The Impact of Moringa oleifera Supplementation on Anemia and other Variables during Pregnancy and Breastfeeding: A Narrative Review
Abstract
1. Introduction
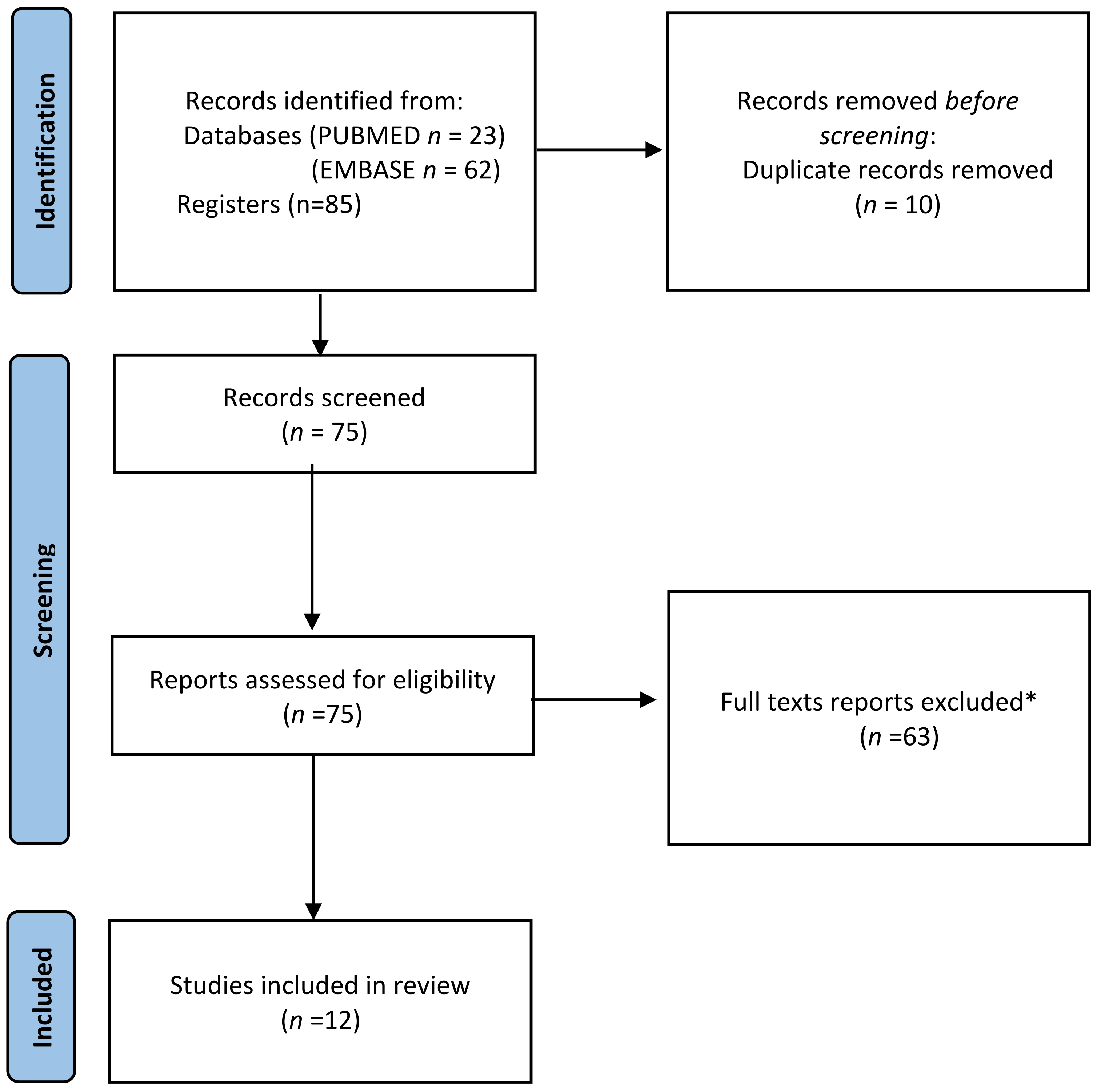
2. Materials and Methods
- (Moringa) AND (pregnancy)
- (‘Moringa’ OR moringa) AND (‘Pregnancy’ OR pregnancy)
- (Moringa) AND (breastfeeding)
- (‘Moringa’ OR moringa) AND (‘Breastfeeding’ OR breastfeeding)
- Population (P): pregnant women, mother–child pairs, and breastfeeding women.
- Exposure (E): Moringa intake.
- Comparison (C): pregnant women not consuming Moringa.
