| Separated/ Widowed | 4 (2.6) | 9 (7.6) | |
| Never married | 5 (3.2) | 5 (4.2) | |
| Family history of cancer in first degree relatives | 6.4 × 10−5 | ||
| No | 84 (53.8) | 92 (78.0) | |
| Yes | 72 (46.2) | 26 (22.0) | |
| Body mass index (kg/m2) | 0.0096 | ||
| Quartile 1 (<22.0) | 43 (27.6) | 29 (24.6) | |
| Quartile 2 (22.0–24.9) | 63 (40.4) | 29 (24.6) | |
| Quartile 3 (25.0–27.9) | 30 (19.2) | 33 (28.0) | |
| Quartile 4 (≥28.0) | 20 (12.8) | 27 (22.9) | |
| Ever-smoker | 0.94 | ||
| No | 88 (56.4) | 64 (55.2) | |
| Yes | 68 (43.6) | 52 (44.8) | |
| Regular alcohol consumption | 0.21 | ||
| No | 81 (53.3) | 50 (44.6) | |
| Yes | 71 (46.7) | 62 (55.4) | |
| Eye colour | 1.28 × 10−8 | ||
| Light brown | 13 (8.3) | 44 (37.3) | |
| Black/dark brown | 143 (91.7) | 74 (62.7) | |
| Skin colour | 0.0012 | ||
| Very white/white | 15 (9.6) | 31 (26.3) | |
| Light tan | 86 (55.1) | 52 (44.1) | |
| Tan/dark Brown/black | 55 (35.3) | 35 (29.7) | |
| Sunburn exposure | 0.0017 | ||
| Never | 65 (41.7) | 69 (58.5) | |
| Seldom | 39 (25.0) | 33 (28.0) | |
| Occasionally | 24 (15.4) | 9 (7.6) | |
| Frequently | 28 (17.9) | 7 (5.9) |
| Serum Concentrations (µg/dL) | p Value | ||||||
|---|---|---|---|---|---|---|---|
| Total (n = 274) | Cases (n = 156) | Controls (n = 118) | |||||
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | ||
| Retinol | 75.3 (26.8) | 73.5 (34.9) | 83.1 (23.5) | 82.4 (28.9) | 65.0 (27.6) | 60.9 (38.3) | 4.1 × 10−9 |
| Lutein | 17.0 (9.0) | 15.4 (10.6) | 19.8 (9.5) | 17.4 (11.1) | 13.3 (6.6) | 11.8 (7.6) | 4.4 × 10−11 |
| Zeaxanthin | 4.6 (2.0) | 4.2 (2.1) | 4.6 (1.8) | 4.3 (2.0) | 4.6 (2.3) | 4.0 (2.5) | 0.27 |
| α-Cryptoxanthin | 88.0 (79.2) | 67.4 (87.7) | 96.3 (88.1) | 71.8 (94.3) | 77.1 (64.3) | 57.3 (76.6) | 0.15 |
| β-Cryptoxanthin | 19.4 (18.9) | 13.3 (17.8) | 22.9 (22.2) | 14.2 (19.7) | 14.8 (11.9) | 11.0 (12.4) | 0.0012 |
| α-Carotene | 1.0 (1.0) | 0.5 (0.7) | 1.2 (1.2) | 0.7 (1.0) | 0.7 (0.7) | 0.5 (0.1) | 5.3 × 10−8 |
| β-Carotene | 9.2 (8.9) | 6.7 (8.3) | 11.8 (9.2) | 9.6 (10.1) | 5.9 (7.2) | 4.2 (4.6) | 5.3 × 10−13 |
| Lycopene | 7.1 (5.2) | 5.6 (6.0) | 7.4 (5.4) | 5.9 (5.8) | 6.8 (5) | 5.3 (5.9) | 0.23 |
| Ubiquinone | 41.7 (18.5) | 38.2 (23.8) | 44.7 (20.2) | 41.8 (23.4) | 37.8 (15.3) | 35.8 (22.0) | 0.0043 |