- Study design (S): cross-sectional studies, case–control studies, cohort studies, propensity matching studies, reviews, and experimental studies.
3. Results
3.1. Moringa and Pregnancy
3.2. Moringa and Breastfeeding
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013–2020; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- UNICEF. Breastfeeding: A Mother’s Gift, for Every Child; Nutrition Section, Programme Division, Data and Analytics Section, Division of Data, Research and Policy, and Division of Communication: New York, NY, USA, 2018; Volume 3. [Google Scholar]
- Mutar, Y.S.; Al-Rawi, K.F.; Mohammed, M.T. Moringa oleifera: Nutritive importance and its medicinal application, as a review. Egypt. J. Chem. 2021, 64, 6827–6834. [Google Scholar] [CrossRef]
- Pareek, A.; Pant, M.; Gupta, M.M.; Kashania, P.; Ratan, Y.; Jain, V.; Pareek, A.; Chuturgoon, A.A. Moringa oleifera: An updated comprehensive review of its pharmacological activities, ethnomedicinal, phytopharmaceutical formulation, clinical, phytochemical, and toxicological aspects. Int. J. Mol. Sci. 2023, 24, 2098. [Google Scholar] [CrossRef] [PubMed]
- Anwar, F.; Latif, S.; Ashraf, M.; Gilani, A.H. Moringa oleifera: A food plant with multiple medicinal uses. Phytother. Res. 2007, 21, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Stohs, S.J.; Hartman, M.J. Review of the safety and efficacy of Moringa oleifera. Phytother. Res. 2015, 29, 796–804. [Google Scholar] [CrossRef]
- Moyo, B.; Masika, P.J.; Hugo, A.; Muchenje, V. Nutritional characterization of Moringa (Moringa oleifera Lam.) leaves. Afr. J. Biotechnol. 2011, 10, 12925–12933. [Google Scholar]
- Saini, R.K.; Sivanesan, I.; Keum, Y. Phytochemicals of Moringa oleifera: A review of their nutritional, therapeutic and industrial significance. 3 Biotech 2016, 6, 203. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Arora, S. Nutritional significance and therapeutic potential of Moringa oleifera: The wonder plant. J. Food Biochem. 2021, 45, e13933. [Google Scholar] [CrossRef]
- Brar, S.; Haugh, C.; Robertson, N.; Owuor, P.M.; Waterman, C.; Fuchs III, G.J.; Attia, S.L. The impact of Moringa oleifera leaf supplementation on human and animal nutrition, growth, and milk production: A systematic review. Phytother. Res. 2022, 36, 1600–1615. [Google Scholar] [CrossRef]
- Basri, H.; Hadju, V.; Zulkifli, A.; Syam, A.; Indriasari, R. Effect of Moringa oleifera supplementation during pregnancy on the prevention of stunted growth in children between the ages of 36 to 42 months. J. Public Health Res. 2021, 10, 2207. [Google Scholar] [CrossRef]
- Kumar, V.; Sinha, A.K.; Makkar, H.P.; De Boeck, G.; Becker, K. Dietary roles of non-starch polysachharides in human nutrition: A review. Crit. Rev. Food Sci. Nutr. 2012, 52, 899–935. [Google Scholar] [CrossRef]
- Fouad, E.A.; Elnaga, A.S.A.; Kandil, M.M. Antibacterial efficacy of Moringa oleifera leaf extract against pyogenic bacteria isolated from a dromedary camel (Camelus dromedarius) abscess. Vet. World 2019, 12, 802. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Gadipudi, A. Iron deficiency anaemia in pregnancy: Developed versus developing countries. Hematology 2018, 6, 101–109. [Google Scholar] [CrossRef]
- Skolmowska, D.; Głąbska, D.; Kołota, A.; Guzek, D. Effectiveness of dietary interventions in prevention and treatment of iron-deficiency anemia in pregnant women: A systematic review of randomized controlled trials. Nutrients 2022, 14, 3023. [Google Scholar] [CrossRef] [PubMed]
- Nadimin, V.H.; As’ad, S.; Buchari, A.; Haruna, I.; Hartono, R. Increasing of nutrition status of pregnant women after supplementation of Moringa leaf extract (Moringa oliefera) in the Coastal Area of Makassar, Indonesia. Indian J. Public Health Res. Dev. 2019, 10, 521–525. [Google Scholar] [CrossRef]