| δ-Tocopherol | 11.9 (5.3) | 11.1 (6.0) | 11.9 (5.6) | 11.2 (6.4) | 11.9 (4.9) | 11.0 (5.6) | 0.76 |
| γ-Tocopherol | 61.4 (29.5) | 57.8 (39.9) | 58.9 (27.6) | 55.4 (34.7) | 64.8 (31.5) | 60.6 (43.5) | 0.10 |
| α-Tocopherol | 1560.4 (508.2) | 1497.7 (541.5) | 1669.5 (565.3) | 1569.1 (540.6) | 1416.1 (377.3) | 1402.6 (504.5) | 5.9 × 10−5 |
| δ-Tocotrienol | 1.3 (1.1) | 1.1 (0.7) | 1.5 (1.1) | 1.3 (0.8) | 1.2 (1.2) | 0.9 (0.6) | 7.2 × 10−6 |
| γ-Tocotrienol | 1.9 (2.2) | 1.3 (1.3) | 2.3 (2.8) | 1.5 (1.7) | 1.3 (0.8) | 1.2 (1.0) | 0.00030 |
| α-Tocotrienol | 1.9 (1.7) | 1.3 (1.4) | 2.3 (2.1) | 1.6 (1.9) | 1.4 (1) | 1.1 (1) | 0.00018 |
| Cases (n = 156) | Controls (n = 118) | |||
|---|---|---|---|---|
| n (%) | n (%) | Crude OR (95% CI) | Adjusted OR (95% CI) a | |
| Retinol | ||||
| ≤Median of 60.9 µg/dL | 20 (12.8) | 59 (50.0) | 1.00 (Ref) | 1.00 (Ref) |
| >Median of 60.9 µg/dL | 136 (87.2) | 59 (50.0) | 6.80 (3.76–12.30) | 6.08 (2.80–13.20) |
| Lutein | ||||
| ≤Median of 11.8 µg/dL | 34 (21.8) | 59 (50.0) | 1.00 (Ref) | 1.00 (Ref) |
| >Median of 11.8 µg/dL | 122 (78.2) | 59 (50.0) | 3.59 (2.12–6.06) | 2.07 (1.07–4.02) |
| Zeaxanthin | ||||
| Tertile 1 (≤3.3 µg/dL) | 34 (21.8) | 41 (34.7) | 1.00 (Ref) | 1.00 (Ref) |
| Tertile 2 (3.3–4.9 µg/dL) | 67 (42.9) | 38 (32.2) | 2.13 (1.16–3.89) | 1.35 (0.63–2.92) |
| Tertile 3 (>4.9 µg/dL) | 55 (35.3) | 39 (33.1) | 1.70 (0.92–3.14) | 1.32 (0.59–2.96) |
| P for trend b | 0.11 | 0.52 | ||
| α-Cryptoxanthin | ||||
| Tertile 1 (≤44.7 µg/dL) | 45 (28.8) | 40 (34.2) | 1.00 (Ref) | 1.00 (Ref) |
| Tertile 2 (44.7–82.9 µg/dL) | 43 (27.6) | 38 (32.5) | 1.01 (0.55–1.85) | 1.23 (0.55–2.73) |
| Tertile 3 (>82.9 µg/dL) | 68 (43.6) | 39 (33.3) | 1.55 (0.87–2.77) | 1.93 (0.91–4.08) |
| P for trend b | 0.13 | 0.084 | ||
| β-Cryptoxanthin | ||||
| Tertile 1 (≤7.8 µg/dL) | 29 (18.6) | 40 (33.9) | 1.00 (Ref) | 1.00 (Ref) |
| Tertile 2 (7.8–16.0 µg/dL) | 56 (35.9) | 39 (33.1) | 1.98 (1.06–3.71) | 1.80 (0.80–4.05) |
| Tertile 3 (>16.0 µg/dL) | 71 (45.5) | 39 (33.1) | 2.51 (1.35–4.66) | 1.96 (0.89–4.32) |
| P for trend b | 0.0045 | 0.11 | ||
| α-Carotene | ||||
| ≤Median of 0.50 µg/dL | 64 (41.0) | 86 (72.9) | 1.00 (Ref) | 1.00 (Ref) |
| >Median of 0.50 µg/dL | 92 (59.0) | 32 (27.1) | 3.86 (2.31–6.47) | 3.27 (1.67–6.38) |
| β-Carotene | ||||
| ≤Median of 4.2 µg/dL | 30 (19.2) | 60 (50.8) | 1.00 (Ref) | 1.00 (Ref) |
| >Median of 4.2 µg/dL | 126 (80.8) | 58 (49.2) | 4.34 (2.54–7.44) | 3.04 (1.53–6.02) |
| Lycopene | ||||
| Tertile 1 (≤3.9 µg/dL) | 47 (30.3) | 40 (34.5) | 1.00 (Ref) | 1.00 (Ref) |
| Tertile 2 (3.9–7.5 µg/dL) | 44 (28.4) | 38 (32.8) | 0.99 (0.54–1.80) | 0.96 (0.44–2.13) |
| Tertile 3 (>7.5 µg/dL) | 64 (41.3) | 38 (32.8) | 1.43 (0.80–2.57) | 0.92 (0.43–1.97) |
| P for trend b | 0.22 | 0.83 | ||
| Ubiquinone | ||||
| Tertile 1 ≤ (29.3 µg/dL) | 32 (20.5) | 40 (34.5) | 1.00 (Ref) | 1.00 (Ref) |
| Tertile 2 (29.3–41.2 µg/dL) | 45 (28.8) | 37 (31.9) | 1.52 (0.80–2.87) | 1.27 (0.57–2.82) |
| Tertile 3 (>41.2 µg/dL) | 79 (50.6) | 39 (33.6) | 2.53 (1.39–4.63) | 2.44 (1.13–5.28) |
| P for trend b | 0.0022 | 0.020 | ||
| δ-Tocopherol | ||||
| Tertile 1 (≤9.7 µg/dL) | 59 (37.8) | 40 (33.9) | 1.00 (Ref) | 1.00 (Ref) |
| Tertile 2 (9.7–13.1 µg/dL) | 45 (28.8) | 39 (33.1) | 0.78 (0.44–1.41) | 0.971 (0.46–2.04) |
| Tertile 3 (>13.1 µg/dL) | 52 (33.3) | 39 (33.1) | 0.90 (0.51–1.61) | 1.08 (0.51–2.28) |
| P for trend b | 0.72 | 0.84 | ||
| γ-Tocopherol | ||||
| Tertile 1 (≤47.2 µg/dL) | 54 (34.6) | 41 (34.7) | 1.00 (Ref) | 1.00 (Ref) |
| Tertile 2 (47.2–74.9 µg/dL) | 66 (42.3) | 38 (32.2) | 1.32 (0.75–2.33) | 1.72 (0.80–3.70) |
| Tertile 3 (>74.9 µg/dL) | 36 (23.1) | 39 (33.1) | 0.70 (0.38–1.29) | 0.60 (0.28–1.31) |
| P for trend b | 0.30 | 0.24 | ||
| α-Tocopherol | ||||
| Tertile 1 (≤1240 µg/dL) | 26 (16.7) | 40 (33.9) | 1.00 (Ref) | 1.00 (Ref) |
| Tertile 2 (1240–1560 µg/dL) | 51 (32.7) | 39 (33.1) | 2.01 (1.05–3.84) | 1.71 (0.75–3.87) |
| Tertile 3 (>1560 µg/dL) | 79 (50.6) | 39 (33.1) | 3.12 (1.67–5.82) | 2.40 (1.08–5.35) |
| P for trend b | 0.00041 | 0.034 | ||
| δ-Tocotrienol | ||||
| Tertile 1 (≤0.7 µg/dL) | 26 (16.7) | 42 (35.6) | 1.00 (Ref) | 1.00 (Ref) |
| Tertile 2 (0.7–1.1 µg/dL) | 39 (25.0) | 43 (36.4) | 1.47 (0.76–2.82) | 1.33 (0.58–3.04) |
| Tertile 3 (>1.1 µg/dL) | 91 (58.3) | 33 (28.0) | 4.45 (2.37–8.37) | 3.70 (1.66–8.25) |
| P for trend b | 1.3 × 10−6 | 0.00079 | ||
| γ-Tocotrienol | ||||
| Tertile 1 (≤0.9 µg/dL) | 43 (27.6) | 41 (34.7) | 1.00 (Ref) | 1.00 (Ref) |
| Tertile 2 (0.9–1.5 µg/dL) | 38 (24.4) | 43 (36.4) | 0.84 (0.48–1.55) | 0.992 (0.45–2.2) |