- Mustapa, Y.; Hadju, V.; Indriasari, R.; Hidayanti, H.; Sirajuddin, S.; Russeng, S.S. The effect of Moringa oleifera to hemoglobin levels of preconception women in the health center Tibawa, district Tibawa, Gorontalo. Open Access Maced. J. Med. Sci. 2020, 8, 104–108. [Google Scholar] [CrossRef]
- Andira, A.; Hadju, V.; Ariyandi, A. The effect of extract Moringa oleifera leaves plus royal jelly on hematocrit level of anaemic pregnant women in Takalar District. Eur. J. Mol. Clin. Med. 2020, 7, 717–723. [Google Scholar]
- Hadju, V.; Marks, G.C.; Nontji, W.; Abdul Hafid, R.; Arundhana, A.I. Moringa oleifera leaf powder supplementation improved the maternal health and birth weight: A randomised controlled trial in pregnant women. Aust. J. Herb. Naturop. Med. 2020, 32, 94–101. [Google Scholar] [CrossRef]
- Manggul, M.S.; Hidayanty, H.; Arifuddin, S.; Ahmad, M.; Hadju, V.; Usman, A.N. Biscuits containing Moringa oleifera leaves flour improve conditions of anemia in pregnant women. Gac. Sanit. 2021, 35, S191–S195. [Google Scholar] [CrossRef]
- Loa, M.; Hidayanty, H.; Arifuddin, S.; Ahmad, M.; Hadju, V. Moringa oleifera leaf flour biscuits increase the index of erythrocytes in pregnant women with anemia. Gac. Sanit. 2021, 35, S206–S210. [Google Scholar] [CrossRef]
- Sari, K.; Sirajuddin, S.; Maddepungeng, M.; Hadju, V.; Saleh, A.; Tanziha, I.; Hastuti, H. Moringa oleifera intake during pregnancy and breastfeeding toward docosahexaenoic acid and arachidonic acid levels in breast milk. Open Access Maced. J. Med. Sci. 2020, 8, 757–761. [Google Scholar] [CrossRef]
- Kominiarek, M.A.; Rajan, P. Nutrition recommendations in pregnancy and lactation. Med. Clin. 2016, 100, 1199–1215. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Child Health and Human Development. Drugs and Lactation Database (LactMed). National Library of Medicine (US): Bethesda, MD, USA, 2006. Available online: https://www.ncbi.nlm.nih.gov/books/NBK501899/ (accessed on 27 May 2023).
- Suhartatik; Hadju, V.; Muis, M.; Ishak, H.; Adriani, M. The effect of Moringa oleifera flour given for mothers breastfeeding against morbidity of baby ages 0–6 months in Jeneponto district. Indian J. Public Health Res. Dev. 2020, 11, 1760–1765. [Google Scholar]
- Hastuti, H.; Hadju, V.; Citrakesumasari, C.; Maddeppungeng, M.; Tanziha, I.; Saleh, A.; Sari, K. The effect of Moringa oleifera on pregnant women and breastfeeding mothers toward social-personal development of children aged 18–23 months in Jeneponto, South Sulawesi. Open Access Maced. J. Med. Sci. 2020, 8, 747–751. [Google Scholar] [CrossRef]
- Pujiastuti, R.S.E.; Salsabila, D.I.B.; Anwar, M.C. Potential of Moringa leaf cookies to increase breastmilk production in postpartum mothers. Open Access Maced. J. Med. Sci. 2022, 10, 207–210. [Google Scholar] [CrossRef]
- Zakaria; Sirajuddin; Veni, H.; Burhanuddin, B.; Rosmini; Suryani, A.; Bohari; Siswanto, A.W. Linear growth of infants aged 0–6 months in breastfeeding mothers who consume Moringa oleifera leaf extract capsules: Randomized controlled double-blind design. Food Res. 2022, 6, 135–143. [Google Scholar] [CrossRef]
- Fungtammasan, S.; Phupong, V. The effect of Moringa oleifera capsule in increasing breast milk volume in early postpartum patients: A double-blind, randomized controlled trial. PLoS ONE 2022, 16, e0248950. [Google Scholar] [CrossRef]
- Bravi, F.; Wiens, F.; Decarli, A.; Dal Pont, A.; Agostoni, C.; Ferraroni, M. Impact of maternal nutrition on breast-milk composition: A systematic review. Am. J. Clin. Nutr. 2016, 104, 646–662. [Google Scholar] [CrossRef]
- Nakul, K.; Nakul, K.P.; Jayashree, M. Effect of maternal nutritional status on the human milk composition. J. Pediatr. Assoc. India 2018, 7, 94. [Google Scholar]
- Mushtaq, B.S.; Hussain, M.B.; Omer, R.; Toor, H.A.; Waheed, M.; Shariati, M.A.; Sergey, P.; Heydari, M. Moringa oleifera in malnutrition: A comprehensive review. Curr. Drug Discov. Technol. 2021, 18, 235–243. [Google Scholar] [CrossRef]
- Foong, S.C.; Tan, M.L.; Foong, W.C.; Marasco, L.A.; Ho, J.J.; Ong, J.H. Oral galactagogues (natural therapies or drugs) for increasing breast milk production in mothers of non-hospitalised term infants. Cochrane Database Syst. Rev. 2020, 5, CD011505. [Google Scholar] [PubMed]
- King, J.; Raguindin, P.F.; Dans, L.F. Moringa oleifera (Malunggay) as a galactagogue for breastfeeding mothers: A systematic review and meta-analysis of randomized controlled trials. Philipp. J. Pediatr. 2013, 61, 34–42. [Google Scholar]
- Estrella, M.C.P.; Jacinto Bias, V., III; David, G.Z.; Taup, M.A. A double-blind, randomized controlled trial on the use of Malunggay (Moringa oleifera) for augmentation of the volume of breastmilk among non-nursing mothers of preterm infants. Phillipp. J. Pediatr. 2000, 49, 3–6. [Google Scholar]
- Magtalas, M.C.; Balbin, P.T.; Cruz, E.C.; Guevarra, R.C.; Cruz, A.; Silverio, C.E.; Lee, K.Y.; Tantengco, O. A systematic review of ethnomedicinal plants used for pregnancy, childbirth, and postpartum care in the Philippines. Phytomed. Plus 2023, 3, 100407. [Google Scholar] [CrossRef]
- Sindhu, S.; Mangala, S.; Sherry, B. Efficacy of Moringa oleifera in treating iron deficiency anemia in women of reproductive age group. Int. J. Physiother. Res. 2013, 3, 15–20. [Google Scholar]
- Estiyani, A.; Suwondo, A.; Rahayu, S.; Hadisaputro, S.; Widyawati, M.N.; Susiloretni, K.A. The effect of Moringa oleifera leaves on change in blood profile in postpartum mothers. Belitung Nurs. J. 2017, 3, 191–197. [Google Scholar] [CrossRef]
- Khoja, K.K.; Aslam, M.F.; Sharp, P.A.; Latunde-Dada, G.O. In vitro bioaccessibility and bioavailability of iron from fenugreek, baobab and moringa. Food Chem. 2021, 335, 127671. [Google Scholar] [CrossRef]
- World Health Organization. Vitamin and Mineral Requirements in Human Nutrition; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Food and Agriculture Organization of the United Nations; International Fund for Agricultural Development; UNICEF; World Food Programme; World Health Organization. The State of Food Security and Nutrition in the World: Safeguarding against Economic Slowdowns and Downturns; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Victoria, G.C.; Adair, L.; Fall, C.; Challal, P.; Ritcher, L.; Sachdev, H. Maternal and child undernutrition 2 maternal and chils undernutrition: Consequences for adult health and human capital. Lancet Community Health 2008, 55, 394–398. [Google Scholar]
- Mariutti, L.R.B.; Rebelo, K.S.; Bisconsin-Junior, A.; de Morais, J.S.; Magnani, M.; Maldonade, I.R.; Madeira, N.R.; TIengo, A.; Maróstica, M.R.; Cazarin, C.B.B. The use of alternative food sources to improve health and guarantee access and food intake. Food Res. Int. 2021, 149, 110709. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rotella, R.; Soriano, J.M.; Llopis-González, A.; Morales-Suarez-Varela, M. The Impact of Moringa oleifera Supplementation on Anemia and other Variables during Pregnancy and Breastfeeding: A Narrative Review. Nutrients 2023, 15, 2674. https://doi.org/10.3390/nu15122674
Rotella R, Soriano JM, Llopis-González A, Morales-Suarez-Varela M. The Impact of Moringa oleifera Supplementation on Anemia and other Variables during Pregnancy and Breastfeeding: A Narrative Review. Nutrients. 2023; 15(12):2674. https://doi.org/10.3390/nu15122674
Chicago/Turabian StyleRotella, Rosita, Jose M. Soriano, Agustín Llopis-González, and María Morales-Suarez-Varela. 2023. "The Impact of Moringa oleifera Supplementation on Anemia and other Variables during Pregnancy and Breastfeeding: A Narrative Review" Nutrients 15, no. 12: 2674. https://doi.org/10.3390/nu15122674
APA StyleRotella, R., Soriano, J. M., Llopis-González, A., & Morales-Suarez-Varela, M. (2023). The Impact of Moringa oleifera Supplementation on Anemia and other Variables during Pregnancy and Breastfeeding: A Narrative Review. Nutrients, 15(12), 2674. https://doi.org/10.3390/nu15122674