| Tertile 3 (>1.5 µg/dL) | 75 (48.1) | 34 (28.8) | 2.10 (1.17–3.79) | 3.02 (1.38–6.59) |
| P for trend b | 0.0098 | 0.0049 | ||
| α-Tocotrienol | ||||
| Tertile 1 (≤0.9 µg/dL) | 39 (25.0) | 44 (37.3) | 1.00 (Ref) | 1.00 (Ref) |
| Tertile 2 (0.9–1.5 µg/dL) | 35 (22.4) | 35 (29.7) | 1.13 (0.60–2.13) | 1.07 (0.49–2.41) |
| Tertile 3 (>1.5 µg/dL) | 82 (52.6) | 39 (33.1) | 2.37 (1.33–4.22) | 2.76 (1.30–5.87) |
| P for trend b | 0.0024 | 0.0066 |
| Smoking Status | Never-Smokers (n = 152) | Ever-Smokers (n = 120) | |||||
|---|---|---|---|---|---|---|---|
| Cases, n (%) | Controls, n (%) | OR (95%) CI a | Cases, n (%) | Controls, n (%) | OR (95%) CI a | P for Interaction b | |
| Lutein | |||||||
| ≤Median of 11.8 µg/dL | 18 (20.5) | 32 (50.0) | 1.00 (Ref) | 7 (10.3) | 27 (51.9) | 1.00 (Ref) | 0.011 |
| >Median of 11.8 µg/dL | 70 (79.5) | 32 (50.0) | 2.75 (1.12–6.73) | 61 (89.7) | 25 (48.1) | 6.43 (1.62–25.50) | |
| β-Cryptoxanthin | |||||||
| Tertile 1 (≤ 7.8 µg/dL) | 21 (23.9) | 23 (35.9) | 1.00 (Ref) | 11 (16.2) | 18 (34.6) | 1.00 (Ref) | 0.0054 |
| Tertile 2 (7.8–16.0 µg/dL) | 28 (31.8) | 20 (31.2) | 1.18 (0.41–3.35) | 25 (36.8) | 17(32.7) | 6.81 (1.37–33.90) | |
| Tertile 3 (>16.0 µg/dL) | 39 (44.3) | 21 (32.8) | 1.08 (0.40–2.95) | 32 (47.1) | 17 (32.7) | 7.50 (1.43–39.50) | |
| β-Carotene | |||||||
| ≤Median of 4.2 µg/dL | 22 (25.0) | 32 (50.0) | 1.00 (Ref) | 9 (13.2) | 26 (50.0) | 1.00 (Ref) | 0.011 |
| >Median of 4.2 µg/dL | 66 (75.0) | 32 (50.0) | 1.88 (0.80–4.41) | 59 (86.8) | 26 (50.0) | 5.61 (1.54–20.40) | |
| Alcohol consumption status | Non-regular alcohol drinkers (n = 131) | Regular alcohol drinkers (n = 133) | |||||
| Cases, n (%) | Controls, n (%) | OR (95%) CI a | Cases, n (%) | Controls, n (%) | OR (95%) CI a | P for interaction b | |
| Lutein | |||||||
| ≤Median of 11.8 µg/dL | 19 (23.5) | 25 (50.0) | 1.00 (Ref) | 12 (16.9) | 31 (50.0) | 1.00 (Ref) | 0.036 |
| >Median of 11.8 µg/dL | 62 (76.5) | 25 (50.0) | 1.81 (0.57–5.76) | 59 (83.1) | 31 (50.0) | 2.71 (1.04–7.10) | |
| β-Cryptoxanthin | |||||||
| ≤Median of 11.0 µg/dL | 39 (48.1) | 25 (50.0) | 1.00 (Ref) | 15 (21.1) | 32 (51.6) | 1.00 (Ref) | 0.044 |
| >Median of 11.0 µg/dL | 42 (51.9) | 25 (50.0) | 1.28 (0.44–3.72) | 56 (78.9) | 30 (48.4) | 2.96 (1.15–7.61) | |
| Ubiquinone | |||||||
| Tertile 1 ≤ (29.3 µg/dL) | 17 (21.0) | 17 (34.0) | 1.00 (Ref) | 16 (22.5) | 21 (33.9) | 1.00 (Ref) | 0.030 |
| Tertile 2 (29.3–41.2 µg/dL) | 36 (44.4) | 16 (32.0) | 1.53 (0.42–5.60) | 14 (19.7) | 20 (32.3) | 0.82 (0.24–2.84) | |
| Tertile 3 (>41.2 µg/dL) | 28 (34.6) | 17 (34.0) | 1.99 (0.48–8.23) | 41 (57.7) | 21 (33.9) | 3.35 (1.10–10.20) | |
| γ-Tocotrienol | |||||||
| Tertile 1 (≤0.9 µg/dL) | 22 (27.2) | 18 (36.0) | 1.00 (Ref) | 26 (36.6) | 23 (37.1) | 1.00 (Ref) | 0.026 |
| Tertile 2 (0.9–1.5 µg/dL) | 16 (19.8) | 16 (32.0) | 2.42 (0.59–9.87) | 11 (15.5) | 22 (35.5) | 0.47 (0.14–1.61) | |
| Tertile 3 (>1.5 µg/dL) | 43 (53.1) | 16 (32.0) | 2.71 (0.73–9.99) | 34 (47.9) | 17 (27.4) | 3.39 (1.08–10.6) | |
| α-Tocotrienol | |||||||
| Tertile 1 (≤0.9 µg/dL) | 20 (24.7) | 18 (36.0) | 1.00 (Ref) | 19 (26.8) | 25 (40.3) | 1.00 (Ref) | 0.021 |
| Tertile 2 (0.9–1.5 µg/dL) | 14 (17.3) | 16 (32.0) | 0.47 (0.11–2.03) | 21 (29.6) | 16 (25.8) | 1.58 (0.52–4.82) | |
| Tertile 3 (>1.5 µg/dL) | 47 (58.0) | 16 (32.0) | 1.46 (0.41–5.13) | 31 (43.7) | 21 (33.9) | 3.06 (1.03–9.10) | |
| OR (95% CI) a | |
|---|---|
| Vitamin A and provitamin A carotenoids index | 1.65 (1.36–1.99) |
| Vitamin E index | 1.47 (1.23–1.75) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loh, W.Q.; Yin, X.; Kishida, R.; Chia, S.E.; Ong, C.N.; Seow, W.J. Association between Vitamin A and E Forms and Prostate Cancer Risk in the Singapore Prostate Cancer Study. Nutrients 2023, 15, 2677. https://doi.org/10.3390/nu15122677
Loh WQ, Yin X, Kishida R, Chia SE, Ong CN, Seow WJ. Association between Vitamin A and E Forms and Prostate Cancer Risk in the Singapore Prostate Cancer Study. Nutrients. 2023; 15(12):2677. https://doi.org/10.3390/nu15122677
Chicago/Turabian StyleLoh, Wei Qi, Xin Yin, Rie Kishida, Sin Eng Chia, Choon Nam Ong, and Wei Jie Seow. 2023. "Association between Vitamin A and E Forms and Prostate Cancer Risk in the Singapore Prostate Cancer Study" Nutrients 15, no. 12: 2677. https://doi.org/10.3390/nu15122677
APA StyleLoh, W. Q., Yin, X., Kishida, R., Chia, S. E., Ong, C. N., & Seow, W. J. (2023). Association between Vitamin A and E Forms and Prostate Cancer Risk in the Singapore Prostate Cancer Study. Nutrients, 15(12), 2677. https://doi.org/10.3390/nu15122677








